Open Access Article
Open Access ArticleRecent advances in green reagents for molecularly imprinted polymers
Xi Wu†
 *a,
Jiajun Du†b,
Mengyao Lia,
Lintao Wua,
Chun Hana and
Feng Sua
*a,
Jiajun Du†b,
Mengyao Lia,
Lintao Wua,
Chun Hana and
Feng Sua
aDepartment of Chemistry, Changzhi University, Changzhi 046011, China. E-mail: wuxi040252768@163.com; Fax: +86-355-2178113; Tel: +86-355-2178113
bDepartment of Medical Information, Chinese PLA General Hospital, 100853, Beijing, China
First published on 2nd January 2018
Abstract
Molecularly imprinted polymers (MIPs) are tailor-made materials with special binding sites. They have been extensively used in various areas because of its maturity. MIP is usually prepared by using a variety of “traditional” organic substances. In recent years, green reagents, such as room temperature ionic liquids (RTILs) and deep eutectic solvents (DESs), have been increasingly used in the preparation of MIPs. These new green reagents are applied for various purposes, i.e. to reduce pollution, to lower the cost and to improve the performance of materials. The RTILs- and DESs-based MIPs have attracted more and more attention because of their superior performance and interesting properties. We hereby propose to review recent advances in the application of RTILs and DESs to MIPs. The current status and perspectives of RTILs- and DESs-based MIPs are discussed in this brief review.
1. Introduction
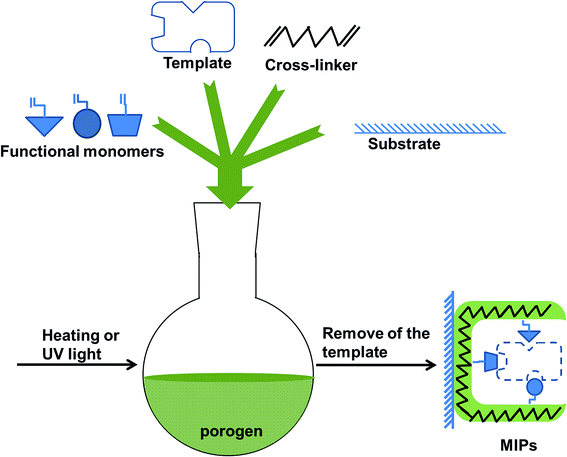
After decades of development, molecularly imprinted technology (MIT) has become a mature technique to prepare polymers with specific binding sites at the molecular level.1,2 This makes molecularly imprinted polymers (MIPs), tailor-made polymers, have excellent recognition ability to templates and their structural analogues. MIPs show many outstanding properties, such as low cost, good mechanical property, chemical stability and resistance to a wide range of pH, solvents, and temperatures. Hence, MIPs have been found applicable in many areas such as chemistry and biology for several years, and widely used in pretreatment of complicated samples in a variety of ways.3,4 MIPs can be synthesized by a variety of substances. Generally speaking, MIPs synthesis protocols must contain following essential ingredients: template, functional monomer, cross-linker, porogen (solvent) and initiator. There are two typical ways to prepare MIPs on the basis of interactions (covalent/non-covalent) between template and functional monomer.5,6 Owing to less restrictions and flexible application, non-covalent imprinting is far more popular than covalent imprinting. Normal procedures for the preparation of MIPs can be described as polymerization of functional monomer and cross-linker around a template in porogen (solvent). The general scheme for preparation of MIPs was shown in Fig. 1. To get MIPs with good performance in a particular environment, a good synthesis scheme is a prerequisite. Obviously, the first question to consider for MIPs preparation is the type of formulation components for MIPs. Up till now, the number of functional monomers, cross-linkers and porogens used in the preparation of MIPs is relatively limited.7 By contrast, the type of templates is multifarious, which includes ions, organic molecules, biomacromolecules and so on. It is implied that exploration of new polymer system is very important to enlarge the application fields of MIPs.Room temperature ionic liquids (RTILs) can be considered as molten salts with melting points close to room temperature.8 It is a new type of reagents with unique and interesting characteristics, such as non-volatility, non-flammability, high ion density, good ionic conductivity, and enhanced dispersibility in inorganic/organic solvents. Hence, RTILs have received extensive attention from chemistry research workers. For example, RTILs can be applied in sample preparation,9 chromatography,10 capillary electrophoretic separation11 and reaction media.12 As a result, RTILs become popular in the molecular imprinting field. At present, owing to their low vapour pressure and high boiling point that facilitates their recycling, RTILs are qualified as green solvents.13 However, some reports show that most RTILs have hazardous toxicity and poor biodegrade ability.14,15 In addition, RTILs are very expensive. These drawbacks limit the application of RTILs to some extent.
To overcome the shortcomings of RTILs, deep eutectic solvents (DESs) have emerged16 as a new generation of green solvents. A DES is usually composed of two components through hydrogen bond interaction.17 The two components are defined as hydrogen bond acceptor (HBA) and hydrogen bond donor (HBD), respectively. Especially since choline chloride (ChCl) has the advantages of low toxicity, biodegradability, being cheap and readily available, it has become one of the most popular HBAs among existing DESs.18–20 For a ChCl-based DES, the most common HBDs are urea, amines, amides, alcohols and organic acids. Furthermore, it is worth pointing out that the DES preparation process is simple and environment-friendly. In a word, DESs share characteristics with RTILs and are more inexpensive and safe. Hence, application of DESs in the field of chemistry has been a hot research topic. Until now, only a small number of investigations on the application of DESs in molecular imprinting have been performed.
Green reagents as RTILs and DESs have been increasingly employed in MIP synthesis protocols and play several different roles in the MIPs preparation process. In current studies, RTILs and DESs can be used as functional monomer, porogen, pore template and so on. Relevant MIPs show excellent performance in many ways. For example, MIPs based on RTILs or DESs have excellent recognition ability to template in aqueous environment.21,22 To find green reagents as replacements of conventional organic solvents has become trends of MIT research. Hence, we focus on the application of RTILs and DESs in preparing MIPs in this brief review. Furthermore, we also focus our interest on the performance of RTILs and DESs-based MIPs in this paper. The assessment of MIPs performance is a significant work and there are many evaluation methods of imprinted materials. For example, the morphologies of MIPs are usually characterized by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The selectivity, specificity and binding capacity of the imprinted materials can be investigated via static equilibrium adsorption experiments and absorption kinetic experiments. In present review, the studies on green reagents-based MIPs and current trends in the area are discussed.
2. RTILs as functional monomer for MIPs
During the process of preparation of MIPs for a specific template, selection of functional monomer is very critical to getting good imprinting effect. So far as we know, small organic molecules, metal ions and biomolecules can be potential templates. However, the type of functional monomer is quite limited. Hence, the investigation of new functional monomers is important for the future development of MIPs. Researches in recent years show that RTILs can interact with multiple molecules such as organic compounds and biomacromolecules by hydrogen bonding, anion-exchange, electrostatic, hydrophobic and π–π interactions.23,24 Regarded as “designer solvents”, RTILs offer a large degree of flexibility, which makes them suitable for different purposes in MIT. Related researches have shown that RTIL-based MIPs have significant performance in aqueous media.Wang et al. proposed a new method of using vinylimidazolium RTIL as a functional monomer for synthesis of chlorsulfuron MIPs.21 In this study, 1-vinyl-3-butylimidazolium chloride ([VBIM]Cl) was first taken as a unique functional monomer as the authors claimed. The novel MIP material was synthesized by bulk polymerization and had shown excellent selectivity and good adsorption/desorption for template. The binding selectivity of the prepared MIPs was evaluated by competitive adsorption using the mixture solution composed of chlorsulfuron and its analogues. The new MIP material showed excellent selectivity of 47.2% for chlorsulfuron, which was higher than that for the analogues. Moreover, it is worth mentioning that low concentration template can be detected by the new material in aqueous medium.
Similarly, a high capacity hollow porous dummy MIPs was fabricated using RTIL as functional monomer by Shi et al.25 The hollow MIPs were prepared using benzoic acid as dummy template, 1-vinyl-3-methylimidazolium chloride ([VMIM]Cl) as functional monomer for recognition of salicylic acid in Actinidia chinensis extract. The highest adsorption capacity (29.75 mg g−1) and imprinting factor (IF) (5.61) of the hollow MIPs can be achieved with template![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) functional monomer
functional monomer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cross-linker at 1
cross-linker at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. The effect of copolymerization with “traditional” functional monomer (4-vinylpyridine, 4-VP) was also tested. The adsorption capacity of template on MIPs decreased to 22.61 mg g−1 when 4-VP was used as functional monomer. In other words, [VMIM]Cl was more efficient than “traditional” functional monomer in this study. The results proved that the resulting MIPs can selectively extraction of trace salicylic acid from complex fruit sample more effectively than MIPs prepared via a conventional formula.
20. The effect of copolymerization with “traditional” functional monomer (4-vinylpyridine, 4-VP) was also tested. The adsorption capacity of template on MIPs decreased to 22.61 mg g−1 when 4-VP was used as functional monomer. In other words, [VMIM]Cl was more efficient than “traditional” functional monomer in this study. The results proved that the resulting MIPs can selectively extraction of trace salicylic acid from complex fruit sample more effectively than MIPs prepared via a conventional formula.
Furthermore, four specific ionic liquids were prepared and used as functional monomers for synthesis of synephrine MIPs by Fan et al.26 The molecular structure of the novel functional monomers named Brønsted acidic imidazolium-typed RTILs was shown in Fig. 2. Obviously, the significant difference among the four RTILs is the side-chain (carboxylic acid group) length. The results showed that MIP using 1-viny-3-carboxybutylimidazolium bromide ([COOHavim]Br) as the monomer had the best adsorption capacity. The authors suggest that interactions between synephrine and RTIL functional monomers are not only the π–π interactions between the phenyl group (synephrine) and imidazolyl (RTILs) but also the hydrogen bond interactions between the amine/hydroxyl group (synephrine) and the carboxylic acid group (RTILs). Moreover, the MIPs prepared with RTIL functional monomers showed good adsorption properties for synephrine in methanol aqueous solution, which is significant for enlarging the applications of MIP in aqueous medium.
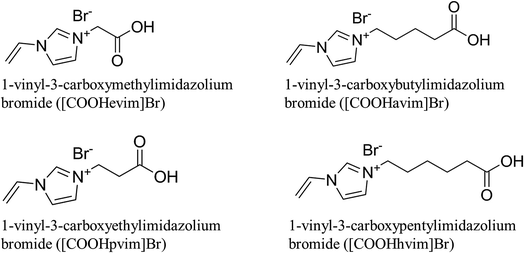 | ||
| Fig. 2 The molecular structure of the new RTIL functional monomers.26 | ||
The effect of different functional groups on RTILs was also investigated by Row et al.27 In their study, the RTIL-modified porous polymers were prepared for effective separation of three tanshinones from Salvia miltiorrhiza Bunge. The preparation process mainly consists of two steps: first, blank polymer was synthesized by using 4-(chloromethyl) styrene as monomer, divinylbenzene (DVB) as cross linker, heptane as porogen and polyvinylpyrrolidone (PVP) ethanol solution as dispersant, respectively. Then, taking 9,10-phenanthrenequinone and RTILs as the template and functional monomers, the IL-modified polymers were obtained by surface imprinting method. Five RTILs were used to modify porous polymers and the chemical structures of all RTILs were shown in Fig. 3. The interactions between three tanshinones and the five resulting polymers, imidazole polymer, methylimidazole polymer, carboxyl-imidazole polymer, amino-imidazole polymer, cyano-imidazole polymer, were investigated. The results showed that the carboxyl-imidazole polymer possessed the best performance. Under optimized conditions, 0.35 mg g−1 of cryptotanshinone, 0.33 mg g−1 of tanshinone I, and 0.27 mg g−1 of tanshinone IIA were obtained from plant extract. This research provides further evidence that functional groups on RTILs have played a significant role in the formation of template–functional monomer complex.
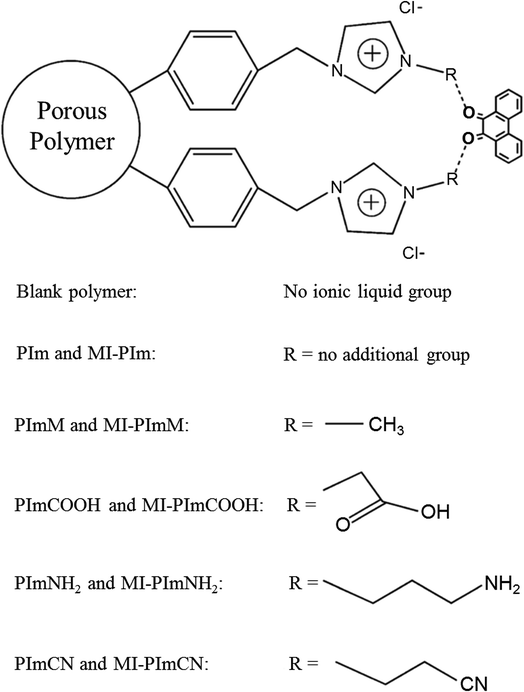 | ||
| Fig. 3 The structures of all RTILs and the relevant porous polymers. Reprinted from ref. 27. | ||
To date, researches are very active on the imprinting of small organic molecules and a large number of relevant studies are published every year. By contrast, preparation of MIPs for large molecules such as polypeptide, enzyme and proteins has been seen as one of the toughest challenges in MIT. One of the reasons is lack of suitable functional monomer for large molecules. The introduction of RTILs is an alternative way to solve this problem. Thymopentin (TP5) is considered as a biomacromolecule that consists of five amino acids. It is difficult to extract and purify TP5 from complex biological samples. Moreover, there are many different kinds of RTILs and it is difficult to find the best RTIL functional monomer for a certain template. In this case, with the help of computational simulation, Wang et al. proposed an approach to synthesize TP5 magnetic MIPs by surface-initiated ATRP polymerization.28 TP5, ethylene glycol dimethacrylate (EDMA), CuCl, Fe3O4–Br and N,N,N′,N′,N′′-pentamethyldiethylene triamine (PMDETA) were used as template, cross-linker, catalyst, initiators and ligand, respectively. Four RTILs, 1-vinyl-3-butyl imidazolium chloride ([VBIM]Cl), 1-vinyl-3-propyl imidazolium chloride ([VPIM]Cl), 1-vinyl-3-ethyl acetate imidazolium chloride and 1-vinyl-3-ethanamide imidazolium chloride, were investigated as candidate functional monomers. In this study, to find the best scheme of imprinting protocol, molecular dynamics (MD) simulations which was frequently used for computational design of MIPs was employed for researching the interactions between template and functional monomer.29 The interaction energies of all candidate functional monomers with TP5 were obtained by MD simulation. In the simulation, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 template–monomer complex in water was taken as a simulated system to reduce computation load. The binding energy (ΔE) of template with each RTIL functional monomer was obtained according to the following formula:
1 template–monomer complex in water was taken as a simulated system to reduce computation load. The binding energy (ΔE) of template with each RTIL functional monomer was obtained according to the following formula:
| ΔE = E(template–functional monomer) − E(template) − E(functional monomer) | (1) |
In the formula, E(template–functional monomer), E(template) and E(functional monomer) were potential energy of the simulated system, potential energy of the template and potential energy of the functional monomer, respectively. The results showed that the binding energy of TP5 with [VBIM]Cl, [VPIM]Cl, 1-vinyl-3-ethanamide imidazolium chloride and 1-vinyl-3-ethyl acetate imidazolium chloride was −16.02, −18.80, −22.18 and −24.37, respectively. It demonstrated that 1-vinyl-3-ethyl acetate imidazolium chloride had higher interaction energy with template. It also proved that 1-vinyl-3-ethyl acetate imidazolium chloride was the best functional monomer for TP5. The optimized template–functional monomer structure was shown in Fig. 4. Then, the TP5 magnetic MIPs were prepared according to the results of simulation. The results showed that the IF of TP5 magnetic MIPs can reach 1.76. The morphological structures of magnetic materials were characterized by TEM and the magnetic MIPs had regular cavities and small size. It means that the RTIL-based magnetic MIPs have good specific recognition to TP5.
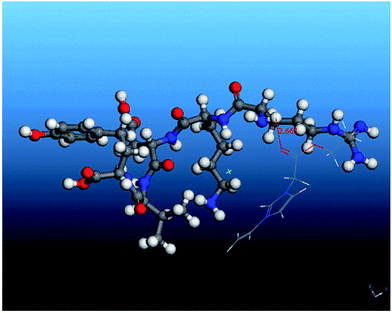 | ||
Fig. 4 The optimized structure of the template–functional monomer complex (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) formed between TP5 and RTIL. Reprinted from ref. 28. 1) formed between TP5 and RTIL. Reprinted from ref. 28. | ||
Furthermore, L-phenylalanine imprinted microspheres were prepared by the same group.30 In this study, a new amino acid RTIL named 1-butyl-3-vinyl imidazolium amino hydrocinnamic acid ([BVIM][Phe]) was synthesized and used not only as a functional monomer but also as a dummy template. Similar to the prior research,28 a theoretical computational method was taken to find the template–functional monomer complex with the highest binding energy. In this study, surface-initiated RAFT polymerization was employed for the preparation of PheIL-MIPs. For the comparison of selectivity of the obtained PheIL-MIPs, the traditional L-Phe MIPs were prepared with 4-VP as the functional monomer under the optimized condition of PheIL-MIPs. The amount of L-Phe bound to PheIL-MIPs and Phe-MIPs was approximately 53 and 28 μmol g−1 and the corresponding IFs were approximately 2.5 and 1.4, respectively. The results demonstrated that the proposed PheIL-MIPs has good affinity and selectivity for L-phenylalanine by using [BVIM][Phe] as functional monomer and dummy template.
RTILs were introduced to prepare bovine serum albumin (BSA) MIPs with an effective molecular interaction between RTILs and BSA by Liu et al.31 In this study, a MWCNTs@BSA-MIPILs film was synthesized by sol–gel technology. Alkoxy-functionalized RTIL 1-(3-trimethoxysilyl propyl)-3-methyl imidazolium chloride ([TMSPMIM]Cl) was used as monomer and multiwall carbon nano-tubes (MWCNTs) was taken as substrate. The synthesis scheme of the MWCNTs@BSA-MIPILs was shown in Fig. 5. The polymer showed good binding ability to BSA and its analogues under optimal conditions. The IFs for BSA, human serum albumin (HAS) and bovine hemoglobin (BHb) were 5.84, 2.24 and 1.04 at pH 9.9, respectively. It is worth reminding that the prepared MWCNTs@BSA-MIPILs film is pH-responsive. The result means that the functionalized RTIL can not only be used as functional monomer but also hydrolysis catalyst in sol–gel system.
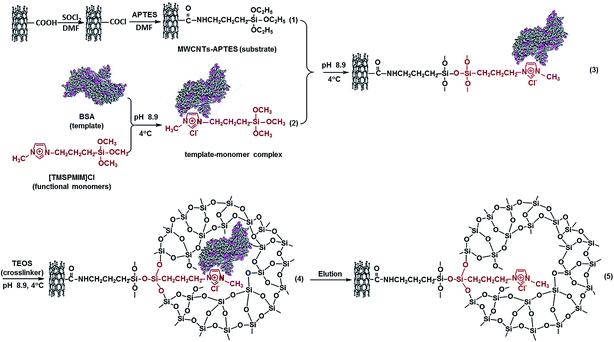 | ||
| Fig. 5 The synthesis scheme of the MWCNTs@BSA-MIPILs. Reprinted from ref. 31. | ||
Qian et al. designed a new RTIL named 1-vinyl-3-amino formylmethyl imidazolium chloride ([VAFMIM]Cl) and used it to prepare BSA molecular imprinted hydrogels (MIHs).32 In this study, RTIL acted both as the co-monomer and stabilizer. More specifically, [VAFMIM]Cl has two functional groups (amide group and imidazolium ring) with a positive charge which can interact with the functional groups of BSA. Hence, it was utilized as monomer along with N-isopropylacrylamide (NIPA) in the study. Furthermore, the influence of [VAFMIM]Cl on the stability of BSA was investigated by circular dichroism. It is indicated that Cl− in [VAFMIM]Cl is responsible for the stability of BSA. Moreover, the results also indicated that the MIHs composed of RTIL exhibited better recognition ability compared to the conventional ones. The IF value of MIH based on [VAFMIM]Cl could reach 2.66, whereas, the MIH based on the conventional monomers, 2-(dimethylamino)ethylmethacrylate (DMAEMA), has a relatively lower IF value (1.56).
Furthermore, the lysozyme-MIPs were prepared by Wang and co-workers33 with [VBIM]Cl as functional monomer, lysozyme as template, N,N′-methylenebisacrylamide (MBA) and MWCNTs as cross-linker and substrate. To prepare lysozyme-imprinted polymer, [VBIM]Cl, 1-vinyl-3-octyl imidazolium chloride ([VOIM]Cl) and vinyl imidazole were studied as candidate functional monomers in this work. The results demonstrated that the [VBIM]Cl-based MIPs had a higher IF in all tested MIPs. The above results imply that RTILs can be used as functional monomer to improve recognition performance of MIPs and it will have a wonderful prospect in the field of large molecule imprinting. More reports about successful applications of RTILs in MIPs are shown in Table 1.
| RTIL | Protocol of preparing MIPs | Comments | Ref. |
|---|---|---|---|
| 1-(Triethoxysilyl) propyl-3-aminopropylimidazole bromide (SilprImN) | Dummy template: BPA | By a sol–gel process, the MIPs was prepared for the simultaneous determination of nine organochlorine pesticides in environmental and food samples | 34 |
| Functional monomer: SilprImN | |||
| Cross-linker: TEOS | |||
| Supported material: activated silica gel | |||
| Solvent: tetrahydrofuran/methanol | |||
| 1-Allyl-3-ethylimidazolium bromide ([AEIM]Br) | Dummy template: phenylephrine | A dummy molecularly imprinted microspheres (DMIMs) were synthesized for the determination of clenbuterol and clorprenaline in urine by aqueous suspension polymerization | 35 |
| Co-monomer: [AEIM]Br/MAA | |||
| Cross-linker: EDMA | |||
| Solvent: chloroform | |||
| Dispersant: PVP aqueous solution | |||
| 1-Allyl-3-ethylimidazolium hexafluorophosphate ([AEIM][PF6]) | Dummy template: 4,4-dichlorobenzhydrol | By aqueous suspension polymerization, a magnetic molecularly imprinted microspheres was synthesized for determination of organochlorine pesticides in environmental water | 36 |
| Co-monomer: [AEIM][PF6]/MAA | |||
| Cross-linker: EDMA | |||
| Magnetic material: Fe3O4@oleic acid | |||
| Solvent: chloroform | |||
| Dispersant: PVP aqueous solution | |||
| 1-Vinyl-3-ethyl acetate imidazolium chloride ([VEAIM]Cl) | Template: thymopentin | By surface-initiated ATRP in aqueous solution, a molecularly imprinted membranes (MIMs) was proposed for isolation and purification of crude thymopentin. The MD simulations were performed for simulating the optimized imprinting protocol | 37 |
| Functional monomer: [VEAIM]Cl | |||
| Cross-linker: MBA | |||
| Ligand: PMDETA | |||
| Catalyst: CuCl | |||
| Solvent: water | |||
| 1-Butyl-3-vinyl imidazolium α-aminohydrocinnamic acid salt ([BVIM][Phe]) | Dummy template/functional monomer: [BVIM][Phe] | By a RAFT approach, IL-based L-Phe MIPs was prepared for determination of L-Phe inhuman serum. The polymerizable IL, [Bvim][Phe], was synthesized and used as dummy template and functional monomer. The MD simulations were utilized for evaluating the template–functional monomer complex | 38 |
| Co-monomer: 4-VP | |||
| Supported material: PDVB microspheres | |||
| Cross-linker: EDMA | |||
| Solvent: acetonitrile–water | |||
| 1-Allyl-3-methyl imidazolium bromide ([AMIM]Br) | Dummy template: FHO | By precipitation polymerization, an IL-based MIPs was utilized as a selective adsorbent for extraction and determination of CFO in urine | 39 |
| Co-monomer: [AMIM]Br/acrylamide | |||
| Cross-linker: DVB | |||
| Solvent: acetonitrile | |||
| 1-Allyl-3-methyl imidazolium X, (X = X = Cl−, PF6−, CF3SO3−, BF4−) ([AMIM]Cl, [AMIM][BF4], [AMIM][PF6], [AMIM][CF3SO3]) | Template: BSA | By free radical polymerization and surface imprinting technique, a biocompatible MWCNTs@BSA-MIPILs with satisfactory selective recognition ability for BSA was prepared. Four ILs with different anion species as monomers were investigated and the materials prepared with PF6− and CF3SO3− based IL monomers were confirmed to have the better performance | 40 |
| Functional monomer: [AMIM][X](X = Cl−, PF6−, CF3SO3−, BF4−) | |||
| Cross-linker: NNMBA | |||
| Substrate: AAm modified MWCNTs | |||
| Solvent: phosphate buffered solution | |||
| Catalyst: TEMED | |||
| 3-Propyl-1-vinyl imidazolium bromide ([C3VIM]Br) | Template: amoxicillin | In this paper, IL was grafted on MWCNT surface by using ionic exchange strategy and the resulting MWCNTs@IL was taken as monomer to prepare MWCNTs@MIP. Then, the MWCNTs@MIP was coated on dendritic Pt–Pd nanoparticle to constructed the electrochemical sensor. The obtained sensor showed sensitive and selective response to amoxicillin | 41 |
| Functional monomer: MWCNTs@[C3VIM]Br | |||
| Cross-linker: EDMA | |||
| Solvent: methanol–water | |||
| 1-Allyl-3-ethyl imidazolium bromide ([AEIM]Br) 1-allyl-3-butyl imidazolium chloride ([ABIM]Cl), 1-allyl-3-hexyl imidazolium chloride ([AHIM]Cl), 1-allyl-3-octyl imidazolium chloride ([AOIM]Cl) | Template: phenolic acid | A new material, ionic liquid-based molecularly imprinted anion-exchange polymer was synthesized and used for separation of phenolic acids from Salicornia herbacea L. extract. Four ILs and four solvent systems were studied and the [AEIM]Br and Ethanol/H2O were considered as the optimal monomer and porogen, respectively | 42 |
| Functional monomer: [AEIM]Br, [ABIM]Cl, [AHIM]Cl, [AOIM]Cl | |||
| Cross-linker: EDMA | |||
| Solvent: ethanol/H2O, isopropanol/H2O, n-butanol/H2O, n-hexanol/H2O | |||
| Mono-6A-deoxy-6-(1-vinyl imidazolium)-β-cyclodextrin tosylate | Template: C-terminal peptides of Cyt C | A novel IL-based functional monomer was synthesized and used for the preparation of Fe3O4@EMIPs by using the combination of epitope and surface imprinting approach. The novel material has a good selectivity, adsorption capacity and recognition ability on Cyt C | 43 |
| Functional monomer: mono-6A-deoxy-6-(1-vinylimidazolium)-β-cyclodextrin tosylate | |||
| Cross-linker: MBA | |||
| Magnetic substrate: Fe3O4@SiO2–MPS | |||
| Solvent: phosphate buffer |
3. RTILs as porogens for MIPs
Porogens play an important role in a scheme for MIPs. For preparation of MIPs, all the components in the scheme should be dissolved in porogens at first and template–monomer complex will form by covalent or non-covalent interactions. It means that the porogens are chosen according to their solubility and its effect on the template–monomer complex formation. Interestingly, MIPs prepared with RTILs as porogens have several special characteristics.According to the previous study,21 MIPs prepared with RTIL as functional monomer have impressive performance in aqueous medium. Yan et al. proposed a new method to prepare dicofol MIPs with a novel synthesized RTIL (1-allyl-3-methyl imidazolium bromide, [AMIM]Br) as porogen.44 The results showed that RTIL played a key function in improving performance of MIPs. On one hand, the Cl− of template could be interacted with the imidazole with positive charge of RTIL by electrostatic interaction. Meanwhile, cross linking could take place between RTIL and functional monomer by using DVB. Hence, RTIL can help to form the specific binding sites in the process of MIPs preparation and the degree of shrinking or swelling of MIPs can be reduced. It is verified that the RTIL-MIPs possess superior properties in polar conditions, and efficient and selective extraction of dicofol from vegetable sample can be achieved by the proposed method. The results showed that the recoveries of dicofol at the spiked levels of 2.3, 23.2, and 232.5 ng g−1 ranged from 86.6% to 101.9% with RSDs of less than 6.5%. Liu et al. also confirmed that shrinking or swelling of MIPs could be reduced by using RTIL as porogens.45 They developed a new MIPs chiral stationary phase for HPLC. The MIP monoliths were prepared with R-mandelic acid as template, 4-VP as functional monomer, EDMA as cross-linker, metal ions as metal pivot and a mixed solvent as porogen. The porogenic system contains 1-butyl-3-methyl imidazolium tetrafluoroborate ([BMIM][BF4]), sulfoxide and dimethylformamide. The authors noted that the use of porogen without RTIL resulted in an MIP monolith with cracks. It indicates that the RTILs have significant influence on MIPs morphology.
It is clear that, as a new porogen, RTILs can influence the MIPs system. However, most studies failed to make a thorough exploration of the effect mechanisms of RTIL. Taking propranolol as a model template, McCluskey et al. made a beneficial trial to explain the functions of the RTILs in the process of synthesis MIPs.46 Four RTILs, [BMIM][BF4], 1-butyl-3-methyl imidazolium hexfluorophosphate ([BMIM][PF6]), 1-hexyl-3-methyl imidazolium hexfluorophosphate ([HMIM][PF6]), 1-octyl-3-methyl imidazolium hexfluorophosphate ([OMIM][PF6]) and one traditional organic solvent, CHCl3, were taken as porogens for propranolol MIPs and the effect of the RTILs on MIP characters and performance was investigated. The results showed that MIPs prepared with CHCl3 (MIPCHCl3) have higher imprinting selectivity (IF = 4.64) than MIPs prepared with [BMIM][PF6] (MIPPF6) (IF = 1.98). The differences between NIP/MIPCHCl3 and NIP/MIPPF6 in physicochemical properties were investigated by means of several analysis methods, such as Brunauer–Emmett–Teller (BET) specific surface area, BET pore volume, positron annihilation lifetime spectroscopy (PALS) diameter of small/large pores, relative number of small/large pores and zeta potential. They found some interesting phenomenons in this study. For example, the polymer morphology can be influenced by template only in the traditional organic solvent systems. The BET specific surface areas of MIPCHCl3 and NIPCHCl3 were 306 and 509 m2 g−1. In contrast, the surface areas of MIP and NIP were essentially identical in the RTIL systems (185 and 180 m2 g−1). Furthermore, the investigation demonstrated that the swelling ratios of MIPs prepared by [BMIM] and [HMIM] based systems were 34% and 33%, respectively. Meanwhile, the [OMIM][PF6]-prepared MIP showed only 6% swelling. The thermal stability of the polymers was increased in the order of [HMIM] ≈ [OMIM] > [BMIM]. It means that the swelling ratio of MIPs decreased and the thermal stability of MIPs increased with increasing alkyl chain length of RTILs. In addition, rebinding studies show that the MIP prepared with OMIM system has a higher IF (2.1) than the MIP prepared with HMIM system (IF = 1.1).
Moreover, Liu et al. proposed a new porogenic system based on RTIL to increase the affinity of MIPs.47 The porogen was a ternary mixture of [BMIM][BF4], dimethyl sulfoxide and macromolecular crowding agent (polymethyl methacrylate) chloroform solution. In this study, with oleanic acid as template, 4-VP as functional monomer, EDMA as cross-linker, a MIP monolith was prepared in the novel porogenic solvent. The type of RTILs is one important polymerization variable and the influence of [BMIM][BF4], [BMIM][PF6], 1-butyl-3-methyl imidazolium hydrogen sulfate ([BMIM][HSO4]) and 1-octyl-3-methyl imidazolium tetrafluoroborate ([OMIM][BF4]) on the performance of MIPs were investigated. The results were shown in Fig. 6. It is clear that the MIPs prepared with [BMIM][BF4]-based porogen has the best affinity to the template. Compared with MIPs made with the [BMIM][BF4] porogen system, the selectivity of the [OMIM][BF4] porogen-based MIPs is limited. It is suggested that the length of the cation alkyl chain may have a significant effect on the interactions between the template and functional monomer. Moreover, it should be noted that the anions in the four RTILs also have an influence on the selectivity of the MIPs. The MIP with [HSO4]− in place of [BF4]− in [BMIM][BF4] showed no selectivity for the template (IF = 1.06). The results showed that the IF value of the polymers decreased in the order of [HSO4]− < [PF6]− < [BF4]−. Furthermore, Wu et al. fabricated a MIP for extraction of four fluoroquilones from real aqueous samples by using a similar porogenic system.2 Comparing with the conventional porogens, the RTIL porogenic system has more advantages. The resulting MIPs exhibited satisfactory selective extraction ability for the target molecules (IF = 3.22) in aqueous samples. The MIPs prepared by acetonitrile and protoporphyrin–chloroform porogenic system showed lower selective extraction ability in acetonitrile and chloroform and the corresponding IF values were 1.76 and 2.34. These studies are further evidence that RTIL-based porogens give special properties to MIPs.
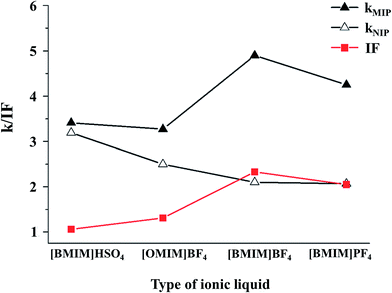 | ||
| Fig. 6 Effect of ionic liquid type on the imprinting factor of the UA-MIP monolith. Reprinted from ref. 47. | ||
According to studies by McCluskey et al., porogens have a significant effect on the process of MIPs preparation.48,49 Taking trans-aconitic acid and cocaine as templates, the conventional porogens, acetonitrile and chloroform, and the RTIL porogens, [BMIM][BF4] and [BMIM][PF6], were investigated. Two polymerization methods, bulk polymerization and precipitation polymerization, were performed at 5 °C and 60 °C. The results showed that the polymers were not formed by using conventional porogens at low temperature no matter what polymerization method was taken. In contrast, the polymers can be yielded by both polymerization methods with RTIL porogens at low temperature. At high temperature, the rate of MIPs polymerization in RTILs is significantly faster than in conventional porogens. Relevant results were shown in Table 2. The results indicated that the polymerization process of MIPs was accelerated by using RTILs as porogen. Subsequently, the impacts of porogens on polymer morphology were also studied and the detailed process is shown in Fig. 7. Under the combined influence of templates, porogens and polymerization methods, the polymers show different morphology characteristics. In the end, this study found that MIPs prepared with RTILs porogens at low temperature have better selective extraction ability.
| Porogen | Reaction temperature [°C] | Volume porogen [mL] | Reaction time [h] | |
|---|---|---|---|---|
| trans-Aconitic acid | Cocaine | |||
| a No reaction.b Not performed. | ||||
| CH3CN | 5 | 5 | —a | —b |
| 25 | —a | —b | ||
| 60 | 5 | 6 | —b | |
| 25 | 18 | —b | ||
| CHCl3 | 5 | 5 | —b | —a |
| 25 | —b | —a | ||
| 60 | 5 | —b | 6 | |
| 25 | —b | 18 | ||
| [BMIM][BF4] | 5 | 5 | 0.75 | 0.5 |
| 25 | 2 | 2 | ||
| 60 | 5 | 2 | 2 | |
| 25 | 8 | 4 | ||
| [BMIM][PF6] | 5 | 5 | 0.5 | 0.75 |
| 25 | 2 | 2 | ||
| 60 | 5 | 2 | 2 | |
| 25 | 8 | 4 | ||
 | ||
| Fig. 7 SEM images of trans-aconitic acid MIPs prepared using different porogens, temperatures, and volumes. Reprinted from ref. 49. | ||
Interestingly, a study proposed by Liu et al. also supports McCluskey's conclusion.50 In Liu's study, a MIP monolith with good permeability was obtained by using ketoprofen or naproxen as template and [BMIM][BF4]/DMSO mixture as porogen. At the same content of porogenic solvent, the RTIL-based MIP monolith has much better column permeability than the MIP monolith prepared with conventional porogens (isooctane/toluene, 20/80, v/v), which suggested that RTIL produced significant effect on morphology of MIPs. Moreover, they found that the polymerization of IL-based MIP monolith was completed in a short time (<1.5 h). It is proved that RTIL can accelerate the rate of polymerization in the preparation of MIPs. Influence of RTIL porogen on the performance of MIP monolith was investigated as well. In this work, high column efficiency of the monolith was achieved. The capacity factor and IF of the RTIL-based MIP monolith for ketoprofen achieved 10.40 and 8.64, respectively. More similar studies on the application of RTIL porogen in the synthesis of MIPs were shown in Table 3.
| RTIL | Protocol of preparing MIPs | Comments | Ref. |
|---|---|---|---|
| 1-Butyl-3-methyl imidazolium hexafluorophosphate ([BMIM][PF6]) | Dummy template: α-chloro-DDT | Taking [BMIM][PF6] as the auxiliary solvent, a RTIL-based MIPs was synthesized by precipitation polymerization. The RTIL-based polymer was utilized as SPE sorbents and it has better selectivity and adsorption capacity to dicofol than conventional SPE sorbents in aqueous medium | 51 |
| Functional monomer: acrylamide (AM) | |||
| Cross-linker: DVB | |||
| Solvent: acetonitrile–Toluene–[BMIM][PF6] | |||
| Template: sulfamoxol | In this study, a silica monolithic support was synthesized in capillary by the sol–gel method at first. Then, the RTIL-based MIPs were coated on the surface of silica monolith. The proposed material was evaluated by CEC. The results demonstrated that the permeability of the RTIL-based MIPs monolithic column was improved and the selectivity of monolithic column was better than MIPs monolithic column without RTIL | 52 | |
| Functional monomer: MAA | |||
| Cross-linker: EDMA | |||
| Supported material: modified silica monolith | |||
| Solvent: acetonitrile–toluene–[BMIM][PF6] | |||
| Template: dibutyl phthalate (DBP) | The MIPs was synthesized by using [BMIM][PF6] as an auxiliary porogen. The results showed that the RTIL-based polymer had excellent enrichment efficiency and selectivity for template | 53 | |
| Functional monomer: MAA | |||
| Cross-linker: EDMA | |||
| Solvent: chloroform–[BMIM][PF6] | |||
| Template: dichlorvos | In this work, a dichlorvos MIPs was prepared by bulk polymerization technique. The shrinking or swelling ratio of polymers decreased by using RTILs as auxiliary porogen. The microstructure of MIPs can keep stable and it is significant to maintain the specific recognition property of MIPs | 54 | |
| Functional monomer: MAA | |||
| Cross-linker: TRIM | |||
| Solvent: acetonitrile–toluene–[BMIM][[PF6]] | |||
| 1-Butyl-3-methyl imidazolium tetrafluoroborate ([BMIM][BF4]) | Dummy template: testosterone | By sol–gel nano casting and sacrificial spacer MIT, the molecularly imprinted silica was obtained. Briefly, the covalent monomer–template complex was synthesized at first. Then, the monomer–template complex was added into a mixture of TEOS and RTIL and the new material was prepared by sol–gel method. The results indicated that [BMIM][BF4] had good porogen properties and solvation qualities | 55 |
| Functional monomer: 3-(triethoxysilyl)propyl isocyanate | |||
| Solvent: [BMIM][BF4] | |||
| Template: Corilagin | A RTIL-mediated MIPs was prepared and evaluated as a SPE sorbent for the pretreatment of corilagin. The results showed that the new MIPs have good specific adsorption capacity for template | 56 | |
| Functional monomer: 4-VP | |||
| Cross-linker: EDMA | |||
| Solvent: DMF–DMSO–[BMIM][BF4] | |||
| Template: norfloxacin | By the proposed formula, a RTIL-mediated MIPs was prepared and was used as HPLC monolith and SPE sorbent. The study showed that the proposed polymer exhibited excellent ability of selective recognition to quinolones | 57 | |
| Functional monomer: MAA | |||
| Cross-linker: EDMA | |||
| Solvent: DMF–DMSO–[BMIM]BF4 | |||
| Template: ketoprofen | A thermo responsive MIP monolith was prepared and evaluated. In this work, the RTIL-based porogen was used to increase the solubility of AMPS. The sensibility of MIP to temperature was also investigated. The novel MIPs has the best specific adsorption capacity for ketoprofen in the transition temperature of 35 °C. It was more than 75 times that at 25 °C | 58 | |
| Functional monomer: AAm–AMPS | |||
| Cross-linker: EDMA | |||
| Solvent: DMSO–[BMIM][BF4] | |||
| Template: methyl gallate | An imprinted monolithic HPLC column was prepared with good performance and permeability by using a RTIL-based ternary porogenic system. In this paper, a polar molecule was taken as template and the RTIL-based monolithic column showed higher imprinting efficiency | 59 | |
| Functional monomer: 4-VP | |||
| Cross-linker: EDMA | |||
| Solvent: DMSO–DMF–[BMIM][BF4] |
4. RTILs used for other purposes in MIT
In the field of MIT, imprinting of macromolecules has always been regarded as a challenging problem. This is because macromolecules, such as protein, DNA and enzymes, have the characteristics of structural complexity and conformational flexibility. Hence, surface imprinting technology was proposed to solve the problem.60 In this strategy, a substrate with effective immobilization capacity is a key to obtaining surface MIPs with high performance and RTILs could play an important role in this field. Qian et al. used BSA as model template to explore the effect of RTIL functionalized material as substrate in surface imprinting technology.61 RTIL functionalized Fe3O4 nanoparticles (Fe3O4@IL) were prepared by a simple method and used as substrate. The scheme of the synthesis Fe3O4@IL@MIP material is shown in Fig. 8. The results show that the resulting magnetic MIP nano-materials have good selectivity and specificity for BSA. The Fe3O4@IL@MIP showed good selectivity towards BSA with IF value of 3.33 and adsorption capacity value of 50.6 mg g−1. This investigation indicated that immobilization of macromolecules using RTIL functionalized substrate in surface imprinting technology would be helpful in preparing MIPs with high capacity and selectivity.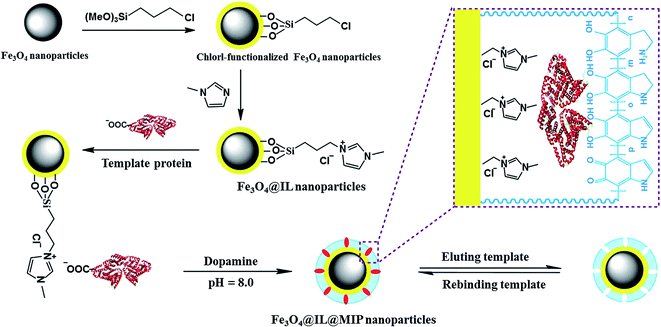 | ||
| Fig. 8 Schematic illustration of the synthesis Fe3O4@IL@MIP nanoparticles. Reprinted from ref. 61. | ||
However, one shortcoming in the application of MIPs is their poor recognition performance to strong hydrophilic templates when using conventional functional monomers such as methacrylic acid (MAA), methyl methacrylate (MMA) and 4-VP. To address this disadvantage, a water-compatible MIP was synthesized by Zhao et al.62 In this work, an RTIL functionalized graphene was prepared using RTIL (1-(3-aminopropyl)-3-methyl imidazolium bromide) and graphene at first. Then, the MIP film was prepared on the surface of RTIL functionalized grapheme which was used as functional monomer and substrate. At last, the composite material was used as electrochemical sensor for imidacloprid sensing. The detailed process is shown in Fig. 9. The resulting imprinted RTIL polymer exhibited high adsorption capacity and good selectivity for imidacloprid in vegetable and fruit samples. The results showed that the peak currents of imidacloprid displayed small change (95.4–107.8%) in the presence of 10-fold interfering substances, indicating that the obtained composite material had good selectivity. It means that some RTILs can interact with hydrophilic molecules and it also indicates the RTIL-based MIPs can have a good performance in aqueous medium.
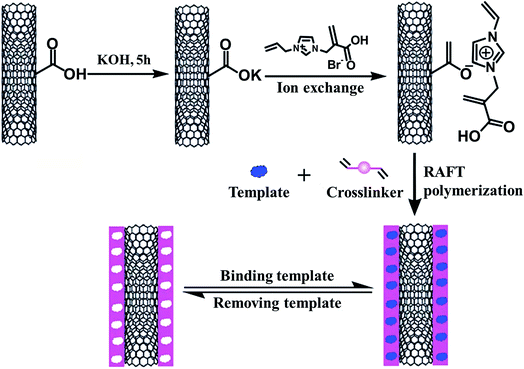 | ||
| Fig. 9 Schematic illustration of preparation of water-compatible surface-imprinted RTIL polymer coated MWNTs. Reprinted from ref. 62. | ||
MIPs coated quantum dots (QDs) is a new kind of material and combines the advantages of the two materials, such as good selectivity and high sensitivity. However, several important problems need to be solved in order to obtain MIPs coated QDs with satisfactory performance. For example, the surface of QDs is lack of appropriate functional groups to form firm chemical bond with MIPs and the application of QDs is limited because of its low fluorescence stability. Hence, a series of MIPs based on RTIL-modified QDs were proposed by Wang et al.63–66 In these researches, the core strategy is that RTILs with carbon–carbon double bonds bind to the surface of CdSe/ZnS QDs. Two RTILs, 1-vinyl-3-octyl imidazolium hexafluorophosphate ([VOIM][PF6]), 1-vinyl-3-butyl imidazolium hexafluorophosphate ([VBIM][PF6]), were investigated. The results showed that related RTIL-modified QDs could form binding groups with MIPs63–65 or carboxylated graphene oxide (GO).66 The MIPs coated IL-modified QDs material with a stable structure can be obtained by the proposed strategy. Furthermore, the fluorescent stability of QDs was also improved because of high thermal and chemical stability of RTILs.
Furthermore, RTILs can be used as pore template in sol–gel processing.67 In this technique, silicate matrix is assembled in proper solvent (pore template) and a highly cross-linked polymer with specific shape and size cavities could be obtained. Moreover, some studies have shown that the shrink or swell of MIPs could be avoided to some extent by using RTILs as solvent.68,69 It is important for functional groups in the polymer to maintain relative position. Wang and co-workers70 proposed an RTIL-mediated, non-hydrolytic sol–gel method to prepare MIP silica-based hybrid monoliths for chiral separation of racemic naproxen. In this research, a hydrophobic RTIL ([BMIM][PF6]) was taken as pore template for non-hydrolytic sol–gel (NHSG) processing and the synthesized product provided a stable solid support for subsequent polymerization. This method avoided the shrink of sol–gel and provided a polymer network with stable structure. As a result, the silica-based hybrid MIP has good selectivity. A resolution value of 8.82 for the separation of (S)-naproxen and (R)-naproxen could be obtained. Subsequently, the same method was applied to preparation of molecularly imprinted silica-based hybrid monoliths and the resulting material was used for chiral separation of zolmitriptan by capillary electrochromatography (CEC).71 In this study, [BMIM][BF4] was utilized to prevent gel shrinkage and also used as pore template. The results showed that the porosity and selectivity of hybrid monoliths were obviously improved. These two researches implied that the RTIL-mediated NHSG strategy is a hopeful method for the preparation of imprinted hybrid materials with high performance.
As mentioned above, some RTILs have hazardous toxicity and poor biodegradability.14,15 This means that RTILs might result in environmental problem in the course of preparation and application of RTILs. To effectively monitor the RTILs in environment, a RTIL surface MIP was prepared by Gao et al.72,73 In these works, the alkyl imidazolium chloride RTILs, 1-butyl-3-methyl imidazolium chloride ([C4MIM]Cl, i.e. [BMIM]Cl) and 1-octyl-3-methyl imidazolium chloride ([C8MIM]Cl, i.e. [OMIM]Cl) were taken as templates. The prepared MIPs were used as solid phase extraction material. The results show that the RTIL surface MIPs have excellent selective adsorption of RTILs ([CnMIM]Cl, n = 2, 4, 6, 8, 12, 16) in complex environmental samples. Obviously, [C4MIM]Cl and [C8MIM]Cl could be regarded as template analogues in such cases. Template analogue technique is generally used to overcome some limiting factors. For example, actual template compound can be very expensive, heat- or light-sensitive or insoluble in polymerisation porogens. Moreover, it can also enhance the affinity of functional monomer to template. An innovative method of preparation for L-phenylalanine (L-Phe) MIPs was proposed by Yang et al.74 In this study, an oil-soluble amino acid IL, 1-butyl-3-methyl imidazolium amino hydrocinnamic acid salt ([BMIM][Phe]), was introduced as template and a surface MIPs was prepared for the selective recognition of L-Phe. The affinity of functional monomer (4-VP) to [BMIM][Phe] and 4-VP to L-Phe were investigated and it was found that the affinity of 4-VP to [BMIM][L-Phe] was much higher. The data showed that MIPs prepared with [BMIM][Phe] were three times selective than the corresponding MIPs prepared with 4-VP. It means that the template analogue technique converted the hydrophilic template imprinting process from water to the organic phase.
Reversible addition–fragmentation chain transfer (RAFT) polymerization was a method of living or controlled radical polymerization and the technique is commonly used to preparation of MIPs. Studies found that the rate of bimolecular termination during the RAFT polymerization was reduced by using RTILs as reaction medium, indicating that the rate of free radical polymerization can be significantly increased.75 Even more remarkably, by using reverse atom transfer radical polymerization (ATRP) and RTILs, the molecular weight distribution of MIPs is more concentrated.76 Based on these methods, Liu and co-workers77 proposed a method based on RAFT polymerization for preparing carprofen MIP monoliths. With the aid of [BMIM][BF4]-based ternary porogen, the obtained MIP monoliths had excellent permeability and satisfactory column efficiency. Furthermore, by morphology analysis, it is verified that the structure of this MIP monolith is uniform.
Due to protein denaturation at higher temperature, protein imprinting is a difficult task in the field of MIT. How to retain the structure of protein at high temperature is a troublesome problem. It is reported that the biocompatible RTIL, choline dihydrogen phosphate (Chol Dhp), was used as a stabilizer during the process of lysozyme-imprinted microspheres preparation.78 It was confirmed that the structural stability of lysozyme can be insured in the polymerization system with 5% RTIL as the stabilizer at 75 °C. The stabilizing effect of Chol Dhp on protein structure is probably the result of its function of hydrophobic and chelate.
Furthermore, RTILs can serve multiple purposes at the same time during the preparation of MIPs. An approach to prepare protein imprinted microspheres was proposed by Zhang et al.79 In this work, an amphiphilic ionic liquid, 1-dodecyl-3-methylimidazolium chloride ([DMIM]Cl), was not only used as stabilizer, but also used as emulsifier. In other words, [DMIM]Cl was used to prevent protein denaturation and applied to form an oil-in-water emulsion system at the same time. There are many examples that RTILs play several different roles in the process of MIPs preparation. These studies introduced the diversity of RTILs in the field of MIT.
In summary, RTILs have brought many benefits to MIPs. For instance, the polymerization of MIPs was achieved with less volatile organic compound solvents, offering economic advantages as well as environmental advantages. Moreover, it is proved that the polymerization process of RTILs-based MIPs could be completed in a short time and that means less energy consumption. Furthermore, the development of RTILs-based MIPs has been giving impetus to the development of protein macromolecule imprinted technology. Besides, the swelling ratio of MIPs can be reduced by using RTIL as porogens. Therefore, the research of RTILs-based MIPs has the character of flexibility and diversity. It is a potential research field that has a wide application view.
5. The application of DES in the preparation of MIPs
DESs are another type of green solvents and it is also classified as one kind of RTILs by some researchers.80 Compared with RTILs, DES has the advantages of low toxicity81 and low price. Moreover, DES is easy to prepare by mixing two or three inexpensive components in a certain proportion. The corresponding components were respectively defined as the HBA and the HBD, since the interaction between them is hydrogen bonds.17 It is worth noting that a broad group of compounds is likely candidates for the HBA or the HBD. This variety of DES provides more flexible choice for various applications, including drug dissolution,82 metal oxides dissolution,20 gas absorption,83 synthesis of nanoparticles,84 organic synthesis85 and synthesis of nanoparticles.86 In view of the fact that DES share similar properties with RTILs, the application of DES for MIPs has just begun to receive considerable attention.Like RTILs, DES performed varied functions in the preparation of MIPs. Liu et al. proposed a novel magnetic DES-MIPs for bovine hemoglobin (BHb) by using DES as functional monomer.22 This is the first study to report the application of DES in MIT. In this study, the DES was prepared by heating the mixture of choline chloride and methacrylic acid (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) at 90 °C and the prepared DES was a colorless and uniform liquid. The Fe3O4 nanoparticles were used as magnetic provider. It was obtained by the solvothermal approach and modified with acrylic acid. BHb, N,N-methylenebisacrylamide (MBAA) and ammonium persulfate (APS)/N,N,N′,N′-tetramethylenediamine (TEMED) were used as template, cross-linker and initiator, respectively. Magnetic DES-MIPs were polymerized at room temperature to prevent heat denaturation of the protein. The detailed preparation procedure is shown in Fig. 10. The reported preparation strategy has the advantages of being simple, low toxicity and low cost. The protein adsorption experiments have shown that the IF of the magnetic DES-MIPs achieved 4.57 under optimum conditions. It is implied that the new material has a good specific recognition property. In order to further investigate the specificity of the new material, the competitive batch rebinding test was performed. As BSA is of similar size with BHb, it was taken as a competitive protein in competitive batch rebinding tests. Moreover, real sample (calf blood) adsorption experiment was carried out to evaluate the practicability of magnetic DES-MIPs. The adsorption capacity of magnetic DES-MIPs for BHb still kept at a high value (150.52 mg g−1). The results demonstrated that the synthesized magnetic DES-MIPs have absorption with good selectivity on the BHb.
2) at 90 °C and the prepared DES was a colorless and uniform liquid. The Fe3O4 nanoparticles were used as magnetic provider. It was obtained by the solvothermal approach and modified with acrylic acid. BHb, N,N-methylenebisacrylamide (MBAA) and ammonium persulfate (APS)/N,N,N′,N′-tetramethylenediamine (TEMED) were used as template, cross-linker and initiator, respectively. Magnetic DES-MIPs were polymerized at room temperature to prevent heat denaturation of the protein. The detailed preparation procedure is shown in Fig. 10. The reported preparation strategy has the advantages of being simple, low toxicity and low cost. The protein adsorption experiments have shown that the IF of the magnetic DES-MIPs achieved 4.57 under optimum conditions. It is implied that the new material has a good specific recognition property. In order to further investigate the specificity of the new material, the competitive batch rebinding test was performed. As BSA is of similar size with BHb, it was taken as a competitive protein in competitive batch rebinding tests. Moreover, real sample (calf blood) adsorption experiment was carried out to evaluate the practicability of magnetic DES-MIPs. The adsorption capacity of magnetic DES-MIPs for BHb still kept at a high value (150.52 mg g−1). The results demonstrated that the synthesized magnetic DES-MIPs have absorption with good selectivity on the BHb.
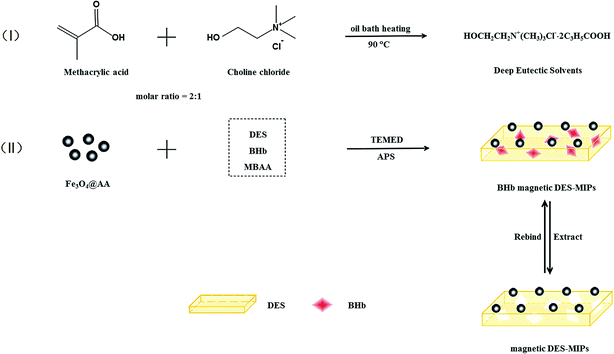 | ||
| Fig. 10 Schematic illustration of the procedure for the magnetic DES-MIPs. Reprinted from ref. 22. | ||
Hydrophilic resins are a class of adsorbents with the advantage of low cost, good porosity, diverse functional groups. It has good adsorption properties for target molecules in aqueous environments. However, poor specific molecular recognition ability restricts its application range. Conversely, specific molecular recognition ability is the crucial advantage of MIPs. Liang et al. developed a molecularly imprinted phloroglucinol–formaldehyde–melamine resin (MIPFMR) by combining hydrophilic resins with MIT.87 As mentioned before, MIPs synthesized in organic solvents have limited compatibility with water and poor molecular recognition of template in aqueous solution. To solve this problem, ethylene glycol–choline chloride DES was used as reaction medium in this study. Compared with materials synthesized in common reaction medium (alcoholic solvent systems), the MIPFMR prepared in DES shows better adsorption capability for the templates, clorprenaline and bambuterol. Although DES has the characteristics of a polar solvent, the adsorptive capacity of MIPFMR was increased. The authors suggest that DES have little impact on the template–monomers hydrogen bonds.
In the studies of DES, ChCl is the most popular HBA and most of the related studies are based on ChCl-based DES. However, ChCl is still relatively expensive. The searching for HBAs which are inexpensive, readily available and of low toxicity has been an important subject in the field of DES. Row et al.88 developed a new type of DES by using betaine and ethylene glycol as HBA and HBD. In this research, DES was synthesized by betaine, ethylene glycol and water with the mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The preparation process of DES-modified MIPs mainly contains three steps. First, the functional monomer 3-aminopropyltriethoxysilane (APTES)-MAA was fabricated under a certain condition. Then, the template (levofloxacin or tetracycline) and the monomer were added to methanol for forming the template–functional monomer complex. At last, the DES, cross-linker (EDMA), initiator (2,2-azobisisobutyronitrile, AIBN) and tetraethoxysilane (TEOS) were added to the template–functional monomer complex solution and the mixture was synthesized at 60 °C for 24 h. The synthesis scheme of DES-modified MIPs is shown in Fig. 11. The selective adsorption capacity of the levofloxacin DES-MIPs and tetracycline DES-MIPs were evaluated and the results show DES-modified MIPs have better adsorption ability than the conventional MIPs and the NIPs. Furthermore, the relevant DES-MIPs showed the highest selectivity recovery for levofloxacin (94.5%) and tetracycline (93.3%) from millet extract with mixture antibiotics, and could remove the interferent effectively. The results suggest that the performance of MIPs could be improved by using DES during MIPs preparation.
1. The preparation process of DES-modified MIPs mainly contains three steps. First, the functional monomer 3-aminopropyltriethoxysilane (APTES)-MAA was fabricated under a certain condition. Then, the template (levofloxacin or tetracycline) and the monomer were added to methanol for forming the template–functional monomer complex. At last, the DES, cross-linker (EDMA), initiator (2,2-azobisisobutyronitrile, AIBN) and tetraethoxysilane (TEOS) were added to the template–functional monomer complex solution and the mixture was synthesized at 60 °C for 24 h. The synthesis scheme of DES-modified MIPs is shown in Fig. 11. The selective adsorption capacity of the levofloxacin DES-MIPs and tetracycline DES-MIPs were evaluated and the results show DES-modified MIPs have better adsorption ability than the conventional MIPs and the NIPs. Furthermore, the relevant DES-MIPs showed the highest selectivity recovery for levofloxacin (94.5%) and tetracycline (93.3%) from millet extract with mixture antibiotics, and could remove the interferent effectively. The results suggest that the performance of MIPs could be improved by using DES during MIPs preparation.
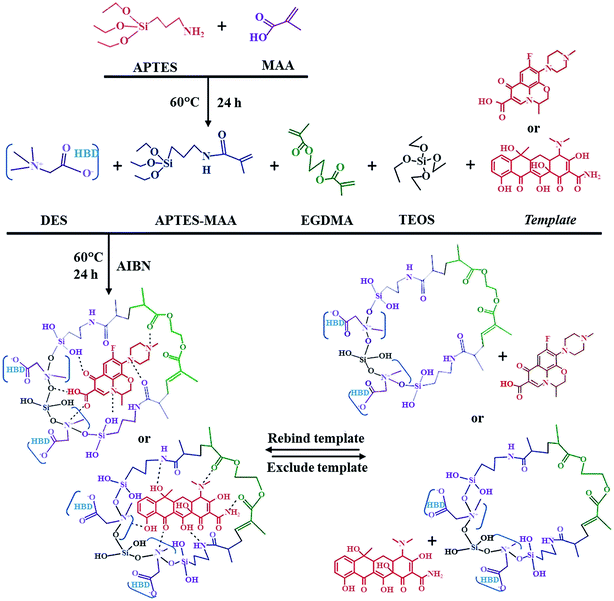 | ||
| Fig. 11 Schematic illustration of the proposed materials formation. Reprinted from ref. 88. | ||
Moreover, an extraction method using DES-based MIPs as sorbent for the determination of levofloxacin green bean extract was proposed by the same team.89 In this study, the preparation method of DES-MIPs was similar with literature.88 The resulting materials also show excellent performance of adsorption ability and selectivity. DES-MIP showed the highest selectivity recovery (95.2%) for levofloxacin the complex matrix. It is proved that DES-modified MIP is still stable for real samples. Furthermore, the influence of various ChCl-based DESs and 1-methylimidazole-based RTILs on the performance of MIPs was also investigated by Row's team.90 Taking rutin, scoparone and quercet in as multi-template, γ-aminopropyltriethoxysilane methacrylic (KH-550-MAA) as multiple functional monomers, EDMA as cross-linker and AIBN as initiator, the DES/RTIL modified hybrid MIPs (HMIPs) were prepared. In this article, three DESs (ChCl–ethylene glycol, ChCl–glycerol and ChCl–1,4-butanediol) and three RTILs (1-ethyl-3-methyl imidazolium bromide ([EMIM]Br), 1-butyl-3-methyl imidazolium bromide ([BMIM]Br) and 1-hexyl-3-methyl imidazolium bromide ([HMIM]Br)) were used for modifying the HMIPs. The results show that the modified HMIPs have good recovery ability and recognition performance toward templates in Herba Artemisiae Scopariae. As described in the study, DES-HMIPs have higher recoveries than RTILs-HMIPs and the ChCl–glycerol modified HMIPs have the best performance among DES-HMIPs with the recoveries of 92.27% (rutin), 87.51% (scoparone) and 80.02% (quercetin), respectively. It is implied that DES has great advantages when applied to MIT. In addition to the functions mentioned above, DES has played multiple roles in the preparation of MIPs. In Row's latest communication,91 DES was synthesized by using choline chloride–caffeic acid–ethylene glycol with a special mole ratio and it was utilized as a functional monomer and template. By the proposed approach, the DES-MIPs were obtained with good recognition ability for the polyphenols in real samples. The research showed that caffeic acid in DES has a great influence on selective recognition ability of MIPs.
Obviously, with the development of MIT, DESs have begun to be applied in the preparation of MIPs because of their particular advantages. DES-based MIPs have good compatibility with water and high molecular recognition of template in aqueous solution. Furthermore, it was proved that DESs could improve the selectivity and affinity of MIPs. However, the DES-based MIPs research is only at the start stage and some relevant mechanisms are still unclear. For this reason, a vast amount of further research is needed to explore the potential value of DES-based MIPs.
6. Conclusion
At present, RTIL-based MIPs and DES-based MIPs have become an active and exciting research area in MIT. The investigations on these new materials have got some progressions up to now. RTIL-based MIPs and DES-based MIPs overcome many shortcomings of traditional MIPs. For example, RTILs and DESs can contribute to improving the effect of macromolecules MIPs. The shrinking or swelling of MIPs is reduced by using RTIL as porogens. Furthermore, it has been proved that RTIL-based MIPs and DES-based MIPs can maintain significant performances in aqueous medium. Comparing with traditional MIPs, the new materials have better specific recognition property. However, studies on the new materials are relatively few, especially DES-based MIPs. The effect mechanism of RTIL and DES in the synthetic process of MIPs has not been studied thoroughly. However, it can be expected that RTIL-based MIPs and DES-based MIPs have a broad application prospects and more relevant reports will be presented in the future.List of abbreviations
| 4-VP | 4-Vinylpyridine |
| AAm | Acrylamide |
| AIBN | 2,2-Azobisisobutyronitrile |
| AMPS | 2-Acrylamide-2-methyl propanesulfonic acid |
| APS | Ammonium persulfate |
| APTES | 3-Aminopropyltriethoxysilane |
| ATRP | Atom transfer radical polymerization |
| BET | Brunauer–Emmett–Teller |
| BHb | Bovine hemoglobin |
| BPA | Bisphenol A |
| BSA | Bovine serum albumin |
| CEC | Capillary electrochromatography |
| CFO | (Z)-3-(Chloromethylene)-6-flourothiochroman-4-one |
| ChCl | Choline chloride |
| Chol Dhp | Choline dihydrogen phosphate |
| CTA-2 | (4-Cyanopentanoic acid)-4-dithiobenzoate |
| Cyt C | Cytochrome C |
| DESs | Deep eutectic solvents |
| DVB | Divinylbenzene |
| EDMA | Ethylene glycol dimethacrylate |
| FHO | (Z)-6-Fluoro-3-(hydroxymethylene)-thiochroman-4-one |
| GO | Raphene oxide |
| HBA | Hydrogen bond acceptor |
| HBD | Hydrogen bond donors |
| KH-550-MAA | γ-Aminopropyltriethoxysilane-methacrylic |
| L-Phe | L-Phenylalanine |
| MAA | Methacrylic acid |
| MBA | N,N′-Methylenebisacrylamide |
| MBAA | N,N-Methylenebisacrylamide |
| MD | Molecular dynamics |
| MIPFMR | Molecularly imprinted phloroglucinol–formaldehyde–melamine resin |
| MIPs | Molecularly imprinted polymers |
| MIT | Molecularly imprinted technology |
| MMA | Methyl methacrylate |
| MWCNTs | Multiwall carbon nanotubes |
| NHSG | Non-hydrolytic sol–gel |
| NNMBA | N,N′-Methylenebis (acrylamide) |
| NIPA | N-Isopropylacrylamide |
| PALS | Positron annihilation lifetime spectroscopy |
| PMDETA | N,N,N′,N′,N′′-Pentamethyldiethylene triamine |
| PVP | Polyvinylpyrrolidone |
| QDs | Quantum dots |
| RAFT | Reversible addition–fragmentation chain transfer |
| RTILs | Room temperature ionic liquids |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| TEMED | N,N,N′,N′-Tetramethylethylenediamine |
| TEOS | Tetraethoxysilane |
| TP5 | Thymopentin |
| [ABIM]Cl | 1-Allyl-3-butyl imidazolium chloride |
| [AEIM]Br | 1-Allyl-3-ethyl imidazolium bromide |
| [AEIM][PF6] | 1-Allyl-3-ethyl imidazolium hexafluorophosphate |
| [AHIM]Cl | 1-Allyl-3-hexyl imidazolium chloride |
| [AMIM]Br | 1-Allyl-3-methyl imidazolium bromide |
| [AOIM]Cl | 1-Allyl-3-octyl imidazolium chloride |
| [BMIM][BF4] | 1-Butyl-3-methyl imidazolium tetrafluoroborate |
| [BMIM]Br | 1-Butyl-3-methyl imidazolium bromide |
| [BMIM]Cl | 1-Butyl-3-methyl imidazolium chloride |
| [BMIM][HSO4] | 1-Butyl-3-methyl imidazolium hydrogen sulfate |
| [BMIM][PF6] | 1-Butyl-3-methyl imidazolium hexfluorophosphate |
| [BMIM][Phe] | 1-Butyl-3-methyl imidazolium aminohydrocinnamic acid salt |
| [BVIM][Phe] | 1-Butyl-3-vinyl imidazolium aminohydrocinnamic acid |
| [C3VIM]Br | 3-Propyl-1-vinyl imidazolium bromide |
| [COOHavim]Br | 1-Viny-3-carboxybutyl imidazolium bromide |
| [COOHevim]Br | 1-Vinyl-3-carboxymethyl imidazolium bromide |
| [COOHhvim]Br | 1-Vinyl-3-carboxypentyl imidazolium bromide |
| [COOHpvim]Br | 1-Vinyl-3-carboxyethyl imidazolium bromide |
| [DMIM]Cl | 1-Dodecyl-3-methyl imidazolium chloride |
| [EMIM]Br | 1-Ethyl-3-methyl imidazolium bromide |
| [HMIM]Br | 1-Hexyl-3-methyl imidazolium bromide |
| [HMIM][PF6] | 1-Hexyl-3-methyl imidazolium hexfluorophosphate |
| [OMIM][BF4] | 1-Octyl-3-methyl imidazolium tetrafluoroborate |
| [OMIM]Cl | 1-Octyl-3-methyl imidazolium chloride |
| [OMIM][PF6] | 1-Octyl-3-methyl imidazolium hexfluorophosphate |
| SilprImN | 1-(Triethoxysilyl) propyl-3-aminopropyl imidazole bromide |
| [TMSPMIM]Cl | 1-(3-Trimethoxysilyl propyl)-3-methyl imidazolium chloride |
| [VAFMIM]Cl | 1-Vinyl-3-aminoformylmethyl imidazolium chloride |
| [VBIM]Cl | 1-Vinyl-3-butyl imidazolium chloride |
| [VBIM][PF6] | 1-Vinyl-3-butyl imidazolium hexafluorophosphate |
| [VEAIM]Cl | 1-Vinyl-3-ethyl acetate imidazolium chloride |
| [VMIM]Cl | 1-Vinyl-3-methyl imidazolium chloride |
| [VOIM]Cl | 1-Vinyl-3-octyl imidazolium chloride |
| [VOIM][PF6] | 1-Vinyl-3-octyl imidazolium hexafluorophosphate |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is sponsored by the Fund for Shanxi Key Subjects Construction and supported by National Natural Science Foundation of China (21402012).References
- Z. H. Wei, X. Wu, B. Zhang, R. Li, Y. P. Huang and Z. S. Liu, J. Chromatogr. A, 2011, 1218, 6498–6504 CrossRef CAS PubMed.
- X. Wu and L. T. Wu, J. Sep. Sci., 2015, 38, 3615–3621 CrossRef CAS PubMed.
- Z. G. Xu, C. Y. Song, Y. L. Hu, J. Wang and G. K. Li, Talanta, 2011, 85, 97–103 CrossRef CAS PubMed.
- W. Boonjob, Y. L. Yu, M. Miro, M. A. Segundo, J. H. Wang and V. Cerda, Anal. Chem., 2010, 82, 3052–3060 CrossRef CAS PubMed.
- R. Schirhagl, Anal. Chem., 2014, 86, 250–261 CrossRef CAS PubMed.
- K. J. Shea and D. Y. Sasaki, J. Am. Chem. Soc., 1991, 113, 4109–4120 CrossRef CAS.
- L. X. Chen, X. Y. Wang, W. H. Lu, X. Q. Wu and J. H. Li, Chem. Soc. Rev., 2016, 45, 2137–2211 RSC.
- T. Welton, Chem. Rev., 1999, 99, 2071–2084 CrossRef CAS PubMed.
- S. Gao, H. Jin, J. You, Y. Ding, N. Zhang, Y. Wang, R. Ren, R. Zhang and H. Zhang, J. Chromatogr. A, 2011, 1218, 7254–7263 CrossRef CAS PubMed.
- D. W. Armstrong, L. He and Y. S. Liu, Anal. Chem., 1999, 71, 3873–3876 CrossRef CAS PubMed.
- C. C. Liu, Q. L. Deng, G. Z. Fang, X. Feng, H. Qian and S. Wang, Anal. Bioanal. Chem., 2014, 406, 7175–7183 CrossRef CAS PubMed.
- M. Bohm, A. A. Tietze, P. Heimer, M. Chen and D. Imhof, J. Mol. Liq., 2014, 192, 67–70 CrossRef.
- A. Martin-Calero, V. Pino and A. M. Afonso, Trends Anal. Chem., 2011, 30, 1598–1619 CrossRef CAS.
- A. Romero, A. Santos, J. Tojo and A. Rodriguez, J. Hazard. Mater., 2008, 151, 268–273 CrossRef CAS PubMed.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123–150 RSC.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 8, 70–71 RSC.
- Q. H. Zhang, K. D. O. Vigier, S. Royer and F. Jerome, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC.
- A. P. Abbott, G. Capper, D. L. Davies, K. J. McKenzie and S. U. Obi, J. Chem. Eng. Data, 2006, 51, 1280–1282 CrossRef CAS.
- M. Karimi, S. Dadfarnia, A. M. H. Shabani, F. Tamaddon and D. Azadi, Talanta, 2015, 144, 648–654 CrossRef CAS PubMed.
- A. P. Abbott, D. Boothby, G. Capper, D. L. Davies and R. K. Rasheed, J. Am. Chem. Soc., 2004, 126, 9142–9147 CrossRef CAS PubMed.
- L. Guo, Q. L. Deng, G. Z. Fang, W. Gao and S. Wang, J. Chromatogr. A, 2011, 1218, 6271–6277 CrossRef CAS PubMed.
- Y. J. Liu, Y. Z. Wang, Q. Z. Dai and Y. G. Zhou, Anal. Chim. Acta, 2016, 936, 168–178 CrossRef CAS PubMed.
- S. F. Yuan, Q. L. Deng, G. Z. Fang, M. F. Pan, X. R. Zhai and S. Wang, J. Mater. Chem., 2012, 22, 3965–3972 RSC.
- R. K. Desai, M. Streefland, R. H. Wijffels and M. H. M. Eppink, Green Chem., 2014, 16, 2670–2679 RSC.
- H. Y. Xiang, M. J. Peng, H. Li, S. Peng and S. Y. Shi, J. Pharm. Biomed. Anal., 2017, 133, 75–81 CrossRef CAS PubMed.
- J. P. Fan, Z. Y. Tian, S. Tong, X. H. Zhang, Y. L. Xie, R. Xu, Y. Qin, L. Li, J. H. Zhu and X. K. Ouyang, Food Chem., 2013, 141, 3578–3585 CrossRef CAS PubMed.
- M. L. Tian, W. T. Bi and K. H. Row, Anal. Bioanal. Chem., 2011, 399, 2495–2502 CrossRef CAS PubMed.
- C. L. Wang, X. L. Hu, P. Guan, L. W. Qian, D. F. Wu and J. Li, Int. J. Polym. Anal. Charact., 2014, 19, 70–82 CrossRef CAS.
- X. Wu, Y. P. Huang and Z. S. Liu, Curr. Org. Chem., 2016, 20, 459–468 CrossRef CAS.
- J. Li, X. L. Hu, P. Guan, D. M. Song, L. W. Qian, C. B. Du, R. Y. Song and C. L. Wang, J. Sep. Sci., 2015, 38, 3279–3287 CrossRef CAS PubMed.
- M. M. Liu, J. Y. Pi, X. J. Wang, R. Huang, Y. M. Du, X. Y. Yu, W. F. Tan, F. Liu and K. J. Shea, Anal. Chim. Acta, 2016, 932, 29–40 CrossRef CAS PubMed.
- L. W. Qian, X. L. Hu, P. Guan, B. Gao, J. Li, C. L. Wang and Y. M. Tang, Talanta, 2014, 121, 56–64 CrossRef CAS PubMed.
- S. F. Yuan, Q. L. Deng, G. Z. Fang, J. H. Wu, W. W. Li and S. Wang, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2014, 960, 239–246 CrossRef CAS PubMed.
- X. Y. Gao, M. F. Pan, G. Z. Fang, W. Jing, S. Y. He and S. Wang, Anal. Methods, 2013, 5, 6128–6134 RSC.
- H. Y. Yan, S. T. Liu, M. M. Gao and N. Sun, J. Chromatogr. A, 2013, 1294, 10–16 CrossRef CAS PubMed.
- F. X. Qiao, M. M. Gao and H. Y. Yan, J. Sep. Sci., 2016, 39, 1310–1315 CrossRef CAS PubMed.
- C. L. Wang, X. L. Hu, P. Guan, D. F. Wu, L. W. Qian, J. Li and R. Y. Song, J. Pharm. Biomed. Anal., 2015, 102, 137–143 CrossRef CAS PubMed.
- J. Li, X. L. Hu, P. Guan, X. Y. Zhang, L. W. Qian, R. Y. Song, C. B. Du and C. L. Wang, RSC Adv., 2015, 5, 62697–62705 RSC.
- Y. N. Yuan, S. R. Liang, H. Y. Yan, Z. Y. Ma and Y. X. Liu, J. Chromatogr. A, 2015, 1408, 49–55 CrossRef CAS PubMed.
- H. Y. Ding, R. F. Chen, M. M. Liu, R. Huang, Y. M. Du, C. Huang, X. Y. Yu, X. H. Feng and F. Liu, RSC Adv., 2016, 6, 43526–43538 RSC.
- G. M. Yang and F. Q. Zhao, Electrochim. Acta, 2015, 174, 33–40 CrossRef CAS.
- W. T. Bi, M. L. Tian and K. H. Row, J. Chromatogr. A, 2012, 1232, 37–42 CrossRef CAS PubMed.
- X. Y. Zhang, N. Zhang, C. B. Du, P. Guan, X. M. Gao, C. Y. Wang, Y. F. Du, S. C. Ding and X. L. Hu, Chem. Eng. J., 2017, 317, 988–998 CrossRef CAS.
- H. Y. Yan, C. Yang, Y. Y. Sun and K. H. Row, J. Chromatogr. A, 2014, 1361, 53–59 CrossRef CAS PubMed.
- L. H. Bai, X. X. Chen, Y. P. Huang, Q. W. Zhang and Z. S. Liu, Anal. Bioanal. Chem., 2013, 405, 8935–8943 CrossRef CAS PubMed.
- K. Booker, C. I. Holdsworth, C. M. Doherty, A. J. Hill, M. C. Bowyer and A. McCluskey, Org. Biomol. Chem., 2014, 12, 7201–7210 CAS.
- C. Zhang, F. Li, S. X. Wang, Z. S. Liu and H. A. Aisa, Anal. Methods, 2015, 7, 10256–10265 RSC.
- K. Booker, M. C. Bowyer, C. I. Holdsworth and A. McCluskey, Chem. Commun., 2006, 16, 1730–1732 RSC.
- K. Booker, M. C. Bowyer, C. J. Lennard, C. I. Holdsworth and A. McCluskey, Aust. J. Chem., 2007, 60, 51–56 CrossRef CAS.
- X. Y. Li, X. X. Chen, D. D. Zhong, Y. P. Huang and Z. S. Liu, RSC Adv., 2014, 4, 50662–50667 RSC.
- H. Y. Yan, N. Sun, Y. H. Han, C. Yang, M. Y. Wang and R. J. Wu, J. Chromatogr. A, 2013, 1307, 21–26 CrossRef CAS PubMed.
- J. X. He, G. Z. Fang, Y. C. Yao and S. Wang, J. Sep. Sci., 2010, 33, 3263–3271 CrossRef CAS PubMed.
- D. D. Zhong, Y. P. Huang, X. L. Xin, Z. S. Liu and H. A. Aisa, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2013, 934, 109–116 CrossRef CAS PubMed.
- Z. X. Xu, G. Z. Fang and S. Wang, Food Chem., 2010, 119, 845–850 CrossRef CAS.
- C. Y. He, Y. Y. Long, J. L. Pan, K. A. Li and F. Liu, Talanta, 2008, 74, 1126–1131 CrossRef CAS PubMed.
- C. Wang, X. J. Li, J. Yang, Y. X. Zhao, Z. S. Liu and H. A. Aisa, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2017, 1041, 98–103 Search PubMed.
- X. L. Sun, J. He, G. R. Cai, A. Q. Lin, W. J. Zheng, X. Liu, L. X. Chen, X. W. He and Y. K. Zhang, J. Sep. Sci., 2010, 33, 3786–3793 CrossRef CAS PubMed.
- X. Sun, C. Y. Zhao, X. H. Wang, Y. P. Huang and Z. S. Liu, Anal. Bioanal. Chem., 2014, 406, 5359–5367 CrossRef CAS PubMed.
- X. M. Zhang, W. M. Yang, N. W. Wang, X. N. Ni, H. J. Wang, Z. Y. Gu, X. Y. Wu and W. Z. Xu, Adv. Polym. Technol., 2015, 36, 220–229 CrossRef.
- X. Qu, F. Wang, Y. Sun, Y. Tian, R. Chen, X. Ma and C. Liu, RSC Adv., 2016, 6, 86455–86463 RSC.
- L. W. Qian, J. X. Sun, C. Hou, J. F. Yang, Y. W. Li, D. Lei, M. X. Yang and S. F. Zhang, Talanta, 2017, 168, 174–182 CrossRef CAS PubMed.
- L. J. Zhao, J. Yang, H. L. Ye, F. Q. Zhao and B. Z. Zeng, RSC Adv., 2017, 7, 4704–4709 RSC.
- H. L. Liu, G. Z. Fang, C. M. Li, M. F. Pan, C. C. Liu, C. Fan and S. Wang, J. Mater. Chem., 2012, 22, 19882–19887 RSC.
- G. Z. Fang, C. Fan, H. L. Liu, M. F. Pan, H. D. Zhu and S. Wang, RSC Adv., 2014, 4, 2764–2771 RSC.
- Q. H. Wang, G. Z. Fang, Y. Y. Liu, D. D. Zhang, J. M. Liu and S. Wang, Food Anal. Method., 2017, 10, 2585–2592 CrossRef.
- H. L. Liu, G. Z. Fang, H. D. Zhu, C. M. Li, C. C. Liu and S. Wang, Biosens. Bioelectron., 2013, 47, 127–132 CrossRef CAS PubMed.
- Y. Liu, M. J. Wang, Z. Y. Li, H. T. Liu, P. He and J. H. Li, Langmuir, 2005, 21, 1618–1622 CrossRef CAS PubMed.
- Z. X. Xu, J. Zhou, D. Y. Zhao, X. G. Qiao and J. M. Yang, J. Food Sci., 2010, 75, C49–C54 CrossRef CAS PubMed.
- Z. X. Xu, J. M. Song, D. Y. Zhao, J. Zhou and X. G. Qiao, Int. J. Polym. Anal. Charact., 2011, 16, 67–77 CrossRef CAS.
- H. F. Wang, Y. Z. Zhu, X. P. Yan, R. Y. Gao and J. Y. Zheng, Adv. Mater., 2006, 18, 3266–3270 CrossRef CAS.
- H. F. Wang, Y. Z. Zhu, J. P. Lin and X. P. Yan, Electrophoresis, 2008, 29, 952–959 CrossRef CAS PubMed.
- X. Gao, R. Li, G. F. Zhu and J. Fan, Monatsh. Chem., 2015, 146, 475–484 CrossRef CAS.
- X. Gao, J. Fan, G. F. Zhu, X. L. Wang and J. J. Wang, J. Sep. Sci., 2013, 36, 3277–3284 CAS.
- L. F. Yang, X. L. Hu, P. Guan, J. Li, D. F. Wu and B. Gao, J. Appl. Polym. Sci., 2015, 132, 42485 Search PubMed.
- M. A. B. H. Susan, T. Kaneko, A. Noda and M. Watanabe, J. Am. Chem. Soc., 2005, 127, 4976–4983 CrossRef CAS PubMed.
- H. Y. Ma, X. H. Wan, X. F. Chen and Q. F. Zhou, J. Polym. Sci., Part A: Polym. Chem., 2003, 41, 143–151 CrossRef CAS.
- L. Ban, X. Han, X. H. Wang, Y. P. Huang and Z. S. Liu, Anal. Bioanal. Chem., 2013, 405, 8597–8605 CrossRef CAS PubMed.
- L. W. Qian, X. L. Hu, P. Guan, B. Gao, D. Wang, C. L. Wang, J. Li, C. B. Du and W. Q. Song, Anal. Bioanal. Chem., 2014, 406, 7221–7231 CrossRef CAS PubMed.
- N. Zhang, X. L. Hu, P. Guan, C. B. Du, J. Li, L. W. Qian, X. Y. Zhang, S. C. Ding and B. P. Li, Chem. Eng. J., 2017, 317, 356–367 CrossRef CAS.
- F. X. Chen, S. L. Xie, J. H. Zhang and R. Liu, Mater. Lett., 2013, 112, 177–179 CrossRef CAS.
- K. Radosevic, M. C. Bubalo, V. G. Srcek, D. Grgas, T. L. Dragicevic and I. R. Redovnikovic, Ecotoxicol. Environ. Saf., 2015, 112, 46–53 CrossRef CAS PubMed.
- H. G. Morrison, C. C. Sun and S. Neervannan, Int. J. Pharm., 2009, 378, 136–139 CrossRef CAS PubMed.
- X. Li, M. Hou, B. Han, X. Wang and L. Zou, J. Chem. Eng. Data, 2008, 53, 548–550 CrossRef CAS.
- H. G. Liao, Y. X. Jiang, Z. Y. Zhou, S. P. Chen and S. G. Sun, Angew. Chem., Int. Ed., 2008, 47, 9100–9103 CrossRef CAS PubMed.
- Z. Chen, B. Zhou, H. Cai, W. Zhu and X. Zou, Green Chem., 2009, 11, 275–278 RSC.
- H. G. Liao, Y. X. Jiang, Z. Y. Zhou, S. P. Chen and S. G. Sun, Angew. Chem., 2008, 120, 9240–9243 CrossRef.
- S. R. Liang, H. Y. Yan, J. K. Cao, Y. H. Han, S. G. Shen and L. G. Bai, Anal. Chim. Acta, 2016, 951, 68–77 CrossRef PubMed.
- X. X. Li and K. H. Row, RSC Adv., 2017, 7, 16997–17004 RSC.
- X. Li and K. H. Row, Anal. Sci., 2017, 33, 611–617 CrossRef CAS PubMed.
- G. Z. Li, W. S. Ahn and K. H. Row, J. Sep. Sci., 2017, 39, 4465–4473 CrossRef PubMed.
- N. Fu, X. Liu, L. Li, B. Tang and K. H. Row, J. Sep. Sci., 2017, 40, 2286–2291 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |