Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Copper-catalyzed direct coupling of benzoxazin-2-ones with indoles for the synthesis of diverse 3-indolylbenzoxazin-2-ones: access to natural cephalandole A†
Rameshwar Prasad Pandit a,
Jae-Jin Shim
a,
Jae-Jin Shim a,
Sung Hong Kimb and
Yong Rok Lee
a,
Sung Hong Kimb and
Yong Rok Lee *a
*a
aSchool of Chemical Engineering, Yeungnam University, Gyeongsan, 38541, Republic of Korea. E-mail: yrlee@yu.ac.kr; Fax: +82-53-810-4631; Tel: +82-53-810-2529
bAnalysis Research Division, Daegu Center, Korea Basic Science Institute, Daegu 41566, Republic of Korea
First published on 5th December 2017
Abstract
A novel and facile copper-catalyzed direct coupling for the synthesis of diverse and functionalized 3-indolyl benzoxazin-2-ones from benzoxazin-2-ones and indoles has been developed. This new methodology offers an easy and rapid approach to a variety of 3-indolylbenzo[b][1,4]oxazin-2-ones in high yield. As an application of this protocol, a gram-scale synthesis of naturally occurring cephalandole A has also been accomplished.
Introduction
Benzoxazines and benzoxazin-2-ones are important heterocyclic compounds found in natural products and biologically active molecules (Fig. 1).1,2 These compounds possess a wide range of pharmaceutical properties such as antihypertensive,3 antifungal,4 antimycobacterial,5 anti-inflammatory,6 bacterial histidine protein kinase inhibitory,7 and D2 receptor antagonist activities.8 In addition, compound 1 exhibits a potent effect of pyruvate kinase activators for the treatment of hereditary nonspherocytic hemolytic anemia and sickle cell anemia9 and compound 2 is useful for the treatment of lung cancer.10 Naturally occurring alkaloid, cephalandole A was originally isolated from Taiwanese orchid Cephalanceopsis gracilis11 and its structure was later revised into 3 by organic structure determination using atomic resolution scanning probe microscopy.12 Moreover, molecules bearing these skeletons have been also used as valuable building blocks for the synthesis of pharmaceuticals and photoactive materials.13,14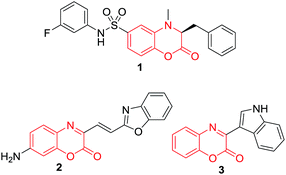 | ||
| Fig. 1 Selected examples of naturally occurring and pharmaceutically active molecules bearing benzoxazin-2-one moiety. | ||
Owing to the importance of benzoxazin-2-ones, several methods for their synthesis have been reported.15,16 The general methods for benzoxazin-2-ones include the domino reaction of o-aminophenol with β-nitroacrylates,17 cleavage of resin-bound pseudooxazolones with 2-aminophenols,18 and TFA-catalyzed tandem reaction of benzoxazoles with 2-oxo-2-arylacetic acids.19 In addition, enantioselective hydrogenation of benzoxazinones and enantioselective addition of indoles to ketimines to give chiral dihydrobenzoxazinones have been accomplished.20,21
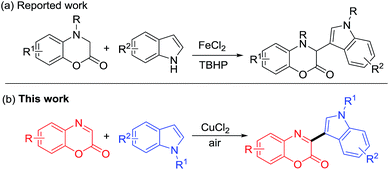
Although several methodologies for the synthesis of benzoxazin-2-ones and dihydrobenzoxazinones have been developed, there are no reports on the direct coupling of benzoxazin-2-ones with indoles for the construction of 3-indolylbenzoxazin-2-ones so far. Recently, an iron-catalyzed oxidative sp3 carbon–hydrogen bond functionalization of dihydrobenzoxazin-2-ones with indoles for the synthesis of 3-indolyldihydrobenzoxazin-2-ones has been described (Scheme 1a).22 As a part of continuing efforts to develop new synthetic protocols for nitrogen heterocycles,23 we herein report the copper-catalyzed direct coupling of benzoxazin-2-ones with indoles for the formation of diverse 3-indolyl benzoxazin-2-ones in air (Scheme 1b).
Results and discussion
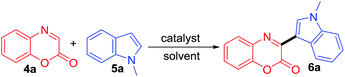
Our initial study commenced with the model reaction between benzoxazin-2-one 4a and N-methylindole 5a for the optimization of reaction condition (Table 1). Various metals were examined as catalysts under several solvents in air. When using 10 mol% of CoCl2, ZnCl2 and NiCl2 at 80 °C for 24 h in dichloroethane, product 6a was isolated in 32, 40, and 41% yields, respectively (entries 1–3, Table 1). Encouraged by these results, we screened other catalysts for the reaction. With 10 mol% of FeCl3 and CuCl2, the yield of 6a increased to 80 and 89% respectively (entries 4–5). However, additional attempt using other copper catalysts such as CuF2, Cu(OAc)2, and Cu(OTf)2, failed to further increase the yield (entries 6–8). Results of solvent screening showed that tetrahydrofuran (THF) was the best solvent (94%) among the solvents such as dioxane (87%), ethanol (66%), and water (60%) (entries 9–12). Changes in the loading of CuCl2 to 5 mol%, 2 mol%, and 13 mol% did not improve the yield of 6a (entries 13–15). In addition, the effect of temperature was next studied. It was found out that decreasing or increasing temperature decreased the yield of 6a (entries 16 and 17). The structure of 6a was determined by spectroscopic analysis. The 1H NMR spectrum of 6a showed a characteristic singlet singlet for indolyl C2 proton at δ 8.60 ppm and N-methyl peak on indolyl moiety at δ 3.85 ppm.| Entry | Catalyst | Solvent | Temp (°C) | Time [h] | Yieldb [%] |
|---|---|---|---|---|---|
| a Reaction conditions: 4a (0.5 mmol), 5a (0.5 mmol), and catalyst (10 or 5 mol%) in solvent (3.0 mL) under air.b Yield of the isolated product 6a after column chromatography. | |||||
| 1 | CoCl2 (10 mol%) | ClCH2CH2Cl | 60 | 24 | 32 |
| 2 | ZnCl2 (10 mol%) | ClCH2CH2Cl | 60 | 24 | 40 |
| 3 | NiCl2 (10 mol%) | ClCH2CH2Cl | 60 | 24 | 41 |
| 4 | FeCl3 (10 mol%) | ClCH2CH2Cl | 60 | 24 | 80 |
| 5 | CuCl2 (10 mol%) | ClCH2CH2Cl | 60 | 24 | 89 |
| 6 | CuF2 (10 mol%) | ClCH2CH2Cl | 60 | 24 | 80 |
| 7 | Cu(OAc)2 (10 mol%) | ClCH2CH2Cl | 60 | 24 | 78 |
| 8 | Cu(OTf)2 (10 mol%) | ClCH2CH2Cl | 60 | 12 | 85 |
| 9 | CuCl2 (10 mol%) | THF | 60 | 12 | 94 |
| 10 | CuCl2 (10 mol%) | Dioxane | 60 | 12 | 87 |
| 11 | CuCl2 (10 mol%) | EtOH | 60 | 12 | 87 |
| 12 | CuCl2 (10 mol%) | H2O | 60 | 12 | 60 |
| 13 | CuCl2(5 mol%) | THF | 60 | 12 | 91 |
| 14 | CuCl2 (2 mol%) | THF | 60 | 24 | 78 |
| 15 | CuCl2 (13 mol%) | THF | 60 | 6 | 82 |
| 16 | CuCl2 (10 mol%) | THF | 50 | 12 | 90 |
| 17 | CuCl2 (10 mol%) | THF | 70 | 10 | 85 |
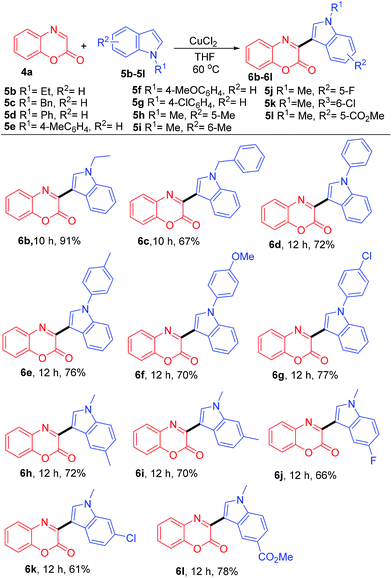
With the optimized reaction condition in hand, we further investigated the substrates scope employing different indoles 5b–5m (Table 2). Reaction of 4a with indoles 5b–5d bearing N-ethyl, N-benzyl, and N-phenyl moieties provided the desired products 6b–6d in 91, 67, and 72% yield, respectively. Treatment of 4a with N-arylated indoles 5e–5g having electron-donating or electron-withdrawing groups on the N-aryl ring, such as 4-Me, 4-OMe, and 4-Cl afforded the corresponding products 6e–6g in 76%, 70%, and 77% yield, respectively. Indoles 5h–5l bearing electron-donating or electron-withdrawing groups on the benzene ring were successful to afford the desired products. For example, reaction with N-methylindoles 5h–5i bearing electron-donating groups like methyl at 5- and 6-position on the aryl ring provided 6h (72%) and 6i (70%), respectively. The reaction of N-methylindoles 5j–5l bearing electron-withdrawing groups (5-F, 6-Cl, and 5-CO2Me) afforded products 6j–6l in 66, 61, and 78% yield, respectively.
| a Reaction condition: 4a (0.5 mmol), 5b–5l (0.5 mmol), CuCl2 (10 mol%), THF (3.0 mL), 60 °C, time (h), and isolated yield. |
|---|
 |
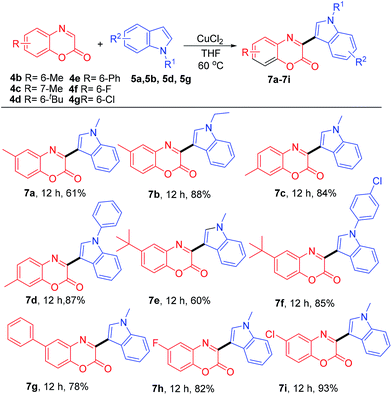
To demonstrate the versatility of this coupling reaction, further reactions between various substituted benzoxazin-2-ones 4b–4g and several N-substituted indoles 5a, 5b, 5d, and 5g were examined (Table 3). The reactions of 4b–4e bearing electron-donating groups such as 6-methyl, 7-methyl, 6-tert-butyl, and 6-phenyl with N-substituted indoles 5a, 5b, 5d, or 5g provided products 7a–7g in the range of 60–88% yield. The reactions of 4f and 4g bearing electron-withdrawing groups of 6-F and 6-Cl with 5a afforded the products 7h and 7i in 82% and 93% yield, respectively.
| a Reaction condition: 4b–4g (0.5 mmol), 5a, 5b, 5d, 5g (0.5 mmol), CuCl2 (10 mol%), THF (3.0 mL), 60 °C, time (h), and isolated yields. |
|---|
 |
The utility of this new methodology for the gram-scale synthesis of naturally occurring cephalandole A (3) was next demonstrated (Scheme 2). Upon treatment of 4a with indole 5m at 60 °C for 12 h in THF, 3 was obtained in 75% yield. This one-pot protocol has several advantages such as higher yield, fewer steps, and lower cost. The synthesized compound was confirmed to be natural product 3 by comparison of its spectroscopic data with those previously reported.22
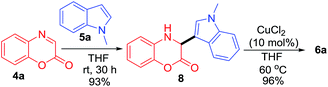
To elucidate the mechanism of this coupling reaction, we performed a control experiment (Scheme 3). The reaction between 4a with 5a in the absence of CuCl2 in THF at room temperature for 30 h provided compound 8 in 93% yield. Further reaction of 8 in the presence of 10 mol% of CuCl2 in THF at 60 °C for 1 h furnished 6a in 96% yield. These results suggest that compound 8 might be the intermediate in the coupling reaction.
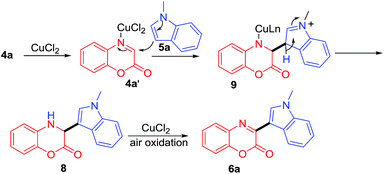
Based on the above experiment, the mechanism for the formation of 6a is proposed as shown in Scheme 4. First, CuCl2 catalyst binds to 4a gives complex 4a′, which subsequently undergoes nucleophilic attack by 5a to give 9. Deprotonation and protonation of 9 would afford intermediate 8, which undergoes air oxidation to give 6a24
Conclusions
In summary, a novel and efficient copper-catalyzed direct coupling of benzoxazin-2-ones with indoles for the synthesis of diverse and functionalized 3-indolylbenzoxazin-2-ones has been developed. This methodology provides a rapid synthetic route to natural cephalandole A and its derivatives. The proposed protocol has a wide substrate scope for both benzoxazin-2-ones and indoles.Experimental
Imino cyclic esters were synthesized in the laboratory according to known procedure.25 All indoles were prepared by either N-alkylation or N-arylation according to known method.26 Solvents were used without further purification. Merck precoated silica gel plates (Art. 5554) with fluorescent indicator were used for analytical TLC. Flash column chromatography was performed using silica gel 9385 (Merck). Melting points are uncorrected and were determined on Fisher-Johns Melting Point Apparatus. 1H NMR and 13C NMR spectra were recorded on a Varian VNS (600 and 150 MHz, respectively) spectrometer in CDCl3 using δ = 7.24 and 77.00 ppm as solvent chemical shift. Chemical shifts (δ) are expressed in units of ppm and coupling constants (J) values are given in Hz. Multiplicities are abbreviated as follows; s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, dd = doublet of doublet and td = triplet of doublet. FT-IR (neat) spectra were recorded on ATR (PerkinElmer Spectrum 2) and HRMS was obtained on JEOL JMS-700 spectrometer at Korean Basic Science Institute.General procedure for synthesis of 3-indolylbenzoxazin-2-ones
To the solution of imino cyclic esters (0.5 mmol) and indoles (0.5 mmol) in THF (3.0 mL), CuCl2 (7 mg, 10 mol%) was added at room temperature and heated at 60 °C for 3–24 h. Upon completion of reaction as indicated by thin layer chromatography, the reaction mixture was concentrated under reduced pressure, and the crude material was purified by column chromatography (hexane/ethyl acetate = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford the desired compounds.
1) to afford the desired compounds.
Gram-scale synthesis of cephalandole A
The solution of 2H-benzo[b][1,4]oxazin-2-one 4a (1.0 gram, 6.80 mmol) and indole 5m (0.81 gram, 6.80 mmol) in THF (15 mL), CuCl2 (48 mg, 10 mol%) was added at room temperature and heated at 60 °C for 12 h. Upon completion of reaction as indicated by thin layer chromatography, the reaction mixture was concentrated under reduced pressure, and the crude material was purified by column chromatography (hexane/ethyl acetate = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford cephalandole A (3, 1.337 gram, 75%); mp 237–239 °C; 1H NMR (600 MHz, acetone-d6) δ 11.07 (s, 1H), 8.89 (dd, J = 6.6, 4.8 Hz, 1H), 8.82 (d, J = 3.0 Hz, 1H), 7.89 (dd, J = 7.8, 1.2 Hz, 1H), 7.59–7.55 (m, 1H), 7.49 (td, J = 7.8, 1.8 Hz, 1H), 7.43 (td, J = 7.8, 1.8 Hz, 1H), 7.35 (dd, J = 7.8, 1.2 Hz, 1H), 7.30–7.26 (m, 2H); 13C NMR (150 MHz, acetone-d6) δ 152.95, 149.01, 146.19, 137.82, 134.53, 133.17, 129.53, 128.83, 127.32, 126.07, 124.13, 124.05, 122.43, 116.71, 112.72, 112.33; ATR-IR (neat) 3285, 1715, 1602, 1530, 1429 cm−1; HRMS (EI) m/z (M+) calcd for C16H10N2O2: 262.0742; found: 262.0740.
1) to afford cephalandole A (3, 1.337 gram, 75%); mp 237–239 °C; 1H NMR (600 MHz, acetone-d6) δ 11.07 (s, 1H), 8.89 (dd, J = 6.6, 4.8 Hz, 1H), 8.82 (d, J = 3.0 Hz, 1H), 7.89 (dd, J = 7.8, 1.2 Hz, 1H), 7.59–7.55 (m, 1H), 7.49 (td, J = 7.8, 1.8 Hz, 1H), 7.43 (td, J = 7.8, 1.8 Hz, 1H), 7.35 (dd, J = 7.8, 1.2 Hz, 1H), 7.30–7.26 (m, 2H); 13C NMR (150 MHz, acetone-d6) δ 152.95, 149.01, 146.19, 137.82, 134.53, 133.17, 129.53, 128.83, 127.32, 126.07, 124.13, 124.05, 122.43, 116.71, 112.72, 112.33; ATR-IR (neat) 3285, 1715, 1602, 1530, 1429 cm−1; HRMS (EI) m/z (M+) calcd for C16H10N2O2: 262.0742; found: 262.0740.
Control experiments
The solution of 4a (147 mg, 1.0 mmol) and N-methylindole 5a (130 mg, 1.0 mmol) in THF (3.0 mL) was stirred at room temperature 30 h. Upon completion of reaction as indicated by thin layer chromatography, the reaction mixture was concentrated under reduced pressure, and the crude material was purified by column chromatography (hexane/ethyl acetate = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 8 as a solid (258 mg, 93%); mp 170–172 °C; 1H NMR (600 MHz, CDCl3) δ 7.67 (d, J = 7.8 Hz, 1H), 7.29 (d, J = 8.4 Hz, 1H), 7.26–7.23 (m, 1H), 7.16–7.13 (m, 1H), 7.07 (dd, J = 7.8, 1.2 Hz, 1H), 7.00 (td, J = 7.8, 1.2 Hz, 1H), 6.98 (s, 1H), 6.87 (td, J = 7.8, 1.2 Hz, 1H), 6.76 (dd, J = 7.8, 1.2 Hz, 1H), 5.33 (s, 1H), 3.70 (s, 4H). 13C NMR (150 MHz, CDCl3) δ 165.15, 141.31, 137.10, 132.83, 127.74, 125.99, 124.99, 122.42, 120.36, 119.99, 119.24, 116.89, 115.04, 109.69, 109.63, 52.56, 32.89; ATR-IR (neat) 3346, 1735, 1529, 1119, 737 cm−1; HRMS (EI) m/z (M+) calcd for C17H14N2O2: 278.1055; found: 278.1058.
1) to afford 8 as a solid (258 mg, 93%); mp 170–172 °C; 1H NMR (600 MHz, CDCl3) δ 7.67 (d, J = 7.8 Hz, 1H), 7.29 (d, J = 8.4 Hz, 1H), 7.26–7.23 (m, 1H), 7.16–7.13 (m, 1H), 7.07 (dd, J = 7.8, 1.2 Hz, 1H), 7.00 (td, J = 7.8, 1.2 Hz, 1H), 6.98 (s, 1H), 6.87 (td, J = 7.8, 1.2 Hz, 1H), 6.76 (dd, J = 7.8, 1.2 Hz, 1H), 5.33 (s, 1H), 3.70 (s, 4H). 13C NMR (150 MHz, CDCl3) δ 165.15, 141.31, 137.10, 132.83, 127.74, 125.99, 124.99, 122.42, 120.36, 119.99, 119.24, 116.89, 115.04, 109.69, 109.63, 52.56, 32.89; ATR-IR (neat) 3346, 1735, 1529, 1119, 737 cm−1; HRMS (EI) m/z (M+) calcd for C17H14N2O2: 278.1055; found: 278.1058.
To the solution of 8 (139 mg, 0.5 mmol) in THF (3.0 mL), CuCl2 (7 mg, 10 mol%) was added at room temperature and heated at 60 °C for 1 h. Upon completion of reaction as indicated by thin layer chromatography, the reaction mixture was concentrated under reduced pressure, and the crude material was purified by column chromatography (hexane/ethyl acetate = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 6a as a solid (132 mg, 96%).
1) to afford 6a as a solid (132 mg, 96%).
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Nano Material Technology Development Program of the Korean National Research Foundation (NRF) funded by the Korean Ministry of Education, Science, and Technology (2012M3A7B4049675) and Priority Research Centers Program (2014R1A6A1031189).Notes and references
- (a) Antibiotics and Antiviral Compounds, ed. K. Krohn, H. A. Kirst and H. Maag, VCH, Weinheim, 1993 Search PubMed; (b) Pharmaceutical Substances, ed. A. Kleemann, J. Engel, B. Kutscher and D. Reichert, Thieme, Stuttgart, New York, 4th edn, 2001 Search PubMed; (c) Houben-Weyl Methods of Organic Chemistry, Hetarenes IV, Six-Membered and Large Hetero-Rings with Maximum Unsaturation, ed. J. Teller and E. Schaumann, Georg Thieme, Stuttgart, 1997, vol. E9a, pp. 141–177 Search PubMed; (d) J. Ilaš, P. S. Anderluh, M. S. Dolenc and D. Kikelj, Tetrahedron, 2005, 61, 7325 CrossRef; (e) B. Achari, S. B. Mandal, P. K. Dutta and C. Chowdhury, Synlett, 2004, 2449 CAS.
- (a) T. Hasui, N. Matsunaga, T. Ora, N. Ohyabu, N. Nishigaki, Y. Imura, Y. Igata, H. Matsui, T. Motoyaji, T. Tanaka, N. Habuka, S. Sogabe, M. Ono, C. S. Siedem, T. P. Tang, C. Gauthier, L. A. DeMeese, S. A. Boyd and S. Fukumoto, J. Med. Chem., 2011, 54, 8616 CrossRef CAS PubMed; (b) H. Matsuoka, N. Maruyama, K. Tsuji, N. Kato, T. Akimoto, Y. Takeda, K. Yano and T. Kuroki, J. Med. Chem., 1997, 40, 105 CrossRef CAS PubMed; (c) S. M. Bromidge, B. Bertani, M. Borriello, S. Faedo, L. J. Gordon, E. Granci, M. Hill, H. R. Marshall, L. P. Stasi, V. Zucchelli, G. Merlo, A. Vesentini, J. M. Watson and L. Zonzini, Bioorg. Med. Chem. Lett., 2008, 18, 5653 CrossRef CAS PubMed; (d) S. M. Bromidge, B. Bertani, M. Borriello, A. Bozzoli, S. Faedo, M. Gianotti, L. J. Gordon, M. Hill, V. Zucchelli, J. M. Watson and L. Zonzini, Bioorg. Med. Chem. Lett., 2009, 19, 2338 CrossRef CAS PubMed; (e) T. Hasui, T. Ohra, N. Ohyabu, K. Asano, H. Matsui, A. Mizukami, N. Habuka, S. Sogabe, S. Endo, C. S. Siedem, T. P. Tang, C. Gauthier, L. A. De Meese, S. A. Boyd and S. Fukumoto, Bioorg. Med. Chem., 2013, 21, 5983 CrossRef CAS PubMed.
- F. Touzeau, A. Arrault, G. Guillaumet, E. Scalbert, B. Pfeiffer, M.-C. Rettori, P. Renard and J.-Y. Mérour, J. Med. Chem., 2003, 46, 1962 CrossRef CAS PubMed.
- K. Waisser, L. Kubicová, V. Buchta, P. Kubanová, K. Bajerová, L. Jirásková, O. Bednařík, O. Bureš and P. Holŷ, Folia Microbiol., 2002, 47, 488 CrossRef CAS.
- (a) K. Waiser, M. Peřina, J. Kuneš, V. Klimešová and J. Kaustová, Il Farmaco, 2003, 58, 1137 CrossRef PubMed; (b) S. Konda, S. Raparthi, K. Bhaskar, R. K. Munagati, V. Guguloth, L. Nagarapu and D. M. Akkerwar, Bioorg. Med. Chem. Lett., 2015, 25, 1643 CrossRef CAS PubMed.
- (a) A. Khalaj, M. Abdollahi, A. Kebriaeezadeh, N. Adibpour, Z. Pandi and S. Rasoulamini, Indian J. Pharmacol., 2002, 34, 184 CAS; (b) I. V. Mashevakaya, L. V. Anikina, Y. B. Vikharev, V. A. Safin, S. V. Koltsova and A. N. Maslivets, Pharm. Chem. J., 2001, 35, 414 CrossRef.
- R. Frechette and M. A. Weidner-Wells, WO Patent Appl. 9717333, 1997.
- L. D. Wise, D. J. Wustrow and T. Belliotti, WO Patent Appl. 9745419, 1997.
- S. U. S.-S. Michael, WO Patent 2012151440, 2012.
- C. Li and G. Xiaoxia, Patent CN 103664919 A, 2014.
- (a) P.-L. Wu, Y.-L. Hsu and C.-W. Jao, J. Nat. Prod., 2006, 69, 1467 CrossRef CAS PubMed; (b) Y.-T. Wu, Y.-L. Hsu and P.-L. Wu, Heterocycles, 2008, 75, 1191 CrossRef CAS.
- (a) J. J. Mason, J. Bergman and T. Janosik, J. Nat. Prod., 2008, 71, 1447 CrossRef CAS PubMed; (b) L. Gross, F. Mohn, N. Moll, G. Meyer, W. M. Abdel-Megeed and M. Jaspars, Nat. Chem., 2010, 2, 821 CrossRef CAS PubMed.
- (a) Q. Chen, M. Chen, C. Yu, L. Shi, D. Wang, Y. Yang and Y. Zhou, J. Am. Chem. Soc., 2011, 133, 16432 CrossRef CAS PubMed; (b) M. Rueping, A. P. Antonchick and T. Theissmann, Angew. Chem., Int. Ed., 2006, 45, 6751 CrossRef CAS PubMed; (c) C. Saitz, H. Rodŕıguez, A. Márquez, A. Cánete, C. Jullian and A. Zanocco, Synth. Commun., 2001, 31, 135 CrossRef CAS; (d) H. Miyabe, Y. Yamaoka and Y. Takemoto, J. Org. Chem., 2006, 71, 2099 CrossRef CAS PubMed.
- (a) R. A. Duval, G. Lewin, E. Peris, N. Chahboune, A. Garofano, S. Drçse, D. Cortes, U. Brandt and R. Hocquemiller, Biochemistry, 2006, 45, 2721 CrossRef CAS PubMed; (b) V. L. Gein, N. A. Rassudikhina, N. V. Shepelina, M. I. Vakhrin, E. B. Babushkina and E. V. Voronina, Pharm. Chem. J., 2008, 42, 519 Search PubMed; (c) X. Li, N. Liu, H. Zhang, S. E. Knudson, R. A. Slayden and P. J. Tonge, Bioorg. Med. Chem. Lett., 2010, 20, 6306 CrossRef CAS PubMed; (d) M. Hu, J. Fan, H. Li, K. Song, S. Wang, G. Cheng and X. Peng, Org. Biomol. Chem., 2011, 9, 980 RSC; (e) S. Bondock, S. Adel, H. A. Etman and F. A. Badria, Eur. J. Med. Chem., 2012, 48, 192 CrossRef CAS PubMed; (f) K. Azuma, S. Suzuki, S. Uchiyama, T. Kajiro, T. Santa and K. Imai, Photochem. Photobiol. Sci., 2003, 2, 443 RSC; (g) T. Nishio, J. Chem. Soc., Perkin Trans. 1, 1990, 565 RSC; (h) S. Nonell, L. R. Feñrrares, A. Caete, E. Lemp, G. Günther, N. Pizarro and A. L. Zanocco, J. Org. Chem., 2008, 73, 5371 CrossRef CAS PubMed.
- (a) Y. M. Lee and Y. S. Park, Heterocycles, 2009, 78, 2233 CrossRef CAS; (b) S. Luo and J. K. De Brabander, Tetrahedron Lett., 2015, 56, 3179 CrossRef CAS; (c) R. I. Storer, D. E. Carrera, Y. K. Ni and D. W. C. MacMillan, J. Am. Chem. Soc., 2006, 128, 84 CrossRef CAS PubMed; (d) M. Rueping, A. P. Antonchick and T. Theissmann, Angew. Chem. Int. Ed., 2006, 45, 6751 CrossRef CAS PubMed; (e) F. Shi, W. Tan, H.-H. Zhang, M. Li, Q. Ye, G.-H. Ma, S.-J. Tu and G.-G. Li, Adv. Synth. Catal., 2013, 355, 3715 CrossRef CAS; (f) A. V. Maikov, K. Vrankova, S. Stoncius and P. Kocovsky, J. Org. Chem., 2009, 74, 5839 CrossRef PubMed; (g) X.-Z. Peng, W.-C. Yuan and X.-M. Zhang, Adv. Synth. Catal., 2010, 352, 2132 CrossRef; (h) Q.-A. Chen, M.-W. Chen, C.-B. Yu, L. Shi, D.-S. Wang, Y. Yang and Y.-G. Zhou, J. Am. Chem. Soc., 2011, 133, 16432 CrossRef CAS PubMed; (i) Q.-A. Chen, K. Gao, Y. Duan, Z.-S. Ye, Y. Yang and Y.-G. Zhou, J. Am. Chem. Soc., 2012, 134, 2442 CrossRef CAS PubMed; (j) L.-Q. Lu, Y.-H. Li, K. Junge and M. Beller, J. Am. Chem. Soc., 2015, 137, 2763 CrossRef CAS PubMed; (k) J. L. Núňez-Rico and A. Vidal-Ferran, Org. Lett., 2013, 15, 2066 CrossRef PubMed.
- (a) C. Trebaul, J. Roncali, F. Garnier and R. Guglielmetti, Bull. Chem. Soc. Jpn., 1987, 60, 2657 CrossRef CAS; (b) R. B. Moffet, J. Med. Chem., 1966, 9, 475 CrossRef; (c) A. Chilin, A. Confente, G. Pastorini and A. Guiotto, Eur. J. Org. Chem., 2002, 1937 CrossRef CAS; (d) D. N. Nicolaides, D. R. Gautam, K. E. Litinas, D. J. Hadjipavlou-Litina and C. A. Kontogiorgis, J. Heterocycl. Chem., 2004, 41, 605 CrossRef CAS; (e) D. N. Nicolaides, R. W. Awad and E. A. Varella, J. Heterocycl. Chem., 1996, 33, 633 CrossRef CAS; (f) I. Yavari, S. Souri, M. Sirouspour and H. Djahaniani, Synthesis, 2006, 3243 CrossRef CAS; (g) N. Zidar and D. Kikelj, Tetrahedron, 2008, 64, 5756 CrossRef CAS; (h) R. Ballini, A. Palmieri, M. A. Talaq and S. Gabrielli, Adv. Synth. Catal., 2009, 351, 2611 CrossRef CAS.
- R. Ballini, A. Palmieri, M. A. Talaq and S. Gabrielli, Adv. Synth. Catal., 2009, 351, 2611 CrossRef CAS.
- S. Gräßle, S. Vanderheiden, P. Hodapp, B. Bulat, M. Nieger, N. Jung and S. Bräse, Org. Lett., 2016, 18, 3598 CrossRef PubMed.
- S. Yan, L. Ye, M. Liu, J. Chen, J. Ding, W. Gao, X. Huang and H. Wu, RSC Adv., 2014, 4, 16705 RSC.
- (a) L.-Q. Lu, Y. Li, K. Junge and M. Beller, J. Am. Chem. Soc., 2015, 137, 2763 CrossRef CAS PubMed; (b) Q.-A. Chen, K. Gao, Y. Duan, Z.-S. Ye, L. Shi, Y. Yang and Y.-G. Zhou, J. Am. Chem. Soc., 2012, 134, 2442 CrossRef CAS PubMed; (c) Q.-A. Chen, M.-W. Chen, C.-B. Yu, L. Shi, D.-S. Wang, Y. Yang and Y.-G. Zhou, J. Am. Chem. Soc., 2011, 133, 16432 CrossRef CAS PubMed; (d) M. Rueping, A. P. Antonchick and T. Theissmann, Angew. Chem. Int. Ed., 2006, 45, 6751 CrossRef CAS PubMed.
- T. Kano, R. Takechi, R. Kobayashi and K. Maruoka, Org. Biomol. Chem., 2014, 12, 724 CAS.
- C. Huo, J. Dong, Y. Su, J. Tang and F. Chen, Chem. Commun., 2016, 52, 13341 RSC.
- (a) R. P. Pandit, S. H. Kim and Y. R. Lee, Adv. Synth. Catal., 2016, 358, 3586 CrossRef CAS; (b) R. P. Pandit, S. H. Kim and Y. R. Lee, Org. Biomol. Chem., 2016, 14, 6996 RSC; (c) R. P. Pandit and Y. R. Lee, Adv. Synth. Catal., 2015, 357, 2657 CrossRef CAS; (d) R. P. Pandit, K. Sharma and Y. R. Lee, Synthesis, 2015, 47, 3881 CrossRef CAS; (e) R. P. Pandit and Y. R. Lee, RSC Adv., 2013, 3, 22039 RSC.
- (a) S. E. Allen, R. R. Walvoord, R. Padilla-Salinas and M. C. Kozlowski, Chem. Rev., 2013, 113, 6234 CrossRef CAS PubMed; (b) S. D. McCann and S. S. Stahl, Acc. Chem. Res., 2015, 48, 1756 CrossRef CAS PubMed.
- P.-L. Shao, J.-Y. Liao, Y. A. Ho and Y. Zhao, Angew. Chem. Int. Ed., 2014, 53, 5435 CrossRef CAS PubMed.
- (a) Y. Wu, X. Peng, B. Luo, F. Wu, B. Liu, F. Song, P. Huang and S. Wen, Org. Biomol. Chem., 2014, 12, 9777 RSC; (b) R. R. Singh and R.-S. Liu, Chem. Commun., 2017, 53, 4593 RSC; (c) F. Damkaci, A. Alawaed and E. Vik, Tetrahedron Lett., 2016, 57, 2197 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra10634c |
| This journal is © The Royal Society of Chemistry 2017 |