Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Reactive molecular dynamics study of the decomposition mechanism of the environmentally friendly insulating medium C3F7CN†
Xiaoxing Zhang a,
Yi Li
a,
Yi Li a,
Dachang Chena,
Song Xiao*a,
Shuangshuang Tiana,
Ju Tanga and
Ran Zhuob
a,
Dachang Chena,
Song Xiao*a,
Shuangshuang Tiana,
Ju Tanga and
Ran Zhuob
aSchool of Electrical Engineering, Wuhan University, BaYi Street No. 299, Wuhan 430072, Hubei Province, China. E-mail: xiaosongxs@gmail.com; Tel: +86 18986238962
bElectric Power Research Institute, China Southern Power Grid, Guangzhou 510623, China
First published on 30th October 2017
Abstract
The extensive use of sulfur hexafluoride (SF6) gas in the power industry has a strong greenhouse effect. Hence, many scholars are committed to studying SF6 alternative gases to achieve green power development. In the past two years, C3F7CN (heptafluoroisobutyronitrile) has attracted the attention of many scholars due to its excellent insulation and environmental protection characteristics as a potential alternative gas. This study theoretically explores the decomposition characteristics of C3F7CN and the C3F7CN/CO2 gas mixture based on the reactive molecular dynamics method and density functional theory. The main decomposition pathways of C3F7CN and the enthalpy of each path at different temperatures were analyzed. The yield of the main decomposition products was obtained under several temperature conditions. The decomposition of C3F7CN mainly produced CF3, C3F7, CN, CNF, CF2, CF, F, and other free radicals and a few molecular products, such as CF4 and C3F8. The C3F7CN/CO2 gas mixture has more excellent decomposition characteristics than that of the pure C3F7CN. The addition of CO2 effectively ensures that the gas mixture has a low liquefaction temperature, which is considerably suitable for use as a gas insulation medium. The relevant research results provide guidance for the further exploration on the electrical properties and practical engineering application of the C3F7CN gas mixture.
1 Introduction
SF6 (sulfur hexafluoride) is the most common insulation medium used in high-voltage (HV) electrical equipment such as gas insulated switchgear (GIS).1 However, SF6 is a greenhouse gas (GHG) regulated under the Kyoto Protocol with a global warming potential (GWP) of 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800.2,3 Although the proportion of greenhouse effect caused by the use of SF6 is not as large as the amount of fossil fuel energy consumed, the global atmospheric content of SF6 has increased by 20% in the past five years and the atmospheric mole fraction of SF6 is 7.28 ppq currently corresponding to a radiative forcing of 0.0041 w m−2.4,5 THe greenhouse effect has caused widespread public concern in recent years.6 Climate deterioration caused by SF6 has gradually become one of the contradictions between power industry development and environmental protection. Hence, the power industry should gradually limit the use of SF6 and search for an environmentally friendly alternative gas for SF6 in gas-insulated equipment.
800.2,3 Although the proportion of greenhouse effect caused by the use of SF6 is not as large as the amount of fossil fuel energy consumed, the global atmospheric content of SF6 has increased by 20% in the past five years and the atmospheric mole fraction of SF6 is 7.28 ppq currently corresponding to a radiative forcing of 0.0041 w m−2.4,5 THe greenhouse effect has caused widespread public concern in recent years.6 Climate deterioration caused by SF6 has gradually become one of the contradictions between power industry development and environmental protection. Hence, the power industry should gradually limit the use of SF6 and search for an environmentally friendly alternative gas for SF6 in gas-insulated equipment.
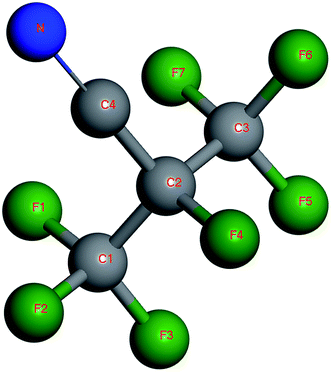
C3F7CN (heptafluoroisobutyronitrile) is a potential alternative gas for SF6 (see Fig. 1 for its molecular structure) with a GWP value of 2100 and Ozone Depression Potential (ODP) value of 0.4 The dielectric strength of C3F7CN is 2.74 times that of SF6.7 It should be used in combination with CO2 or other buffer gases with low liquefaction temperature due to its high liquefaction temperature (i.e., −4.7 °C). In addition, the toxicity of the C3F7CN/CO2 gas mixture with C3F7CN volume fraction below 10% is lower than that of pure SF6. Thus, the former is harmless to humans.8 These physical and chemical properties indicate that C3F7CN is excellent in terms of environmental protection, insulation and safety, and has immense potential to replace SF6.
In the past two years, many scholars have proven that the C3F7CN/CO2 gas mixture has excellent insulation and arc extinguishing performance through numerous tests.3,7–10 For engineering applications, a gas insulated line (GIL) with a rated voltage of 420 kV and a current transformer (CT) with a rated voltage of 245 kV using the C3F7CN/CO2 gas mixture as insulating medium were tested in 2015.3,8 In addition, the investigation of the decomposition properties of a gas-insulating medium is an important component to comprehensively evaluate its performance. On the one hand, the insulation defects caused by aging under long-term operating conditions in electrical equipment will lead to partial discharge (PD) or flashover and is accompanied by the decomposition of the insulating medium. The local overheating fault can also lead to the decomposition of the insulating medium. On the other hand, the decomposition characteristics of the insulating medium are closely related to its self-recovery characteristics and arc-extinguishing properties.
At present, researches on the decomposition characteristics of C3F7CN have achieved noteworthy results. Kieffel et al. investigated the pyrolysis characteristics of the C3F7CN/CO2 gas mixture. They found the initial decomposition temperature of C3F7CN was 650 °C, thereby mainly producing decomposition products, such as CO, CF3CN, and C2F5CN.8 Andersen et al. investigated the atmospheric chemistry of C3F7CN and evaluated its environmental effects based on density functional theory and infrared spectroscopy.11 Our team has explored the decomposition mechanism of C3F7CN in the presence of trace water impurity through quantum chemical calculation. The ionization parameters of C3F7CN and various decomposition products were analyzed from the perspective of molecular structure, thereby providing a theoretical reference for the further study of the C3F7CN decomposition characteristics.12
At present, only a few studies have been conducted on the decomposition characteristics of the C3F7CN/CO2 gas mixture. Our previous studies on the decomposition mechanism of C3F7CN were from the perspective of individual molecule and did not consider the decomposition process in multi-molecular and gas mixture systems. In this paper, we constructed the multi-molecular and C3F7CN/CO2 gas mixture model to study the decomposition process of C3F7CN using the reactive force field molecular dynamics (ReaxFF-MD) method and density functional theory (DFT). The decomposition paths, reaction enthalpy, and products distribution of the C3F7CN gas mixture at different temperatures were firstly investigated. Relevant results provide guidance for further exploration on electrical properties and practical engineering application of C3F7CN gas mixture.
2 Theoretical methods and computational details
2.1 ReaxFF-MD simulation details
ReaxFF is based on bond order to describe the dissociation and formation of covalent bonds at the atomic level13 and is a beneficial tool for studying chemical reactions and predicting the performance of new materials. During the past more than ten years, ReaxFF has been used to treat a variety of elements and multifunctional systems and is suitable for describing polyatomic systems.14–16 The energy expression of ReaxFF is presented as follows:| Esystem = Ebond + Eover + Eunder + Eval + Epen + Etors + Econj + EvdWaals + ECoulomb, | (1) |
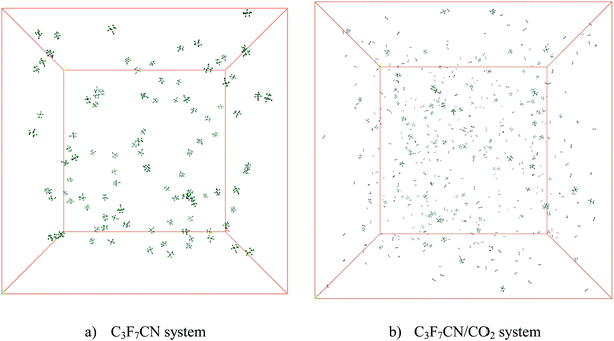
Two periodic cubic boxes were built to explore the decomposition of C3F7CN and the C3F7CN/CO2 gas mixture (see Fig. 2). The C3F7CN system contains 100 C3F7CN molecules with a density of 0.00811 g cm−3. The length of the box is 159 Å. At present, many scholars have tested the insulation characteristics of C3F7CN/CO2 mixture and found that mixtures with 20% C3F7CN displayed dielectric strengths comparable to SF6.4,7 In order to reveal the decomposition properties of mixtures in this scale, we built the C3F7CN/CO2 system which contains 100 C3F7CN molecules and 400 CO2 molecules with a density of 0.00351 g cm−3. The length of the box is 260 Å. The initial density corresponds to the actual density of C3F7CN under 25 °C and 0.1 Mpa. The system was minimized with the NVE ensemble for 5 ps at 5 K and equilibrated with the NVT ensemble thereafter for 10 ps at 1000 K using a time step of 0.1 fs.16 The NVT simulations were then conducted at different temperatures for 1000 ps to explore the decomposition process of C3F7CN and C3F7CN/CO2 gas mixture. The temperature was controlled by the method of a Berendsen thermostat with a 0.1 ps damping constant.17 All the ReaxFF-MD simulations in this paper were performed based on the ReaxFF module of ADF (Amsterdam Density Functional).18
2.2 DFT calculation details
The first principle of calculations based on the density functional theory (DFT) was performed to obtain the precise dissociation energy of C3F7CN at different temperatures. This theory is widely used in the study of chemical reaction mechanisms, and can describe any given chemical system with high accuracy.19–21The spin unrestricted DFT calculation in the current study was performed based on the Dmol 3 module of the Materials studio 8.0. The meta-generalized approximation (mGGA) method treated by M06L functions is used to describe the exchange-correlation energy. This local density functional does well for predicting geometries and vibrational frequencies and gives great performance for a combination of main-group thermochemistry and thermochemical kinetics.22,23 The double numerical atomic orbital augmented by d-polarization (DNP) is used as the basis set. Geometry optimizations were performed with the following convergence parameters: (1) energy convergence tolerance of 1.0 × 10−5 Ha, (2) maximum force of 0.002 Ha Å−1, and (3) maximum displacement of 0.005 Å. It should be pointed that spin contamination (unrestricted DFT is much less affected by it than unrestricted Hartree–Fock theory24) and wave function unstability may occur in spin unrestricted calculation. The self-consistent-field (SCF) solution stability is checked to ensure the accuracy of calculation results.
The enthalpy correction is presented as follows to obtain the precise energy of the system at a given temperature:25
| ΔE = ∑(Eproduct + ΔEproduct) − ∑(Ereactant + ΔEreactant), | (2) |
3 Results and discussion
3.1 Decomposition of the pure C3F7CN
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 K.26–28 The current study analyzed the decomposition mechanism of C3F7CN and its gas mixture at different temperatures to clarify the particle composition and product change. It should be noted that the time scale of ReaxFF MD simulation is normally limited to several dozens of nanoseconds due to the computational cost.28,29 Hence, we enhance the temperatures to accelerate the simulation process to allow reactions can be observed.
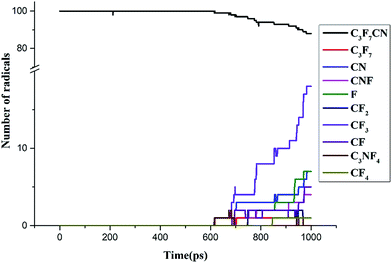
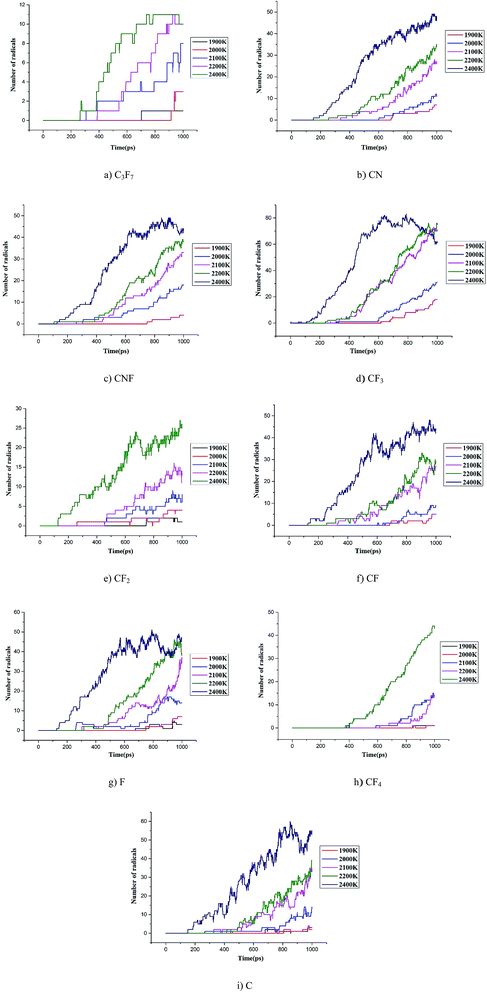
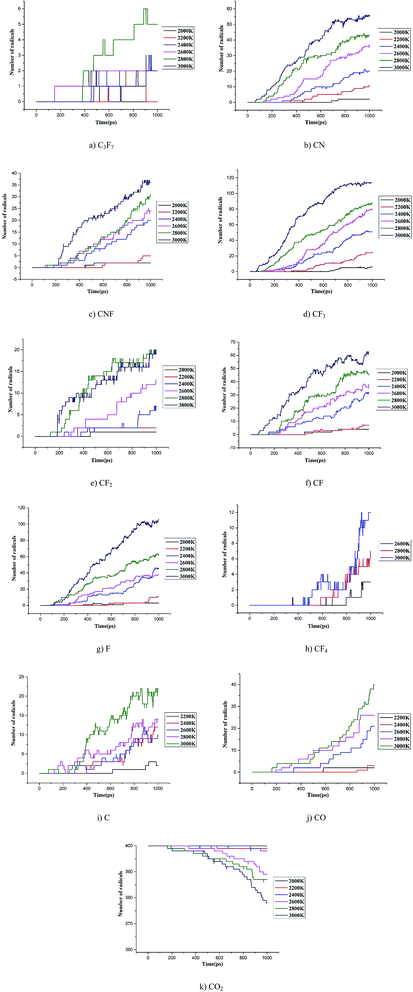
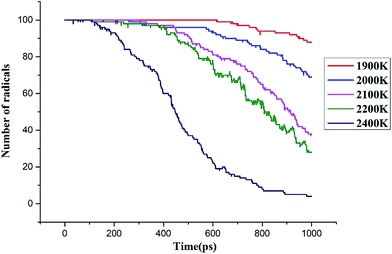
000 K.26–28 The current study analyzed the decomposition mechanism of C3F7CN and its gas mixture at different temperatures to clarify the particle composition and product change. It should be noted that the time scale of ReaxFF MD simulation is normally limited to several dozens of nanoseconds due to the computational cost.28,29 Hence, we enhance the temperatures to accelerate the simulation process to allow reactions can be observed.First, the multi-molecular ReaxFF molecular dynamic simulations of C3F7CN were performed and demonstrated that the C3F7CN molecule began to decompose at 1900 K. Fig. 3 shows the time evolution of the major species of the C3F7CN decomposition at 1900 K. Table 1 shows the generation time of the major species and corresponding decomposition pathways. At 1900 K, the C3F7CN decomposition mainly produces free radicals, such as CF3, C3F7, CN, CNF, CF2, CF, CF3CFCN(C3NF4), and F. It is important to note that some free radicals, such as CN, are harmful free radicals and could produce toxic molecules. The content and toxicity of the decomposition products of C3F7CN need further test study before engineering application.
| Major species | Generation time (ps) | Reaction | ΔEa (kcal mol−1) | |
|---|---|---|---|---|
| a T = 1900 K, at mGGA-M06L level with ZPE and enthalpy corrections. | ||||
| C3NF4, CF3 | 615 | A | C3F7CN → CF3 + CF3CFCN | 72.67 |
| C3F7 | 702.5 | B | C3F7CN → C3F7 + CN | 101.33 |
| C4F6N | — | C | C3F7CN → (CF3)2CCN + F | 89.17 |
| CN, CF | 685.625 | D | CF3CFCN → CF3 + CF + CN | 174.66 |
| CF2, F | 748.125 | E | CF3 → CF2 + F | 79.04 |
| CNF | 748.125 | F | CN + F → CNF | −118.48 |
| CF4 | 845.625 | G | CF3 + F → CF4 | −107.64 |
| C | — | H | CF → C + F | 121.23 |
C3F7CN began to decompose at 615 ps, thereby producing CF3CFCN and CF3. This reaction path is required to absorb 72.67 kcal mol−1. Another decomposing path began at 702.5 ps, thereby producing C3F7 and CN and needs to absorb 101.33 kcal mol−1. In addition, the decomposition of CF3CFCN is at 685.625 ps, thereby generating CF3, CF, and CN. CF3 can further decompose to CF2 and F by absorbing 79.04 kcal mol−1, CF can further decompose to C and F by absorbing 121.23 kcal mol−1. CF4 is one of the stable decomposition products, which is formed by CF3 and F and releases −107.64 kcal mol−1. In addition, the DFT calculation results show that the energy required for path A is lower than that for path B, which means that path A is more likely to occur than path B. And the ReaxFF-MD results show that the number of generated CF3 increases rapidly during 700–1000 ps, which indicates the results of ReaxFF are consistent with DFT calculations and the performance of ReaxFF is reliable.
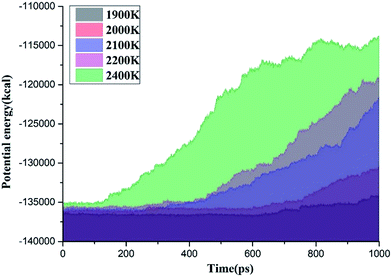
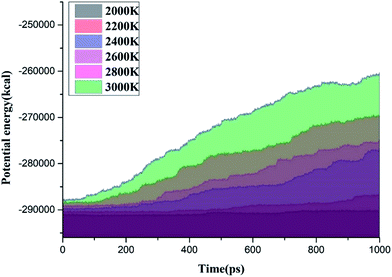
Fig. 4 shows the time evolution of the potential energy between 1900 K and 2400 K. The potential energy of the system increases during the reaction process at different temperatures, thereby indicating that the entire decomposition process of C3F7CN needs to absorb energy. The growth rate of the potential energy is evidently accelerated with the increase of temperature, indicating that the increase of the ambient temperature will lead to the accelerated decomposition of C3F7CN. When the ambient temperature reaches 2400 K, the growth rate of the potential energy in the time range of 125 ps to 600 ps is significantly higher than that of 600–1000 ps. This result indicates that the reactions in the system before 600 ps are mainly absorbed energy. Moreover, exothermic reactions may occur in the system after 600 ps, thereby leading to the considerably gradual growth of the overall potential energy.
Fig. 5 illustrates the time evolution of the C3F7CN molecules between 1900 K and 2400 K. The decomposition rate and quantity of the C3F7CN molecules are evidently accelerated with the increase of temperature. For example, only 8 C3F7CN molecules decomposed at 1900 K, whereas 96 C3F7CN molecules decomposed at 2400 K at the end of the molecular dynamic simulation.
 | ||
| Fig. 5 Time evolution of the C3F7CN molecules decomposition when temperature is increased from 1900 to 2400 K (C3F7CN system). | ||
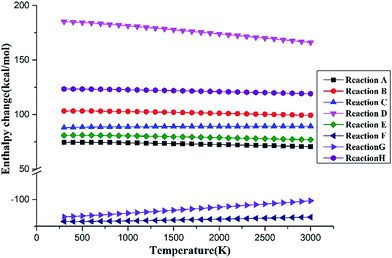
To further analyze the effect of temperature on the decomposition of C3F7CN, the enthalpy of the main reaction paths in the temperature range of 300–3000 K is calculated based on DFT (see Fig. 6). As the temperature increases, the reaction enthalpy of paths A, B, D, and E decreases, that is, the temperature increase is favorable for the reaction progress. The enthalpy change of path D is the most evident, thereby indicating that the increase of temperature has a significant effect on the decomposition of CF3CFCN. The reaction enthalpy change of paths C and H is not significantly with increasing temperature. Paths F and G are exothermic reaction processes and the energy released decreases as the temperature increases.
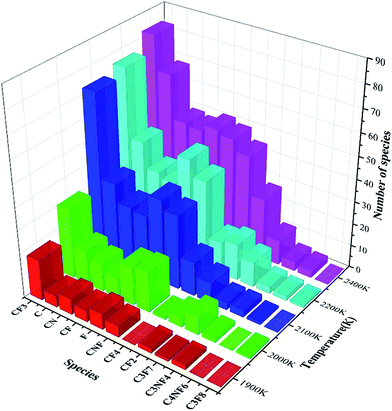
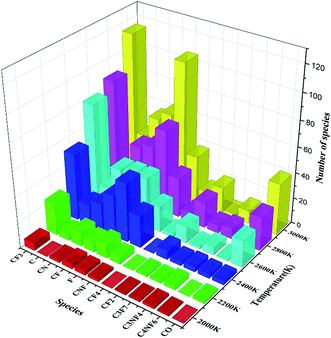
The statistical results in Fig. 8 show that the content of CF3 at each temperature is the highest among all the products, while the contents of CF2, CN, CNF, and F are similar. The contents of C3F7, C3NF4, and C4NF6 are lower compared with those of other products. C4NF6 is generated by the dissociation of the F atom in the C3F7CN molecule from the central C atom (C2 atom as shown in Fig. 1), thereby requiring the absorption of 89.17 kcal mol−1. In addition, a few small stable molecules, such as CF4 and C3F8, are found during the simulation. The generation rate of CF4 in the time range of 600–1000 ps at 2400 K increased and the amount of CF3 and F during the time range reduced (as shown in Fig. 7). Given that the generation path of CF4 (path G in Table 1) is exothermic, the gradual growth of the overall potential energy of the system is actually due to the large amount of CF4 produced.
3.2 Decomposition of the C3F7CN/CO2 mixture
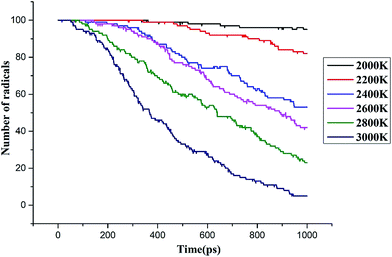 | ||
| Fig. 10 Time evolution of the C3F7CN molecules decomposition when temperature is increased from 2000 to 3000 K (C3F7CN/CO2 system). | ||
The potential energy of the system shows an increasing trend in the range of 2200–3000 K. When the ambient temperature is above 2400 K, the potential energy of the system increases. When the ambient temperature is 2000 K, the potential energy of the system does not change substantially, thereby exhibiting a relationship with the decomposition degree when C3F7CN is low. The decomposition rate of C3F7CN is accelerated with the increase of temperature, thereby showing consistency with the conclusion of the pure C3F7CN system.
Similar to the pure C3F7CN system, the content of the major decomposition products shows an increasing trend with the increase of temperature. The content of CF3 in the products is the highest at each temperature. When the temperature is below 2800 K, the yields of CN, CNF, CF, and F are similar. When the temperature is above 2800 K, the content of F in the system increases. A new decomposition of products, namely, CO, is produced due to the addition of CO2. The decomposition of CO2 accelerated at temperatures over 2600 K and 40 molecules of CO are produced at 3000 K. In the C3F7CN/CO2 system, the yield of CF4 reduces. Only 12 CF4 molecules were produced at 3000 K at the end of the simulation, which is lower than that of the pure C3F7CN system. In addition, the generation rate of CF3 and F after 800 ps at 3000 K slowed down, which is related to the formation of CF4.
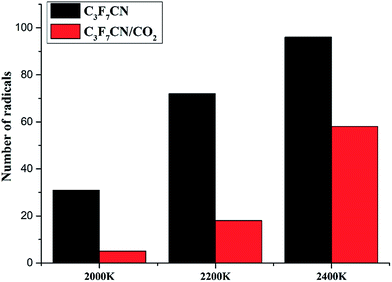
Table 2 shows that the final amount of the decomposition products in the C3F7CN/CO2 gas mixture system is lower than that in the pure C3F7CN system at the same temperature. Moreover, the addition of CO2 inhibits the formation of CF4 and C, thereby making it difficult for the system to precipitate carbon and produce products with relatively poor insulation properties. This result is beneficial to protect the insulation properties of the system.
| Major species | Temperature | |||||
|---|---|---|---|---|---|---|
| 2000 K | 2200 K | 2400 K | ||||
| C3F7CN | C3F7CN/CO2 | C3F7CN | C3F7CN/CO2 | C3F7CN | C3F7CN/CO2 | |
| C3F7 | 8 | 1 | 11 | 2 | 11 | 3 |
| CF3 | 31 | 6 | 76 | 25 | 83 | 52 |
| CF2 | 4 | 1 | 16 | 2 | 27 | 7 |
| CF | 9 | 4 | 33 | 8 | 48 | 32 |
| CN | 12 | 2 | 35 | 11 | 49 | 21 |
| CNF | 18 | 2 | 39 | 5 | 49 | 25 |
| F | 17 | 3 | 46 | 11 | 51 | 46 |
| C3NF4 | 2 | 2 | 3 | 4 | 4 | 4 |
| C4NF6 | 0 | 1 | 2 | 0 | 3 | 3 |
| CF4 | 1 | 0 | 14 | 0 | 44 | 0 |
| C | 15 | 0 | 46 | 5 | 68 | 16 |
Therefore, the C3F7CN/CO2 gas mixture is relatively suitable for using as a gas-insulated medium relative to pure C3F7CN. The mechanism of the action of CO2 over C3F7CN to avoid its decomposition process in function of temperature needs further study.
4 Conclusion
This study analyzed the decomposition characteristics of C3F7CN and its gas mixture based on ReaxFF-MD method and density functional theory. The main decomposition pathways, reaction enthalpy, and the composition of the main decomposition products of C3F7CN at different temperatures were revealed. The decomposition characteristics of C3F7CN and C3F7CN/CO2 gas mixture were compared and analyzed.The decomposition of C3F7CN mainly produces CF3, C3F7, CN, CNF, CF2, CF, F, and other free radicals and a few molecular products, such as CF4 and C3F8. The decomposition rate of C3F7CN and generation rate of the products increase with the increase of ambient temperature. The C3F7CN/CO2 gas mixture has more excellent decomposition characteristics than that of pure C3F7CN at the same condition, which is relatively suitable for use as a gas-insulated medium in electrical equipment.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The current work is supported by the science and technology project of China Southern Power Grid (No. CSGTRC-K163010)References
- A. Beroual and A. Haddad, Recent Advances in the Quest for a New Insulation Gas with a Low Impact on the Environment to Replace Sulfur Hexafluoride (SF6) Gas in High-Voltage Power Network Applications, Energies, 2017, 10(8), 1216 CrossRef.
- X. Zhang, Y. Li and S. Xiao, et al., Decomposition mechanism of C5F10O: An environmentally friendly insulation medium, Environ. Sci. Technol., 2017, 51(17), 10127–10136 CrossRef CAS PubMed.
- J. Reilly, R. Prinn and J. Harnisch, et al., Multi-gas assessment of the Kyoto Protocol, Nature, 1999, 401(6753), 549 CrossRef CAS.
- Y. Kieffel, T. Irwin and P. Ponchon, et al., Green Gas to Replace SF6 in Electrical Grids, IEEE Power and Energy Magazine, 2016, 14(2), 32–39 CrossRef.
- M. P. Sulbaek Andersen, M. Kyte and S. T. Andersen, et al., Atmospheric Chemistry of (CF3)2CFCN: A Replacement Compound for the Most Potent Industrial Greenhouse Gas, SF6, Environ. Sci. Technol., 2017, 51(3), 1321–1329 CrossRef CAS PubMed.
- Z. Lv, D. Zhao and S. Xu, Facile synthesis of mesoporous melamine-formaldehyde spheres for carbon dioxide capture, RSC Adv., 2016, 6(64), 59619–59623 RSC.
- J. G. Owens, Greenhouse gas emission reductions through use of a sustainable alternative to SF6, IEEE Electrical Insulation Conference (EIC), Montréal, Canada, 2016, pp. 535–538 Search PubMed.
- Y. Kieffel, Characteristics of g3-an alternative to SF6, IEEE International Conference on Dielectrics(ICD), Montpellier, France, 2016, vol. 2, pp. 880–884 Search PubMed.
- H. E. Nechmi, A. Beroual and A. Girodet, et al., Fluoronitriles/CO2 Gas Mixture as Promising Substitute to SF6 for Insulation in High Voltage Applications, IEEE Trans. Dielectr. Electr. Insul., 2016, 23(5), 2587–2593 CrossRef.
- C. Preve, R. Maladen and D. Piccoz, Method for validation of new eco-friendly insulating gases for medium voltage equipment, IEEE International Conference on Dielectrics (ICD), Montpellier, France, 2016, vol. 1, pp. 235–240 Search PubMed.
- M. P. S. Andersen, M. Kyte, S. T. Andersen, C. J. Nielsen and O. J. Nielsen, Atmospheric Chemistry of (CF3)2CF–CN: A Replacement Compound for the Most Potent Industrial Greenhouse Gas, SF6, Environ. Sci. Technol., 2017, 51(3), 1321–1329 CrossRef PubMed.
- X. Zhang, Y. Li and S. Xiao, et al., Theoretical study of the decomposition mechanism of environmentally friendly insulating medium C3F7CN in the presence of H2O in a discharge, J. Phys. D: Appl. Phys., 2017, 50(32), 325201 CrossRef.
- A. C. T. V. Duin, S. Dasgupta and F. Lorant, et al., ReaxFF: A Reactive Force Field for Hydrocarbons, J. Phys. Chem. A, 2001, 105(41), 9396–9409 CrossRef.
- J. Zhang, Y. Si and C. Leng, et al., Molecular dynamics simulation of Al–SiO2 sandwich nanostructure melting and low-temperature energetic reaction behavior, RSC Adv., 2016, 6(64), 59313–59318 RSC.
- S. Bhoi, T. Banerjee and K. Mohanty, Insights on the combustion and pyrolysis behavior of three different ranks of coals using reactive molecular dynamics simulation, RSC Adv., 2016, 6(4), 2559–2570 RSC.
- D. Hong, L. Liu and Y. Huang, et al., Chemical effect of H2O on CH4 oxidation during combustion in O2/H2O environments, Energy Fuels, 2016, 30(10), 8491–8498 CrossRef CAS.
- H. J. C. Berendsen, J. P. M. Postma and W. F. V. Gunsteren, et al., Molecular dynamics with coupling to an external bath, J. Chem. Phys., 1984, 81(8), 3684–3690 CrossRef CAS.
- G. Te Velde, F. M. Bickelhaupt and E. J. Baerends, et al., Chemistry with ADF, J. Comput. Chem., 2001, 22(9), 931–967 CrossRef CAS.
- T. C. Ngo, D. Q. Dao and M. T. Nguyen, et al., A DFT analysis on the radical scavenging activity of oxygenated terpenoids present in the extract of the buds of Cleistocalyx operculatus, RSC Adv., 2017, 7(63), 39686–39698 RSC.
- Q. Zhou, X. Su and W. Ju, et al., Adsorption of H2S on graphane decorated with Fe, Co and Cu: a DFT study, RSC Adv., 2017, 7(50), 31457–31465 RSC.
- P. Zhang, X. Hou and J. Mi, et al., Curvature Effect of SiC Nanotubes and Sheet for CO2 Capture and Reduction, RSC Adv., 2014, 4(90), 48994–48999 RSC.
- Y. Zhao and D. G. Truhlar, The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals, Theoretical Chemistry Accounts: Theory, Computation, and Modeling, 2008, 120(1), 215–241 CrossRef CAS.
- Y. Zhao and D. G. Truhlar, A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions, J. Chem. Phys., 2006, 125(19), 194101 CrossRef PubMed.
- A. S. Menon and L. Radom, Consequences of spin contamination in unrestricted calculations on open-shell species: effect of Hartree-Fock and Møller-Plesset contributions in hybrid and double-hybrid density functional theory approaches, J. Phys. Chem. A, 2008, 112(50), 13225–13230 CrossRef CAS PubMed.
- X. Zhang, S. Xiao and J. Zhang, et al., Influence of humidity on the decomposition products and insulating characteristics of CF3I, IEEE Trans. Dielectr. Electr. Insul., 2016, 23(2), 819–828 CrossRef.
- R. Ono and T. Oda, Measurement of gas temperature and OH density in the afterglow of pulsed positive corona discharge, J. Phys. D: Appl. Phys., 2008, 41(3), 035204 CrossRef.
- Y. Fu, M. Rong and K. Yang, et al., Calculated rate constants of the chemical reactions involving the main byproducts SO2F, SOF2, SO2F2 of SF6 decomposition in power equipment, J. Phys. D: Appl. Phys., 2016, 49(15), 155502 CrossRef.
- H. Tanaka, D. Tanahashi and Y. Baba, et al., Finite-difference time-domain simulation of partial discharges in a gas insulated switchgear, High Voltage, 2016, 1(1), 52–56 CrossRef.
- Z. Chen, W. Sun and L. Zhao, High-temperature and High-pressure Pyrolysis of Hexadecane: A Molecular Dynamic Simulation Based on Reactive Force Field (ReaxFF), J. Phys. Chem. A, 2017, 121(10), 2069 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra09959b |
| This journal is © The Royal Society of Chemistry 2017 |