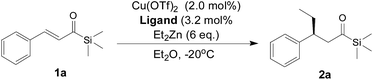
Open Access Article
Open Access ArticleEnantioselective synthesis of chiral acylsilanes by copper/HZNU-Phos-catalyzed asymmetric conjugate addition of diethyzinc to α,β-unsaturated acylsilanes†
Ji-Yuan Lva,
Zheng Xua,
Zhan-Jiang Zhenga,
Li Li*a,
Yu-Ming Cuia,
Jian Caoa,
Ke-Fang Yanga and
Li-Wen Xu *ab
*ab
aKey Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education, Hangzhou Normal University, No 1378, Wenyi West Road, Science Park of HZNU, Hangzhou 311121, P. R. China. E-mail: liwenxu@hznu.edu.cn
bSuzhou Research Insititue, State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences (CAS), P. R. China
First published on 1st December 2017
Abstract
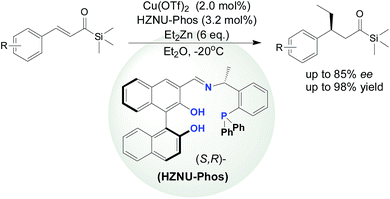
The catalytic asymmetric copper-catalyzed conjugate addition of diethylzinc to α,β-unsaturated acylsilanes was found to proceed smoothly in moderate to good yields and promising enantioselectivities (up to 85% ee) in the presence of the multifunctional HZNU-Phos with both a phosphine center and BINOL-based diol moiety that played a crucial role in the achievement of the best enantioselectivity for this reaction.
Since Brook reported the synthesis of acylsilanes in 1957,1 much effort has been devoted to the synthesis and synthetic utility of acylsilanes in organic chemistry because acylsilanes can be considered as unusual carbonyl compounds bonded with a silicon-based bulky group at the carbonyl sp2 carbon atom.2 Additionally, acylsilanes are a fascinating class of carbonyl compounds, and as such, growing attention has been paid to the utilization of acylsilanes in a diverse range of transformations. Therefore, it is an increasingly attractive moiety in organic synthesis, as demonstrated by the steric and the electronic effects as well as the unique reactivity pattern of the bulky trisubstituted silyl groups.3 As a family of functional acylsilanes, the α,β-unsaturated acylsilanes are very attractive building blocks for organic synthesis.4 While various addition reactions to α,β-unsaturated acylsilanes toward the synthesis of corresponding β-functionalised acylsilanes over past decades,5 to the best of our knowledge, there is rare examples on the conjugate addition of organometallic reagents to α,β-unsaturated acylsilanes, and asymmetric versions still remain sparse. The chemistry is still remarkably underdeveloped in the case of catalytic asymmetric conjugate addition reaction with α,β-unsaturated acylsilanes. There remains, however, a formidable and exciting challenge associated with the endeavor on the development of highly chemo- and stereo-selective β-functionalization process. It occurred to us that copper catalysis could be used to promote conjugate addition of organometallic reagents, such as diethylzinc, to α,β-unsaturated acylsilanes. Although enormous efforts have been performed in the asymmetric 1,4-conjugate addition of diethylzinc to α,β-unsaturated carbonyl compounds,6 the development of new variants of conjugate addition reaction with excellent enantioselectivity remains a challenge (Fig. 1).
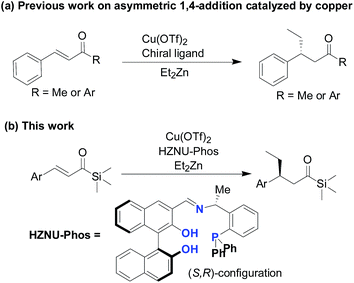 | ||
| Fig. 1 Copper-catalyzed 1,4-conjugate addition of diethylzinc to α,β-unsaturated carbonyl compounds: (a) typical examples of asymmetric 1,4-conjugate addition catalysed by copper6 and (b) copper-catalyzed conjugate addition of diethylzinc to α,β-unsaturated acylsilanes. | ||
Although recent advances have been made in the field of transformations of acylsilanes, this type of conjugate addition reaction continues to present a formidable challenge because it generally generate mixtures of 1,4-adducts and 1,2-adducts (Fig. 2),2 α-hydroxysilanes,7 silyl enol ethers,8 silyl ethers or other side-products depending on the Brook rearrangement reaction (migration of a silyl group from carbon center to an oxygen atom).9 We recently developed a synthetic methodology for the enantioselective copper-catalyzed conjugate addition of diethylzinc and trapping of the zinc enolate employing a multifunctional N,O,P-ligand (HZNU-Phos) bearing multiple stereogenic centers,10 which operates with high level of enantioselectivity for chalcone and 4-phenylbutenone and its analogues. The copper/HZNU-Phos catalyst system exhibited the highest catalytic performance to date in term of enantioselectivity (up to >99% ee) and efficiency (TON = 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600).
600).
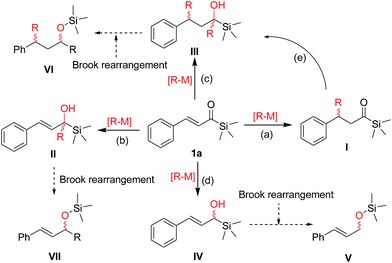 | ||
| Fig. 2 Possible products from the conjugate addition reaction of organometallic reagents [R-M] to α,β-unsaturated acylsilanes. | ||
Herein, using our multifunctional HZNU-Phos ligand mentioned above, we would like to report a copper-catalyzed conjugate addition of diethylzinc to α,β-unsaturated acylsilanes,11 which provides a catalytic method to give various acylsilanes with good enantioselectivity.
Initially, the evaluation of enantioselectivity for the catalytic asymmetric conjugate addition of diethylzinc to α,β-unsaturated acylsilane 1a were made in the presence of various ligands. As shown in Fig. 3, different types of chiral phosphine ligands and multifunctional heteroatom-containing ligands were used in this work.12 All these phosphine ligands with different groups can catalyze the conjugate addition of diethylzinc to α,β-unsaturated acylsilane 1a smoothly with good conversions. It can be seen that varied enantioselectivities were achieved from different ligands (Table 1), and the BINOL-derived multifunctional phosphine ligand (L6, called HZNU-Phos) showed its great impact on the enantioselective conjugate addition reaction. Interestingly, the ligand L5 gave no enantioselectivity compared to that with L6, which supported the important role of two phenol groups in this reaction. In contrast, the BINAP (2,2′-bis(diphenylphophino)-1,1′-binaphthyl) and other phosphine ligands evaluated in this work proved substantially no or low enantioselectivity (<67% ee). The BINOL-derived phosphine L19 that has been used as a highly efficient ligand in copper-catalyzed conjugate addition of Et2Zn, reported firstly by Endo and Shibata,13 proved to be poor ligand in this reaction, which gave the desired product 2a in low enantioselectivity. In addition, there is no activity or enantioselectivity for L5, L9 (Fei-Phos), L13, or L18 (Tao-Phos), albeit these ligands exhibited excellent enantioselectivity in other organic transformations, which further suggested the crucial role of multifunctional group and multiple stereogenic centers on the (S,R)-HZNU-Phos ligands in the copper-catalyzed conjugate addition of diethylzinc to α,β-unsaturated acylsilane 1a. Although the pybox ligands have been used in this reaction, the use of pybox L11 (2,6-bis((S)-4-phenyl-4,5-dihydrooxazol-2-yl)pyridine) and pybox L12 (2,6-bis((S)-4-isopropyl-4,5-dihydrooxazol-2-yl)pyridine) resulted in low to moderate enantioselectivities in this reaction (up to 53% ee). And interestingly, the substituent on the pybox has a large effect on the enantioselectivity. These screenings of ligands appeared to us as that the (S,R)-HZNU-Phos ligand (L6) was still the best choice for the copper-catalyzed conjugate addition of diethylzinc to α,β-unsaturated acylsilane 1a (Table 1).
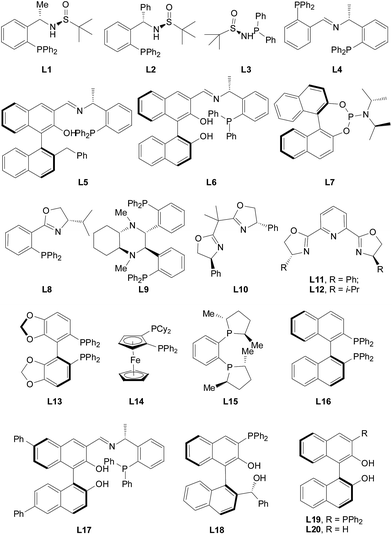 | ||
| Fig. 3 Representative ligands evaluated in the copper-catalyzed asymmetric conjugate addition of diethylzinc to α,β-unsaturated acylsilane 1a. | ||
| Entry | Ligand | Solvent | T (°C) | Yielda (%) | eeb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: α,β-unsaturated acylsilane 1a (0.5 mmol), Cu(OTf)2 (2 mol%), chiral ligand (3.2 mol%). The conversions were detected by GC-MS.b The enantiomeric excess (ee) was determined by chiral HPLC analysis. | |||||
| 1 | L1 | Et2O | −20 °C | 36 | −57 |
| 2 | L2 | Et2O | −20 °C | 48 | −20 |
| 3 | L3 | Et2O | −20 °C | 20 | −9 |
| 4 | L4 | Et2O | −20 °C | 25 | −11 |
| 5 | L5 | Et2O | −20 °C | 79 | 0 |
| 6 | L6 | Et2O | −20 °C | 80 | 85 |
| 7 | L7 | Et2O | −20 °C | 33 | 15 |
| 8 | L8 | Et2O | −20 °C | 28 | 11 |
| 9 | L9 | Et2O | −20 °C | 50 | 0 |
| 10 | L10 | Et2O | −20 °C | 65 | −28 |
| 11 | L11 | Et2O | −20 °C | 77 | −9 |
| 12 | L12 | Et2O | −20 °C | 40 | −53 |
| 13 | L13 | Et2O | −20 °C | 35 | 0 |
| 14 | L14 | Et2O | −20 °C | 56 | −20 |
| 15 | L15 | Et2O | −20 °C | 75 | −67 |
| 16 | L16 | Et2O | −20 °C | 78 | −60 |
| 17 | L17 | Et2O | −20 °C | 37 | −25 |
| 18 | L18 | Et2O | −20 °C | 59 | 0 |
| 19 | L19 | Et2O | −20 °C | 65 | −11 |
| 20 | L20 | Et2O | −20 °C | 27 | −9 |
After the screening of chiral ligands for catalytic conjugate addition of diethylzinc to α,β-unsaturated acylsilane 1a, it was found that the effect of solvents and additive on reaction enantioselectivity were also important. As shown in Table 2, no enantioselectivity was detected in THF, and the difference in enantioselectivity for other solvents was largely, in which the diethyl ether was further confirmed as the best solvent in this reaction (Entry 4). Notably, we have also found that Me2Zn, Ph2Zn, and Et3Al were not suitable organometallic nucleophiles in this copper-catalyzed conjugate addition reaction of 1a (see Table S1 of ESI†).
| Entry | Solvent | Additive | Time (h) | Yield | eeb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.5 mmol), Cu(OTf)2 (2 mol%), (S,R)-HZNU-Phos (3.2 mol%). The conversions were completed (>99%) that detected by GC-MS.b The enantiomeric excess (ee) was determined by chiral HPLC analysis. | |||||
| 1 | THF | — | 9 | 75 | 0 |
| 2 | DCM | — | 9 | 83 | 71 |
| 3 | Toluene | — | 9 | 85 | 69 |
| 4 | Et2O | — | 9 | 80 | 85 |
| 5 | Et2O | KHF2 | 5 | 65 | 76 |
| 6 | Et2O | K2CO3 | 5 | 67 | 76 |
| 7 | Et2O | KHSO4 | 5 | 75 | 78 |
| 8 | Et2O | KH2PO4 | 5 | 68 | 76 |
| 9 | Et2O | Cinchonidine | 5 | 55 | 40 |
| 10 | Et2O | ZnCl2 | 5 | 23 | 11 |
In addition, the desired product was obtained with almost the same level of enantioselectivity when general inorganic bases, such as KHF2, K2CO3, KHSO4, or KH2PO4, were used as additive in this reaction (Table 2, Entries 5–8). However, further improvement with chiral cinchona alkaloid, such as cinchonidine, and with Lewis acid (ZnCl2), were proved to be unsuccessfully because of inferior enantioselectivity in these cases (40% ee and 11% ee respectively, entries 9 and 10). Overall, the adduct 2a could be obtained in 85% ee when the reaction run in Et2O in the presence of Cu(OTf)2/HZNU-Phos (L6) at −20 °C. Therefore, these reaction conditions would be the most suitable for the catalytic asymmetric conjugate addition of diethylzinc to α,β-unsaturated acylsilane at present.
Having identified an efficient chiral phosphine ligand with suitable reaction conditions, we continued to evaluate the substrate scope of the catalytic asymmetric copper-catalyzed asymmetric conjugate addition of diethylzinc to α,β-unsaturated acylsilanes. As shown in Table 3, a variety of aromatic groups on α,β-unsaturated acylsilanes were examined for this reaction, and the corresponding products 2 were obtained in moderate to good yields with promising enantioselectivities (Table 3). Most of α,β-unsaturated acylsilanes resulted in moderate to good ee values. However, the substituted groups on the ortho-position of phenyl ring that derived from aromatic aldehydes were proved to a disadvantageous factor in the enantioselective conjugate addition cycloaddition. For example, the variation of phenyl of substrate 1a to ortho-halide substituted phenyl derivatives were generally found to be underwent conjugate addition in low enantioselectivities but with good isolated yields (Entries 7–10 of Table 3). Furthermore, the use of meta-Cl substituted α,β-unsaturated acylsilane instead of ortho-Cl substituted α,β-unsaturated acylsilane also underwent selective conjugate addition with good enantioselectivity and excellent yields (Entry 11, 98% yield and 68% ee). Unfortunately, the X-ray data of product 3 are not available at present. Notably, in the past years, electronic circular dichroism (ECD) has been proved to be a reliable and alternative option to determine absolute configurations of enantioenriched molecules.14 In order to determine the absolute configuration of the chiral acylsilane product 3, we compared the calculated CD spectrum and experimental CD spectrum of the acylsilane compound 3d (see ESI, Fig. S1–S5†), in which the (R)-configuration of the stereogenic sp3 carbon center on chiral acylsilane product 3d is proposed to be more possibly than that of acylsilane product 3d with (S)-configuration based on the calculated results (Fig. S4†) as well as the experimental CD spectra of chiral 4-pheny-hexan-2-one (S-configuration, Fig. S1†).10
| Entry | R | Yieldb (%) | eec (%) |
|---|---|---|---|
| a Reaction conditions: α,β-unsaturated acylsilane 1 (0.5 mmol), Cu(OTf)2 (2 mol%), chiral ligand is (S,R)-HZNU-Phos (3.2 mol%).b Isolated yield.c The enantiomeric excess (ee) was determined by HPLC analysis on a chiral stationary phase (see ESI for details). | |||
| 1 | H | 3a: 56 | 85 |
| 2 | p-Me | 3b: 40 | 80 |
| 3 | p-Et | 3c: 65 | 72 |
| 4 | p-Cl | 3d: 60 | 66 |
| 5 | p-CF3 | 3e: 60 | 52 |
| 6 | p-Br | 3f: 60 | 72 |
| 7 | o-F | 3g: 32 | 26 |
| 8 | o-Cl | 3h: 68 | 9 |
| 9 | o-Br | 3i: 70 | 34 |
| 10 | o-CF3 | 3j: 40 | 35 |
| 11 | m-Cl | 3k: 98 | 68 |
In summary, we have investigated the catalytic asymmetric conjugate addition of diethylzinc to α,β-unsaturated acylsilanes. This conjugate addition reaction was performed smoothly in moderate to good yields and promising enantioselectivities (up to 85% ee). The preliminary study suggested that the multifunctional HZNU-Phos play a crucial role in this asymmetric conjugate addition reaction, in which both phosphine center and BINOL-based diol moiety were indispensable functional groups to the achievement of the best enantioselectivity for this reaction at present, in which the experimental results further indicate the powerful potential of HZNU-Phos in the asymmetric catalysis. Further studies on the investigation of catalytic performance of HZNU-Phos in catalytic asymmetric transition metal-catalyzed organic transformations are currently underway and will be reported in due future.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This Project was supported by the National Natural Science Foundation of China (No. 21472031, 21703051, and 21773051), Zhejiang Provincial Natural Science Foundation of China (LZ18B020001, LY16E030009, LY17B030005, and LY17E030003), and Science and Technology Department of Zhejiang Province (2015C31138). The authors also thank Dr Z. R. Qu, Dr C. Q. Sheng, Dr K. Z. Jiang, and Dr Q. H. Pan (all at HZNU) for their technical and analytical support.Notes and references
- A. G. Brook, J. Am. Chem. Soc., 1957, 79, 4373 CrossRef CAS.
- H. J. Zhang, D. L. Priebbenow and C. Bolm, Chem. Soc. Rev., 2013, 42, 8540 RSC.
- (a) P. C. Bulman-Page, M. J. McKenzie, S. S. Klair and S. Rosenthal, in The Chemistry of Organosilicon Compounds, Part 2, ed. S. Patai and Z. Rappoport, Wiley, Chichester, UK, 1998, vol. 2, p. 1599 Search PubMed; (b) P. C. Bulman-Page, M. J. McKenzie, S. S. Klair and S. Rosenthal, Patai's Chemistry of Functional Groups, Wiley, 2009, DOI:10.1002/9780470682531.pat0189; (c) P. C. Bulman-Page and M. J. McKenzie, in Science of Synthesis, ed. I. Fleming, Thieme, Stuttgart, 2001, vol. 4, p. 513 Search PubMed; (d) H. Qi and D. P. Curran, in Comprehensive Organic Functional Group Transformations, ed. A. R. Katrinsky, O. Meth-Cohn, C. W. Rees and C. J. Moody, Pergamon, Oxford, UK, 1995, p. 409 Search PubMed; (e) M. A. Brook, Silicon in Organic, Organometallic, and Polymer Chemistry, John Wiley & Sons, New York, 2000 Search PubMed.
- (a) H. J. Reich, M. J. Kelly, R. E. Olson and R. C. Holtan, Tetrahedron, 1983, 39, 949 CrossRef CAS; (b) H. J. Reich and M. J. Kelly, J. Am. Chem. Soc., 1982, 104, 1119 CrossRef CAS.
- (a) R. L. Danheiser and D. M. Fink, Tetrahedron Lett., 1985, 26, 2509 CrossRef CAS; (b) R. L. Danheiser and D. M. Fink, Tetrahedron Lett., 1985, 26, 2513 CrossRef CAS; (c) A. Ricci, A. Degl'Innocenti, G. Borselli and G. Reginato, Tetrahedron Lett., 1987, 28, 4093 CrossRef CAS; (d) K. Narasaka, H. Kusama and Y. Hayashi, Tetrahedron, 1992, 48, 2059 CrossRef CAS.
- For recent examples, see: (a) N. Mistry and S. P. Fletcher, Adv. Synth. Catal., 2016, 358, 2489 CrossRef CAS; (b) T. T. Yang, Y. L. Zhang, P. Cao, M. Wang, L. Li, D. Li and J. Liao, Tetrahedron, 2016, 72, 2707 CrossRef CAS; (c) L. Han, Y. Lei, P. Xing, X. L. Zhao and B. Jiang, J. Org. Chem., 2015, 80, 3752 CrossRef CAS PubMed; (d) B. H. Lipshutz, S. L. Huang, W. W. Y. Leong, G. F. Zhong and N. A. Isley, J. Am. Chem. Soc., 2012, 134, 19985 CrossRef CAS PubMed; (e) K. Dohi, J. Kondo, H. Yamada, R. Arakawa and S. Sakaguchi, Eur. J. Org. Chem., 2012, 7143 CrossRef CAS; (f) M. Magrez, J. Wencel-Delord, A. Alexakis, C. Crevisy and M. Mauduit, Org. Lett., 2012, 14, 3576 CrossRef CAS PubMed; (g) L. Palais, L. Babel, A. Quintard, S. Belot and A. Alexakis, Org. Lett., 2010, 12, 1988 CrossRef CAS PubMed; (h) M. Welker, S. Woodward, L. F. Veiros and M. J. Calhorda, Chem.–Eur. J., 2010, 16, 5620 CrossRef CAS PubMed; (i) M. Sada, T. Furuyama, S. Komagawa, M. Uchiyama and S. Matsubara, Chem.–Eur. J., 2010, 16, 10474 CrossRef CAS PubMed; (j) M. Sada and S. Matsubara, J. Am. Chem. Soc., 2010, 132, 432 CrossRef CAS PubMed; (k) J. Wencel-Delord, A. Alexakis, C. Crevisy and M. Mauduit, Org. Lett., 2010, 12, 4335 CrossRef CAS PubMed; (l) A. Quintard and A. Alexakis, Adv. Synth. Catal., 2010, 352, 1856 CrossRef CAS; For representative reviews, see: (m) A. Alexakis, J. E. Bäckvall, N. Krause, O. Pàmies and M. Diéguez, Chem. Rev., 2008, 108, 2796 CrossRef CAS PubMed.
- (a) P. F. Cirillo and J. S. Panek, J. Org. Chem., 1990, 55, 6071 CrossRef CAS; (b) B. F. Bonini, M. Comes-Franchini, M. Fochi, G. Mazzanti, C. Nanni and A. Ricci, Tetrahedron Lett., 1998, 39, 6737 CrossRef CAS; (c) E. P. Lodge and C. H. Heathcock, J. Am. Chem. Soc., 1987, 109, 3353 CrossRef CAS.
- A. Tsubouchi, N. Sasaki, S. Enatsu and T. Takeda, Tetrahedron Lett., 2013, 54, 1264 CrossRef CAS.
- (a) A. G. Brook, Acc. Chem. Res., 1974, 7, 77 CrossRef CAS; (b) E. J. Corey and S. Lin, J. Am. Chem. Soc., 1996, 118, 8765 CrossRef CAS; (c) R. Unger, T. Cohen and I. Marek, Eur. J. Org. Chem., 2009, 1749 CrossRef CAS; (d) R. Unger, F. Weisser, N. Chinkov, A. Stanger, T. Cohen and I. Marek, Org. Lett., 2009, 11, 1853 CrossRef CAS PubMed; (e) R. Unger, T. Cohen and I. Marek, Tetrahedron, 2010, 66, 4874 CrossRef CAS; (f) R. B. Lettan II, T. E. Reynolds, C. V. Galliford and K. A. Scheidt, J. Am. Chem. Soc., 2006, 128, 15566 CrossRef PubMed; (g) R. B. Lettan II, C. V. Galliford, C. C. Woodward and K. A. Scheidt, J. Am. Chem. Soc., 2009, 131, 8805 CrossRef PubMed; (h) M. Honda, T. Nakajima, M. Okada, K. Yamaguchi, M. Suda, K.-K. Kunimoto and M. Segi, Tetrahedron Lett., 2011, 52, 3740 CrossRef CAS; (i) B. Liu and C. D. Lu, J. Org. Chem., 2011, 76, 4205 CrossRef CAS PubMed; (j) C. Wang, Z. Gan, J. Lu, X. Wu and Z. Song, Tetrahedron Lett., 2011, 52, 2462 CrossRef CAS.
- F. Ye, Z. J. Zheng, W. H. Deng, L. S. Zheng, Y. Deng, C. G. Xia and L. W. Xu, Chem.–Asian J., 2013, 8, 2242 CrossRef CAS PubMed.
- A. Nikolaev and A. Orellana, Org. Lett., 2015, 17, 5796 CrossRef CAS PubMed.
- Z. Xu and L. W. Xu, Chem. Rec., 2015, 15, 925 CrossRef CAS PubMed.
- K. Endo, M. Ogawa and T. Shibata, Angew. Chem., Int. Ed., 2010, 49, 2410 CrossRef CAS PubMed.
- For recent reviews, see: (a) G. Pescitelli, L. D. Bari and N. Berova, Chem. Soc. Rev., 2011, 40, 4603 RSC; (b) D. Slade, D. Ferreira and J. P. J. Marais, Phytochemistry, 2005, 66, 2177 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data, crystallographic data in CIF and NMR spectra. See DOI: 10.1039/c7ra11919d |
| This journal is © The Royal Society of Chemistry 2017 |