Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Electronic and photocatalytic properties of N/F co-doped anatase TiO2†
Yafei Zhao a,
Wei Wanga,
Can Lib and
Liang He*a
a,
Wei Wanga,
Can Lib and
Liang He*a
aNational Laboratory of Solid State Microstructures, School of Electronic Science and Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing 210093, China. E-mail: heliang@nju.edu.cn
bCenter for Coordination Bond Engineering, College of Materials Science and Engineering, China Jiliang University, Hangzhou 310018, China
First published on 5th December 2017
Abstract
The crystal structure, formation energies, electronic structures and effective masses of charge carriers of N/F co-doped anatase TiO2 were investigated by first principle calculations. We have found that the incorporation of the N atom will be easier in the presence of the F dopant. Second, the impurity band of N/F co-doped TiO2 has no spin polarization and smaller effective mass, which improves the photogenerated carriers' mobility and separation. Moreover, it has a lower band edge energy, which increases the oxidation ability of the photogenerated holes. Thus, we explained the mechanism of the enhancement of photocatalytic efficiency of N/F co-doped TiO2 observed from experiments.
1. Introduction
TiO2 has attracted much interest and been widely utilized in photoelectrochemical devices, photovoltaic solar cells and the atmospheric environment, because of its low cost, nontoxicity, chemical inertness, oxidizing potential and high photocatalytic activity.1–4 However, practical applications of TiO2 have been restricted by its large intrinsic band gap. Thus, it can only be excited by ultra-violet (UV) light (about 5% of the solar spectrum).5–7Doping nonmetal or metal elements is considered to be a promising method to reduce the absorption threshold of semiconductor-based photocatalysts (TiO2, ZnO etc.) and to move it from UV to visible light (about 45% of the solar spectrum).8–12 However, it is generally found that single element doped TiO2 has limited visible light photocatalytic performance.13–18 Such as, although much work has suggested that N doping of TiO2 extends the optical absorption edge into the visible light region, increased photocatalytic efficiency is still limited.15,19 On the other hand, co-doping two elements in TiO2 demonstrates remarkable enhancement of photocurrent and photocatalyst effect, such as B and N co-doping,20 C and N co-doping,21,22 C and F co-doping,23 N and S co-doping,24,25 and N and F co-doping.26–30 Among them, N and F co-doping are particular interesting, because the formation of the impurity levels (ILs) effectively lowering the bulk band, and charge compensation effect between N and F atoms reducing the recombination rate of photogenerated electron–hole pairs.9
However, there is almost no relevant theoretical study performed on the influence of photogenerated carrier effective mass and ORR over the enhanced photocatalytic efficiency of the N/F co-doped TiO2. Thus, a systematically first principles study was conducted on the crystal structures, formation energy, electronic structures and effective masses of charge carriers. The results show that the Ti-rich growth condition is favorable to all doped TiO2; meanwhile, the incorporation of the N atom into the TiO2 will be promoted in the presence of the F dopant. Moreover, N/F co-doped TiO2 not only greatly improves the photogenerated carriers' mobility and separation, but also increases the oxidation ability of the photogenerated holes; especially Ns–Fs co-doped TiO2. This explains why ˙OH radical dominates the process of photocatalytic degradation of methylene blue.
2. Computational methods
First-principle density functional theory (DFT) calculations are performed using the CASTEP code. Norm-conserving pseudopotentials and Perdew–Burke–Ernzerhof for Solids (PBESOL) function of the generalized gradient approximation (GGA) are used for the electron–ion interactions and exchange-correlation potential, respectively.31,32 All simulations are carried out for a 2 × 2 × 1 supercell with 16 Ti atoms and 32 O atoms. In the N-doped system, we only consider the N atom substitutes the O atom (Ns), which is consistent with the previous experimental results.15,26 For co-doped TiO2 model, N atom and F atom are simultaneously introduced into the supercell of TiO2, they substitutes the O atom (Ns or Fs) or occupy the interstitial position (Ni or Fi). This is resulted in four different kinds of N/F co-doped TiO2, namely, Ns–Fs-, Ns–Fi-, Ni–Fs- and Ni–Fi-co-doped TiO2. In addition, we have considered the physical doping position of N and F atoms, and calculated the total energies of all possible configurations (based on the doped distance between N and F), as shown in ESI Fig. S1.† Only the most stable configurations for each case of doped TiO2 are shown in Fig. 1. The doping concentration (atom ratio) of the N or F ranges is chosen as around 2.08%, similar to the concentration used in the experiments.26,29,33,34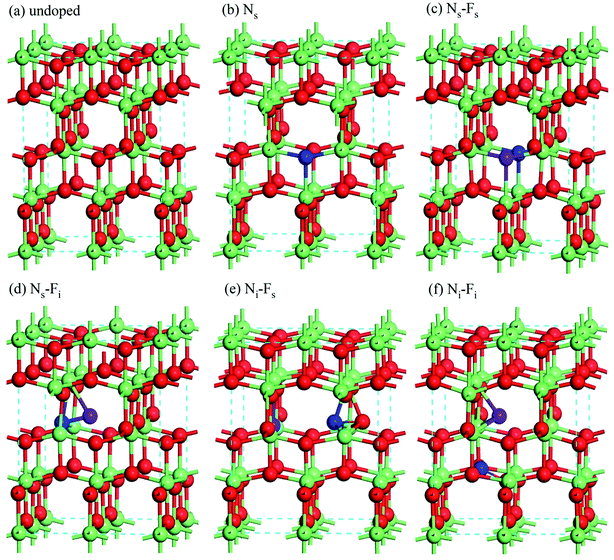 | ||
| Fig. 1 The crystal structures of undoped and doped TiO2, where green, red, blue and purple balls are the Ti, O, N and F atoms, respectively. | ||
The cutoff energy with 750 eV and 4 × 4 × 3 k-point sampling set are sufficiently large for the systems considered. The convergence tolerance of energy, maximum displacement, and maximum force are 5.0 × 10−6 eV per atom, 5.0 × 10−4 Å, and 0.01 eV Å−1, respectively. On the other hand, DFT calculations underestimate the band gap, and DFT+U, as the improvement of standard DFT can overcome this situation. In this work, U values was selected 7.0 eV for Ti elements based on the calculated band-gap for anatase TiO2 as a function of the U parameter in our previous work.35 And, the energy for the band gap (Eg) of undoped anatase TiO2 is 3.05 eV, which is consisted with the experimental value (3.20 eV) and other DFT+U and HSE06 calculated values.36,37
We calculated the Ef to assess the stability of all the doped systems:
| Es = Edoped − Eundoped − mμN − nμF + xμO | (1) |
| μTi + 2μO = μ(TiO2) | (2) |
| μN + 2μO = μ(NO2) | (3) |
| 3μF + μO = μ(OF3) | (4) |
Under O-rich condition, μO is the chemical potential of the ground-state of the O2 molecule; μTi, μN and μF are obtained by the eqn (2), (3) and (4), respectively. Under Ti-rich condition, the μTi is the chemical potential of Ti bulk, while μO, μN and μF are calculated by the eqn (2), (3) and (4), respectively. The simulation bond lengths of the O2, NO2 and OF3 molecules are close to the experimental value.38
3. Result and discussion
3.1 Crystal structure and formation energies
Table 1 lists the calculated lattice constants of the undoped and doped TiO2. The lattice constants of undoped TiO2 is consistent with the previous experimental results.39 Overall, the effect of dopants and various doped models on the lattice constants and volume is very minor, within 0.74% and 1.37%, respectively. The reason may be that the atomic radius of the dopant is similar to that of the O atom. However, further observation from Fig. 1 found that dopants significantly affect the local structure and thus the electronic properties dramatically.| System | TiO2 | Ns | Ns–Fs | Ns–Fi | Ni–Fs | Ni–Fi | |
|---|---|---|---|---|---|---|---|
| a | 7.551 | 7.587 | 7.564 | 7.573 | 7.565 | 7.602 | |
| b | 7.551 | 7.543 | 7.607 | 7.554 | 7.595 | 7.550 | |
| c | 9.627 | 9.615 | 9.589 | 9.696 | 9.655 | 9.695 | |
| V | 548.91 | 550.25 | 551.74 | 554.67 | 554.74 | 556.45 | |
| Ef | Ti-rich | — | −1.79 | −6.92 | −3.73 | −4.77 | −0.01 |
| O-rich | — | 5.77 | 4.01 | 4.68 | 3.63 | 5.89 | |
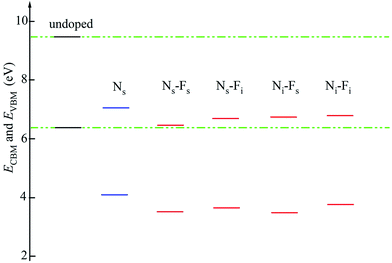
| EF | 6.41 | 4.08 | 4.09 | 3.65 | 4.10 | 5.19 | |
| ECBM | 9.46 | 7.06 | 6.47 | 6.70 | 6.72 | 6.79 | |
| EVBM | 6.41 | 4.08 | 3.53 | 3.65 | 3.48 | 3.74 | |
| Eg | 3.05 | 2.98 | 2.94 | 3.05 | 3.24 | 3.05 | |
| Emax | 3.05 | 1.88 | 2.39 | 3.05 | 2.61 | 1.49 | |
| m* | CBM | 0.35 | 0.38 | 0.40 | 0.42 | 0.46 | 0.39 |
| IL | — | 5.43 | 1.21 | — | 2.73, 1.08 | 1.63, 2.10, 2.27 | |
| VBM | 0.92 | 1.28 | 0.95 | 1.30 | 1.25 | 1.21 | |
We have calculated the Ef to assess the stability of dopants into TiO2, as shown in Table 1. All of them are positive (or negative) under O-rich (or Ti-rich) conditions. Negative Ef suggests that dopant tends to enter the TiO2 lattice. Thus, this confirms that the N(F) atoms prefer to substitute the O atom under Ti-rich conditions, since more oxygen vacancies are present in this case.29,34 Meanwhile, the Ef of these doped systems have the relative relationships: Ef(Ns–Fs) < Ef(Ni–Fs) < Ef(Ns–Fi) < Ef(Ns) < Ef(Ni–Fi) under Ti-rich conditions. Thus, Ns–Fs co-doped TiO2 is the most stable system, due to its lowest Ef(−6.92 eV). Moreover, the Ef of all co-doped TiO2 (except for Ni–Fi co-doped TiO2) is smaller than N doped TiO2 indicates that the incorporation of the N atoms into the TiO2 will be easier to achieve in the presence of the F dopant. Further, considering the relationship between Ef and μO, a variety of N/F co-doped TiO2 can be synthesized by controlling the flow of O2 and supplying enough energy.
3.2 Electronic properties
To demonstrate how doped atoms modify the electronic properties of TiO2, the band structures and partial density of states (PDOS) of individual Ti, O, N and F atoms of all the systems are shown in Fig. 2 and 3, respectively. And the Eg, the Fermi level (EF), the maximum energy gap in the band gap (Emax), the energy of conduction band minimum (CBM) (ECBM) and valence band maximum (VBM) (EVBM) are listed in Table 1. | ||
| Fig. 2 The band structures of undoped and doped TiO2, where the blue full (red dash) lines represent the spin up (spin down) states and the horizontal dashed lines denote the Fermi level (EF). | ||
The band gap of TiO2 is mostly controlled by O-2p and Ti-3d states, but the ILs of the dopants can also modify it. Thus the dopants can fine tune the band gap of the doped system. The details of the band gap tuning can be described as the followings.
For Ns doped TiO2 (Fig. 2b and 3b), the valence band now has the half-filled Ns-2p state which is just above the original VBM, while the conduction band (CB) remain unchanged. Thus, the valence band (VB) lifts toward to the CB and the Eg is reduced by 0.07 eV. Similar to this, the filled IL of the Ns–Fs co-doped TiO2 (Fig. 2c and 3c) is also above the VBM. Thus, the Eg is lowered to 2.94 eV compared to undoped TiO2. For the case of Ns–Fi co-doped TiO2 (Fig. 2d and 3d) and Ni–Fi co-doped TiO2 (Fig. 2f and 3f), the ILs overlap the original VB and CB, thus the band gap remains the same. For the case of Ni–Fs co-doped TiO2 (Fig. 2e and 3e), the filled ILs locate in the middle of the band gap. And the VB is lowered due to missing one O atom. Thus the band gap increases compared to the undoped TiO2. This verifies that N and F atoms can modulate the electronic structure of the TiO2, by changing the position and amount of ILs. Furthermore, the spin polarization states present in the Ns doped TiO2 is not observed in the co-doped system due to the charge compensation effect between N and F atom.
Overall, the doped system only reduces the band gap below 0.11 eV. In other words, the maximum red-shifted optical absorption edge is only 15.3 nm. However, the ILs in the band gap not only reduce the Eg, but also separate and promote photo-excited electrons pumping from the VB into the CB. These characteristics are likely to improve visible light photocatalytic activity. More details will be discussed later.
3.3 Effective mass of photogenerated carriers
We all know that the limited number of density of states and the low charge carrier mobility in the flat ILs limits its role as a stepping stones for electronic transition, and the electrons are easily annihilated by recombination with holes. The carrier mobility has been calculated according to the equation μ = eτ/m*, where τ is the mean free time, m* is the effective mass (both related to the curvature of the band level).40,41 Smaller efficient masses mean higher carrier mobility. The m* of all the systems were presented in Table 1. The results show that there is a flat IL (m* = 5.43) in the Ns doped TiO2 that limits the separation of photogenerated carriers. In contrast, all co-doped systems have smaller m*, especially Ns–Fs co-doped TiO2 (m* = 1.21). Thus, this IL suppresses the recombination rate. We believe that Ns–Fs co-doped TiO2 will have the highest photocatalytic efficiency.3.4 Band edge energy and photocatalytic properties
The band edges energy (EVBM and ECBM) determines the redox potential of the semiconductor photocatalyst. Fig. 4 and Table 1 indicate that the EVBM and ECBM of all doped TiO2 move down to the low energy region (more than 2.33 eV), compared with the undoped TiO2. Such as, the oxidizability of photogenerated holes at the VBM is enhanced 2.33–2.93 eV, while the reducibility of photogenerated electrons at the CBM is reduced 2.40–2.99 eV. Moreover, the band edges energy of N/F co-doped TiO2 move more to the lower energy region compared with Ns doped TiO2. So, they have greater ability to modify the redox potentials; especially the Ns–Fs co-doped TiO2.On the other hand, O2− ion and ˙OH radical all are important oxidants in the process of photocatalytic degradation of methylene blue. The degradation mechanism is shown in Fig. 5. On the surface of TiO2, O2− ions are reduced by the photogenerated electrons from the O2, while ˙OH radicals are oxidized by the photogenerated holes from the OH−. Thus, all doped systems tend to produce more ˙OH than O2− during the photocatalytic process. For that, as discussed above, we conclude that Ns–Fs co-doped model can explain the mechanism of enhanced photocatalytic activity in the recent experiments that the ˙OH radical played a leading role during the visible light photocatalytic process.33,42–45 Meanwhile, volumes were 550.25 and 551.74 Å3 for Ns doped and Ns–Fs co-doped TiO2. This expanded surface area also promotes the photocatalytic ability of Ns–Fs co-doped TiO2.
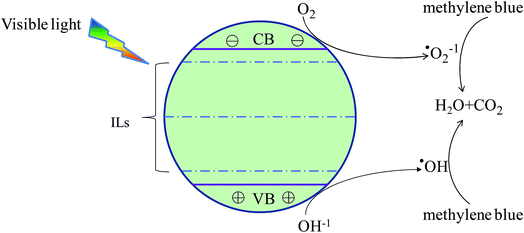 | ||
| Fig. 5 The mechanism for photocatalytic degradation of MB by doped TiO2 under visible light irradiation. | ||
4. Conclusions
In summary, the crystal structure, formation energy, electronic structures and effective masses of charge carriers of N doped and N/F co-doped TiO2 were investigated by first-principles calculations. We have found that the Ti-rich growth condition is beneficial to all doped TiO2. Meanwhile, the incorporation of the N atom into the TiO2 will be easier to achieve in the presence of the F dopant. N/F co-doped TiO2 has no spin polarization, smaller effective mass and lower band edge energy. They, especially Ns–Fs co-doped TiO2, not only greatly improve the photogenerated carriers' mobility and separation, but also increase the oxidation ability of the photogenerated holes. Thus, we have explained the mechanism of the enhancement of photocatalytic efficiency of N/F co-doped TiO2 observed by experiments. Moreover, this work also provides new insights into synthesis and design of the various-doped TiO2 by controlling the flow of O2 during sample preparation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Key Research and Development Program of China (No. 2016YFA0300803), the National Basic Research Program of China (No. 2014CB921101), the National Natural Science Foundation of China (No. 61474061, 61674079). Jiangsu Shuangchuang Program and the Natural Science Foundation of Jiangsu Province of China (No. BK20140054). Computational resources were provided by the Jilin University.References
- A. L. Linsebigler, G. Lu and J. T. Yates, Chem. Rev., 1995, 95, 735 CrossRef CAS.
- M. R. Hoffmann, S. T. Martin, C. Wonyong and D. W. Bahnemann, Chem. Rev., 1995, 95, 69 CrossRef CAS.
- I. Nakamura, S. Kutsuna, T. Ihara, S. Sugihara and K. Takeuchi, J. Mol. Catal. A: Chem., 2000, 161, 205 CrossRef CAS.
- Y. Q. Wang, R. R. Zhang, J. B. Li, L. L. Li and S. W. Lin, Nanoscale Res. Lett., 2014, 9, 46 CrossRef PubMed.
- X. P. Cao, D. Li, W. H. Jing, W. H. Xing and Y. Q. Fan, J. Mater. Chem., 2012, 22, 15309 RSC.
- S. U. M. Khan, M. A. Shahry and W. B. Ingler Jr, Science, 2002, 297, 2243 CrossRef CAS PubMed.
- H. Wang and J. P. Lewis, J. Phys.: Condens. Matter, 2006, 18, 421 CrossRef CAS.
- C. Li, Y. F. Zhao, Y. Y. Gong, T. Wang and C. Q. Sun, Phys. Chem. Chem. Phys., 2014, 16, 21446 RSC.
- C. D. Valentin and G. Pacchioni, Catal. Today, 2013, 206, 12 CrossRef.
- Y. F. Zhao, C. Li, J. Y. Hu, Y. Y. Gong, L. Y. Niu and X. J. Liu, Phys. Lett. A, 2016, 380, 910 CrossRef CAS.
- J. Q. Wen, X. Li, W. Liu, Y. P. Fang, J. Xie and Y. H. Xu, Chin. J. Catal., 2015, 36, 2049 CrossRef CAS.
- W. L. Yu, J. F. Zhang and T. Y. Peng, Appl. Catal., B, 2016, 181, 220 CrossRef CAS.
- Y. F. Zhao, C. Li, S. Lu and L. J. Yan, Chem. Phys. Lett., 2016, 647, 36 CrossRef CAS.
- J. G. Yu, P. Zhou and Q. Li, Phys. Chem. Chem. Phys., 2013, 15, 12040 RSC.
- C. D. Valentin, E. Finazzi, G. Pacchioni, A. Selloni, S. Livraghi, M. C. Paganini and E. Giamello, Chem. Phys., 2007, 338, 44 CrossRef.
- C. Y. Jimmy, J. G. Yu, W. Ho, Z. T. Jiang and L. Z. Zhang, Chem. Mater., 2002, 14(9), 3808 CrossRef.
- J. Xu, B. F. Yang, M. Wu, Z. P. Fu, Y. Lv and Y. X. Zhao, J. Phys. Chem. C, 2010, 114, 15251 CAS.
- Y. Y. Wu, Y. M. Ding, X. F. Xia, X. Liu and H. X. Li, Appl. Surf. Sci., 2016, 364, 829 CrossRef CAS.
- M. S. Akple, J. X. Low, Z. Y. Qin, S. Wageh, A. A. Al-Ghamdi, J. G. Yu and S. W. Liu, Chin. J. Catal., 2015, 36, 2127 CrossRef CAS.
- J. Georgieva, E. Valova, S. Armyanov, D. Tatchev, S. Sotiropoulos, I. Avramova, N. Dimitrova, A. Hubin and O. Steenhaut, Appl. Surf. Sci., 2017, 413, 284 CrossRef CAS.
- J. S. Zhou, F. Z. Li, C. Du, J. M. Liu, Y. Z. Wang, W. Li, G. N. He and Q. Y. He, RSC Adv., 2016, 6, 84457 RSC.
- L. Hao, Z. W. Wang, Y. Q. Zheng, Q. Q. Li, S. J. Guan, Q. Zhao, L. J. Cheng, Y. Lu and J. Z. Liu, Appl. Surf. Sci., 2017, 391, 275 CrossRef CAS.
- P. Zhou, J. H. Wu, W. L. Yu, G. H. Zhao, G. J. Fang and S. W. Cao, Appl. Surf. Sci., 2014, 319, 167 CrossRef CAS.
- K. Shen, X. Xue, X. Y. Wang, X. Y. Hu, H. W. Tian and W. Zheng, RSC Adv., 2017, 7, 23319 RSC.
- P. Zhou, J. G. Yu and Y. X. Wang, Appl. Catal., B, 2013, 45, 142 Search PubMed.
- C. D. Valentin, E. Finazzi and G. Pacchioni, Chem. Mater., 2008, 20, 3706 CrossRef.
- B. P. Dhamanir, A. Kumar, A. K. Srivastava and J. S. Tawale, Res. Chem. Intermed., 2017, 43, 387 CrossRef.
- J. W. J. Hamilton, J. A. Byrne, P. S. M. Dunlop, D. D. Dionysiou, M. Pelaez, K. O'Shea, D. Synnott and S. C. Pillai, J. Phys. Chem. C, 2014, 118, 12206 CAS.
- S. H. Shin, H. H. Chun and W. K. Jo, Materials, 2015, 8, 31 CrossRef PubMed.
- J. Y. Cheng, J. Chen, W. Lin, Y. D. Liu and Y. Kong, Appl. Surf. Sci., 2015, 332, 573 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- D. R. Hamann, M. Schluter and C. Chiang, Phys. Rev. Lett., 1979, 43, 1494 CrossRef CAS.
- Y. Lv, Z. P. Fu, B. F. Yang, J. Xu, M. Wu, C. Q. Zhu and Y. X. Zhao, Mater. Res. Bull., 2011, 46, 361 CrossRef CAS.
- N. Janpetch, C. Vanichvattanadecha and R. Rujiravanit, Cellulose, 2015, 22, 3321 CrossRef CAS.
- C. Li, J. C. Li, J. S. Lian and Q. Jiang, J. Appl. Phys., 2009, 106, 094102 CrossRef.
- M. E. Arroyo-de Dompablo, A. M. Garcia and M. Taravillo, J. Chem. Phys., 2011, 135, 054503 CrossRef CAS PubMed.
- P. Deak, B. Aradi and T. Frauenheim, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 155207 CrossRef.
- http://www.webelements.com.
- M. Horn, C. F. Schwerdtfeger and E. P. Meagher, Z. Kristallogr., 1972, 136, 273 CrossRef CAS.
- A. R. West, Basic Solid State Chemistry, John. Wiley, Chichester, 1999, p. 49 Search PubMed.
- T. H. Wang, Y. F. Zhou and Q. Jiang, J. Phys. Chem. C, 2013, 117, 12873 CAS.
- Q. Guo, Z. H. Zhang, X. P. Ma, K. Jing, M. L. Shen, N. Yu, J. H. Tang and D. D. Dionysiou, Sep. Purif. Technol., 2017, 175, 305 CrossRef CAS.
- A. E. Giannakas, E. Seristatidou, Y. Deligiannakis and I. Konstantinou, Appl. Catal., B, 2013, 132, 460 CrossRef.
- A. V. Katsanaki, A. G. Kontos, T. Maggos and M. Pelaez, Appl. Catal., B, 2013, 140, 619 CrossRef.
- D. Li, N. Ohashi, S. Hishita, T. Kolodiazhnyi and H. Haneda, J. Solid State Chem., 2005, 178, 3293 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra10546k |
| This journal is © The Royal Society of Chemistry 2017 |