Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Role of lanthanum vacancy on the structural, magnetic and magnetocaloric properties in the lacunar perovskite manganites La0.8−x□xNa0.2MnO3 (0 ≤ x ≤ 0.15)
S. Choura-Maatara,
R. M’nassri *b,
W. Cheikhrouhou-Koubaaa,
M. Koubaaa,
A. Cheikhrouhoua and
E. K. Hlilc
*b,
W. Cheikhrouhou-Koubaaa,
M. Koubaaa,
A. Cheikhrouhoua and
E. K. Hlilc
aLT2S Lab (LR16 CNRS 01), Digital Research center of Sfax, Sfax Technopark. Cité El Ons, B.P. 275, 3021 Sfax, Tunisia
bUnité de Recherche Matériaux Avancés et Nanotechnologies (URMAN), Institut Supérieur des Sciences Appliquées et de Technologie de Kasserine, Kairouan University, BP 471 Kasserine 1200, Tunisia. E-mail: rafik_mnassri@yahoo.fr
cInstitut Néel, CNRS, Université Grenoble Alpes, BP 166, F-38042 Grenoble cedex 9, France
First published on 27th October 2017
Abstract
Lacunar La0.8−x□xNa0.2MnO3 (0 ≤ x ≤ 0.15) ceramics where □ is a lanthanum deficiency were synthesized via a sol–gel method. Magnetic phase transitions and magnetocaloric effects of the ceramics have been systematically studied. Structural studies using X-ray diffraction show that all compounds crystallize in the rhombohedral structure with an R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c space group. The lanthanum deficiency does not modify the crystal structure of the pristine compound (x = 0) but results in a slight change of the lattice parameters. The unit cell volume and Mn–O–Mn angle decrease with increasing deficiency content whereas the Mn–O bond length increases. This situation weakens the double-exchange interaction and hence reduces the Curie temperature (TC). By analyzing the temperature and field dependence of magnetization, we find that the La0.8−x□xNa0.2MnO3 family exhibits a second order magnetic phase transition whose critical temperature is tunable near room temperature. A considerable magnetic entropy change is observed in La0.8−x□xNa0.2MnO3 near room temperature. The maximum value of the relative cooling power (RCP) is found to be ∼268 J kg−1 in La0.65□0.15Na0.2MnO3 at 5 T. This value of RCP is about ∼65.3% of that obtained in gadolinium metal, known as one of the most important materials for magnetic refrigeration, at the same magnetic field change of 5 T. With increasing vacancy our manganites exhibit a stable magnetocaloric effect in a wide temperature range. The results suggest that La0.8−x□xNa0.2MnO3 and its composite materials could be expected to have effective applications for magnetic refrigeration near room temperature.
c space group. The lanthanum deficiency does not modify the crystal structure of the pristine compound (x = 0) but results in a slight change of the lattice parameters. The unit cell volume and Mn–O–Mn angle decrease with increasing deficiency content whereas the Mn–O bond length increases. This situation weakens the double-exchange interaction and hence reduces the Curie temperature (TC). By analyzing the temperature and field dependence of magnetization, we find that the La0.8−x□xNa0.2MnO3 family exhibits a second order magnetic phase transition whose critical temperature is tunable near room temperature. A considerable magnetic entropy change is observed in La0.8−x□xNa0.2MnO3 near room temperature. The maximum value of the relative cooling power (RCP) is found to be ∼268 J kg−1 in La0.65□0.15Na0.2MnO3 at 5 T. This value of RCP is about ∼65.3% of that obtained in gadolinium metal, known as one of the most important materials for magnetic refrigeration, at the same magnetic field change of 5 T. With increasing vacancy our manganites exhibit a stable magnetocaloric effect in a wide temperature range. The results suggest that La0.8−x□xNa0.2MnO3 and its composite materials could be expected to have effective applications for magnetic refrigeration near room temperature.
1. Introduction
Reversible magnetic-field-driven thermal changes are known as magnetocaloric effects (MCE) and have been proposed for magnetic refrigeration (MR) technology.1 The latter is a technology used to warm and cool in response to the application and removal of an external magnetic field, and it receives comprehensive attention due to its several apparent advantages over traditional vapor compression based cooling technologies: high cooling efficiency, environmentally friendly technology and convenient for miniaturization.2–4 The MCE refers to an induced temperature change (ΔTad) and/or entropy change (ΔS) caused by introducing a magnetic field change to a magnetic material.5,6 This effect originates from the coupling of a magnetic field with magnetic moments in a solid. Magnetic entropy change is known to achieve relatively high values at finite temperatures near ferromagnetic and antiferromagnetic phase transition. Theoretical predictions and experimental results for various classes of material like manganites confirm that the magnetic entropy change is larger for the first than for the second order phase transition. However, physical properties in manganites are strongly affected by the modification of the average cationic size,7 cationic disorder,8 doping level,9 grain boundary engineered10 particle size11 and oxygen stoichiometry.12 The induce distortion of the MnO6 octahedron thereby changes the distance and angle between the Mn–O–Mn ions. Moreover, a strong electron–phonon interaction arising from the Jahn–Teller splitting of the outer Mn d level13 and the oxygen deficiency plays a role in the electrical and magnetic properties of lanthanum manganites. Also, it is well known that any magnetic transitions, or even changes in magnetic properties with temperature or magnetic field, are accompanied by transformation or, at least, some spontaneous deformation of the crystal lattice. These distortions are evidenced by several techniques such as Raman spectroscopy.14 The observation of structural changes in doped manganites in the presence of a magnetic stimulus can be explained by the orthorhombic distortions, which can be tuned by an applied magnetic field. Based on the interplay between the double exchange and Jahn–Teller electron–phonon coupling, this phenomenon is interpreted as spectroscopic evidence of the strong coupling between the magnetic field and the lattice leading to an additional tunable charge carrier localization in the double exchange model. In the context of MCE compounds, the changes in the microstructure of the material can produce local inhomogeneities around the Mn atoms, and a distribution of the Mn–Mn interatomic distances affecting the exchange interactions. These alterations would change the value of the Curie temperature (TC) in the sense that instead of a unique value for TC, a more reliable picture considers also a distribution function for this magnitude in the prepared sample. The latter distribution of TC values can make a modification to the nature of the transition and induces a broadening of the magnetic entropy change plots.15 The technical parameter called the relative cooling power (RCP) also depends on the width of the transition temperature interval16 and determines the applicability of materials for solid-state cooling applications. In developing MCE materials with large magnetocaloric properties, many community efforts are currently devoted to MCE materials that can generate a large adiabatic temperature change (ΔTad) and isothermal entropy change (ΔS); however, these MCE materials can require very high magnetic fields, which further limits practical purposes. There are a number of different magnetocaloric materials available for use in magnetic refrigeration such as pure Gd and manganites; these are systematically described in several reviews in the literature.1,5,17,18 Recently, Bahl et al.19 have demonstrated the potential of manganites as working magnetocaloric substances for application in devices. For this purpose, lanthanum manganites and their derivatives have secured a prominent position in the MCE phenomenon as they possess high magnetic moments and are ferromagnetic in nature.5,20,21 This class of material has good wear resistance, high thermal and chemical stability, uncomplicated preparation, low cost and energy-efficiency, grain growth to a desired size via heat treatment and, more importantly, the ability to control the magnetic transition temperatures close to room temperature by substitutions which make the material useful for solid-state cooling applications.11,22 These oxides, inexpensive and abundant, are known only to a limited extent, and a search for new refrigerant materials and new synthesis routes leading to a stronger magnetocaloric effect is still desired. However, only a small number of investigations have been proposed to discuss the deficiency effect in MCE-manganite materials. The presence of a cationic vacancy on the A-site of the perovskite lattice cell is an alternative way to introduce a modification in the average ionic radius 〈rA〉 of the A site and also an additional change in the Mn4+ content. This change affects the lattice parameters and in particular the Mn–O–Mn networks and can thus enhance the super-exchange interactions and consequently, TC may be diminished.23,24 In continuation of previous work from our laboratory, we have investigated the effect of a cationic vacancy in the A-site on the physical properties of manganites.25–27 This investigation is also of interest to the magnetocaloric behaviors in lacunar systems. In the present study, we synthesized novel lacunar manganite La0.8−x□xNa0.2MnO3 with different A-site vacancy amounts by the Pechini-type sol–gel process and studied the structural, magnetic and magnetocaloric effects due to the presence of La3+ concentration deficiencies.2. Materials production
Polycrystalline La0.8−x□xNa0.2MnO3 samples where □ is a lanthanum vacancy were prepared by a modified Pechini-type sol–gel method. High purity (99.9%) precursors La2O3, Na2CO3 and MnO2 were taken as starting materials in an appropriate stoichiometric ratio. The precursor solution was prepared by dissolving the starting materials in a concentrated nitric acid solution in order to convert them into nitrates. During this procedure, the solution was continuously heated and stirred using a magnetic stirrer at 60 °C in order to accelerate the dissolution. After that, one adds citric acid (CA) and ethylene glycol (EG) as chelating agents until a completely homogeneous and transparent solution is obtained. These chelators serve to adjust the viscosity of the complex solution and control the moving velocity of the metal cations, resulting in the gelation of the reaction mixture. The product mixture was heated at 80 °C under constant stirring and the polyesterification reaction between citric acid and ethylene glycol takes place. After slowly drying the solution at 130 °C, a residue of high viscosity is formed and a transparent gel is developed during the heating process. In a later step, the obtained gel is heated to 300 °C with a heating rate of 10 °C per minute with the purpose of having the propagation of a combustion which transforms the freezing gel into fine powder. The resulting powders were then calcined at 450 °C in a furnace for 6 h, in order to expel the organics from the material. The final black powders were then pressed into a pellet (of about 1 mm thickness and 13 mm diameter) followed by sintering at 900 and 950 °C for 24 hours in air with intermediate regrinding and repelling.3. Physical measurements
To understand the structural properties of the samples under study, XRD measurements were performed at room temperature. The lattice structure and cell parameters of the La0.8−x□xNa0.2MnO3 system were characterized by diffraction (XRD) data, recorded on a PANalytical X’PERT Pro MPD diffractometer, using θ/2θ Bragg–Brentano geometry with diffracted beam monochromatized CuKα radiation (λ = 1.5406 Å). The diffraction patterns were collected by steps of 0.017° over the angle range 10–100°/5–90° for x = 0 and x = 0.05/x = 0.1 and x = 0.15. Structural analysis was carried out using the standard Rietveld procedure28 built-in FULLPROF software29 which minimizes the difference between the observed and simulated powder diffraction patterns. Magnetization measurements versus temperature and versus the magnetic applied field up to 5 T were carried out using BS1 and BS2 extraction type magnetometers. The used magnetometers are employed respectively for magnetic measurements at high and low temperatures equipped with a super conducting coil developed at the NEEL Institute. These magnetometers use an extraction technique and can produce a field of 7 T for BS1 and 11 T for BS2. The sample temperature is controlled by circulating helium gas. These two instruments are automated by a computer system that allows the registration of digital data for each successive measurement. M(T) data were obtained under a magnetic applied field of 0.05 T in the field cooled mode (FC). Isothermal M(μ0H) data were measured up to 5 T. The MCE results were deduced from the magnetization measurements versus magnetic applied field up to 5 T at several temperatures.4. Results and discussion
4.1. Structural study
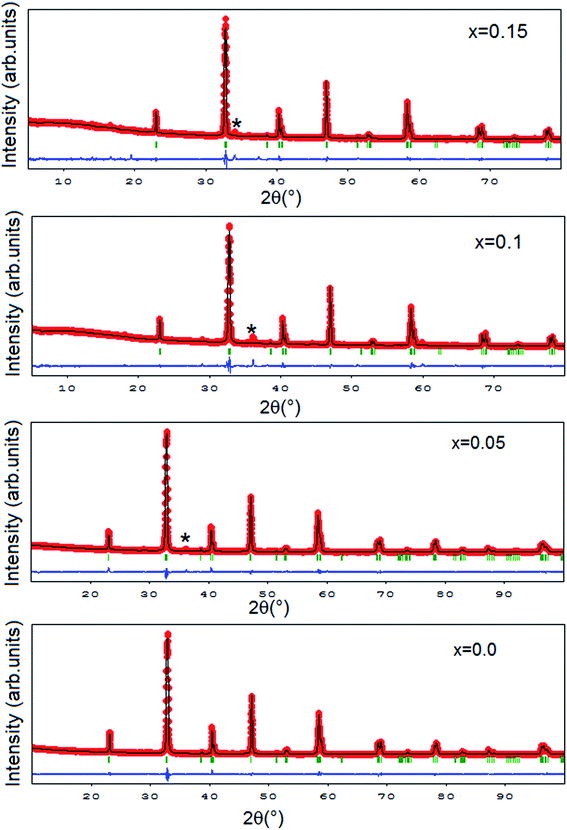
The X-ray powder diffraction patterns registered at 300 K for La0.8−x□xNa0.2MnO3 compounds are depicted in Fig. 1 and indicate that all the samples have the same perovskite structure. The diffraction peaks can be indexed in the rhombohedral setting of the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c space group (Z = 6) in which La/Na atoms are located at the 6a (0, 0, 1/4) position, Mn atoms at the 6b (0, 0, 0) position, and O atoms at the 18e (x, 0, 1/4) position. Fig. 1 shows typical XRD patterns for La0.8−x□xNa0.2MnO3 samples including the observed and calculated profiles as well as the difference profile. The data were refined by the Rietveld technique using FULLPROF software.30 The refinement produced satisfactory agreement factors and lattice parameters which are listed in Table 1. In fact, we noticed the existence of diffraction peaks with very small intensities in the XRD patterns for the lacunar samples. This is due to the presence of small amounts of Mn3O4, which is frequently encountered after the final step in the synthesis of mixed-valence manganites. Given the small concentration of Mn3O4, we assume that the secondary phase does not have any significant effect on the perovskite stoichiometry according to ref. 31.
c space group (Z = 6) in which La/Na atoms are located at the 6a (0, 0, 1/4) position, Mn atoms at the 6b (0, 0, 0) position, and O atoms at the 18e (x, 0, 1/4) position. Fig. 1 shows typical XRD patterns for La0.8−x□xNa0.2MnO3 samples including the observed and calculated profiles as well as the difference profile. The data were refined by the Rietveld technique using FULLPROF software.30 The refinement produced satisfactory agreement factors and lattice parameters which are listed in Table 1. In fact, we noticed the existence of diffraction peaks with very small intensities in the XRD patterns for the lacunar samples. This is due to the presence of small amounts of Mn3O4, which is frequently encountered after the final step in the synthesis of mixed-valence manganites. Given the small concentration of Mn3O4, we assume that the secondary phase does not have any significant effect on the perovskite stoichiometry according to ref. 31.
| x = 0 | x = 0.05 | x = 0.1 | x = 0.15 | |
|---|---|---|---|---|
| a (Å) | 5.488 (3) | 5.483 (2) | 5.481 (9) | 5.480 (9) |
| b (Å) | 5.488 (3) | 5.483 (2) | 5.481 (9) | 5.480 (9) |
| c (Å) | 13.330 (4) | 13.310 (4) | 13.319 (1) | 13.317 (8) |
| Volume (Å3) | 347![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 740 (9) 740 (9) |
347![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180 (1) 180 (1) |
346![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 628 (1) 628 (1) |
346![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 399 (7) 399 (7) |
| dMn–O (Å) | 1.949 | 1.950 | 1.951 | 1.951 |
| θMn–O–Mn (°) | 166.09 | 164.79 | 164.31 | 164.19 |
| χ2 | 2.85 | 2.53 | 2.80 | 3.05 |
| W/W0 (10−2) | 9.6 | 9.571 | 9.548 | 9.54 |
| C (μB) | 2.465 | 2.237 | 3.233 | 3.261 |
| Θp (K) | 335 | 328 | 326 | 323 |
| μtheff (μB) | 4.712 | 4.565 | 4.415 | 4.217 |
| μexpeff (μB) | 4.44 | 4.23 | 5.085 | 5.107 |
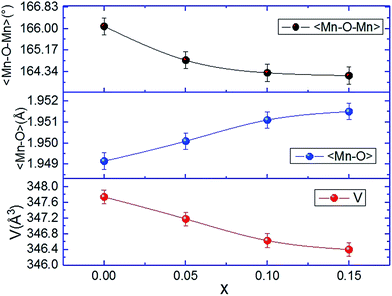
Lanthanum vacancies did not affect the space group of the parent compound La0.8Na0.2MnO3. With increasing La-deficiency amount, the room temperature unit cell volume is systematically decreased (see Fig. 2). Basically, the introduction of the vacancy (x) in the A site implies a partial conversion of Mn3+ to Mn4+ ions according to the formula La0.8−x3+□xNa0.4+Mn0.8−3x3+Mn0.2+3x4+O32− and surely a change in the average ionic radius 〈A〉 of this site. In our case, the increase of the lanthanum-deficiency content (x) leads to an increase of the Mn tetravalent ion number with a smaller radius (rMn4+ = 0.53 Å and rMn3+ = 0.65 Å (ref. 32)). This increase in Mn4+ content induced a decrease in the average size of the B-site cation. Also, and for electrostatic considerations, a vacancy has an average radius 〈rv〉 ≠ 0 and leads to a change in the average ionic radius. These two effects explain the decrease of the unit cell volume. A similar result has been observed in other lacunar manganite systems such as Pr0.7−x□xSr0.3MnO3 (ref. 33) and La0.65−x□xCa0.35MnO3.34
It is known that the magnetic state of a manganite system is very sensitive to changes in the Mn–O–Mn bond lengths and angles. For this reason, the average Mn–O bond length and Mn–O–Mn bond angle are extracted from the Rietveld refinement at room temperature and are depicted in Fig. 2. It is clear that the decrease of unit cell volume with the increase of La-deficiency is accompanied by an increase of 〈Mn–O〉 length and a decrease of 〈Mn–O–Mn〉 bond angle of MnO6 (corresponding to the small tilts of oxygen octahedra which accommodate the elongation of the bond). Both variations compensate one another to diminish the internal strain induced by the La-deficiency amount, thereby enlarging the octahedral distortion. Since the exchange interaction between Mn–Mn depends on both the bond angle and the bond distance, the decrease in bond angle and the increase in bond length decrease the Mn–Mn exchange interaction which leads to a lower magnetic ordering temperature TC and produces a change in the thermomagnetic properties as evident in the magnetic data which follows.
4.2. Magnetic properties
The normalized magnetization–temperature curves, measured at a constant magnetic field equal to 0.05 T, after field cooling (FC) mode for all samples, are depicted in Fig. 3-a. The latter show clearly a transition from a paramagnetic (PM) to a ferromagnetic (FM) state with decreasing temperature. This type of transition has already been observed in other lacunar manganites such as La0.6Sr0.2Ba0.2−x□xMnO3 (ref. 27) and Pr0.7−x□xSr0.3MnO3.33 A reasonable estimation of the Curie temperature TC can be obtained by determining the temperature of the minimum differential quotient of magnetization (dM(T)/dT). Fig. 3-b shows that the magnitudes and shapes of the dM/dT curves are varied depending on the vacancy level, which renders some indications regarding the sample homogeneity. These curves reveal a strong variation of magnetization around the Curie temperature TC, defined as the temperature at which the (dM/dT − T) curve reaches a minimum. This indicates that there is a possible large magnetic entropy change around TC. A slight shift in TC towards lower temperatures is also observed with increasing the lanthanum-deficiency content (x). The inset of Fig. 3-b shows the Curie temperatures TC of La0.8−x□xNa0.2MnO3 for different x amounts. The decrease in TC is obviously related to an increase in the rate of Mn4+, since the antiferromagnetic super-exchange interaction Mn4+–O–Mn4+ is strengthened with the ferro-double exchange interaction Mn3+–O–Mn4+. However, Table 1 contains an evaluation of the charge carrier bandwidth W through the empirical formula W ∝ cos(θ/2)/Mn–O3.5 (where θ = 180° is the tilt angle in the Mn–O–Mn plane). It is found that the bandwidth W decreases with increasing lanthanum-deficiency. This decrease reduces the overlap between the O-2p and the Mn-3d orbitals, which in turn decreases the exchange coupling of Mn3+–Mn4+ as well as the magnetic ordering temperature TC.13The inverse of the susceptibility χ−1 ∼ 1/M (calculated from the data of Fig. 3-a) is shown in Fig. 3-c, as a function of temperature in the PM region (T > TC) for x = 0.05 and x = 0.15. It can be fitted by a Curie Weiss law34 χ = C/(T − Θp) where C is the Curie constant and Θp is the paramagnetic Weiss temperature. From the linearity of the χ−1 curve for all of our materials, the paramagnetic Curie temperature θp is found to be ∼335, 328, 326 and 323 K for x = 0, 0.05, 0.1 and 0.15, respectively. θp decreases with x content following the same trend as TC. The positive values of θp reveal the existence of a ferromagnetic exchange interaction between the nearest neighbors in the paramagnetic region. Moreover, the obtained values are slightly higher than the corresponding TC. This may be due to the existence of short-range FM correlation in the PM state which is related to the presence of a magnetic inhomogeneity.35 Fitting the linear 1/χ(T) data to the Curie Weiss law introduces C values for all samples. Using these values, we obtained the experimental effective paramagnetic moment μexpeff. It is noted that the μexpeff values for x = 0, 0.05, 0.1 and 0.15 are 4.44, 4.23, 5.085 and 5.107 μB respectively. Assuming orbital momentum to be quenched in Mn3+ and Mn4+, commonly, the theoretical effective moment for each case is determined by the equation: 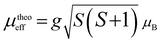 where g = 2 is the gyromagnetic factor and S is the spin of the cation (the S values are 3/2 for Mn4+ and 2 for Mn3+). The moment of the La0.8−x□xNa0.2MnO3 system is expected to originate from the moments of the Mn ion only; thus, the calculated effective paramagnetic moment per formula unit can be written as:
where g = 2 is the gyromagnetic factor and S is the spin of the cation (the S values are 3/2 for Mn4+ and 2 for Mn3+). The moment of the La0.8−x□xNa0.2MnO3 system is expected to originate from the moments of the Mn ion only; thus, the calculated effective paramagnetic moment per formula unit can be written as:
 | (1) |
Considering the Mn3+ and Mn4+ magnetic moments (μtheff(Mn4+) ≈ 3.87 μB and μtheff(Mn3+) ≈ 4.9 μB), μtheoeff was calculated for all compounds as 4.712, 4.565, 4.415 and 4.217 μB for x = 0.00, 0.05, 0.1 and 0.15, respectively. These values are not consistent with the experimental results suggesting the formation of FM clusters of Mn3+–Mn4+ double exchange pairs in the paramagnetic region.36 The discrepancy between the experimental and theoretical values implies that some short-range FM couplings might have been developed in the PM region contributing to the additional magnetic moments.
Fig. 4 presents the isothermal magnetization curves for La0.8−x□xNa0.2MnO3 (0 ≤ x ≤ 0.15) These M(μ0H) measurements are measured near the magnetic transition with increasing magnetic field in a wide temperature range. It can be seen that M(μ0H) versus magnetic applied field up to 5 T recorded at 280 K shows a typical ferromagnetic nature for La0.8−x□xNa0.2MnO3. The magnetization increases sharply with magnetic applied field for μ0H = 1 T and then saturates. It can be seen that magnetization decreased as the lanthanum-deficiency in the La0.8−x□xNa0.2MnO3 sample increased. This verifies that a vacancy gets incorporated into each sample proportionally to the amount of the x deficiency. This should be related to the observation of the enlarged Mn–O bond length and reduced Mn–O–Mn bond angle for our system. The inset of Fig. 4 shows isothermal magnetization curves for the La0.75□0.05Na0.2MnO3 sample, measured under an applied magnetic field ranging from 0–5 T and at a temperature ranging from 200–380 K. At T < TC, the M(μ0H) curves are non-linear corresponding to the FM state and become linear at T > TC corresponding to the PM state.
4.3. Magnetocaloric properties
According to thermodynamics, magnetic entropy change ΔS caused by the variation of applied magnetic field from 0 to μ0Hmax is given by:
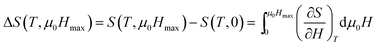 | (2) |
From the magnetization measurements made at discrete field and temperature intervals ΔS can be approximately evaluated by the following expression:
 | (3) |
In this expression, Mi and Mi+1 are the experimental values of magnetization measured, under an applied magnetic field μ0H, at the temperatures Ti and Ti+1, respectively.
The magnetic entropy change, −ΔS, induced by the magnetic field change was calculated using the M(μ0H) data. In the neighborhood of room temperature, the La0.8−x□xNa0.2MnO3 system is significant compared with alkaline-earth-doped lanthanide manganites such as La–A (Ca, Ba and Sr)–MnO3.37 Fig. 5 shows the temperature dependence of −ΔS for different applied magnetic field changes for x = 0, 0.1 and 0.15. The obtained negative sign of the magnetic entropy change means that heat is released by the adiabatic change of the magnetic field confirming the ferromagnetic behavior of our lacunar samples. It can be seen that ΔS depends on both T and μ0H. The magnetic entropy change increases to a maximum value (ΔSmax) when the temperature approaches Curie temperature and reaches a maximum value around TC. There is, however, a notable change in the shape of the −ΔS(T) curves, specifically, increasing lanthanum-deficiency content gives an increase in the width (see Fig. 5) and alters the overall peak structure to have a relatively flat shape more typical of magnetocaloric materials with second order phase transitions. The largest changes in magnetic entropy take place near TC, which is a property of simple ferromagnets due to the efficient ordering of magnetic moments induced by the magnetic field at the ordering temperature. Basically, a broad peak on the ΔS(T) curves appears near the respective TC, unlike the sharp peak typical of La2/3Ca1/3MnO3,38 where the phase transition is of the first order accompanied by hysteresis.39
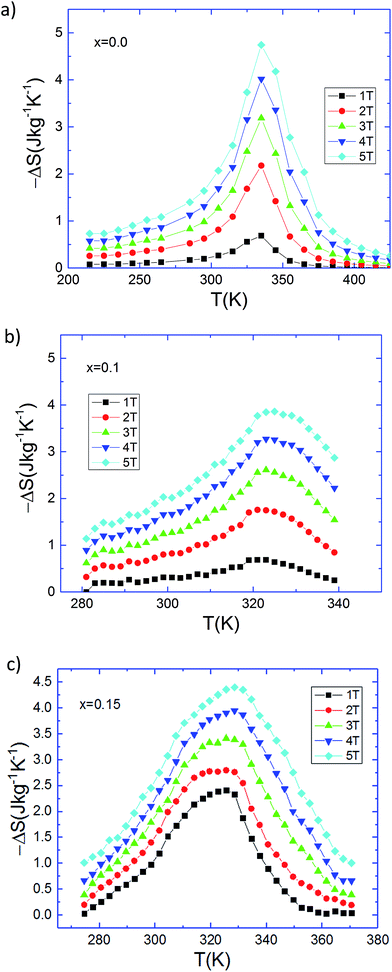 | ||
| Fig. 5 Magnetic entropy change versus temperature for the La0.8Na0.2−x□xMnO3 samples under several magnetic applied field changes: (a) x = 0.00, (b) x = 0.10, and (c) x = 0.15. | ||
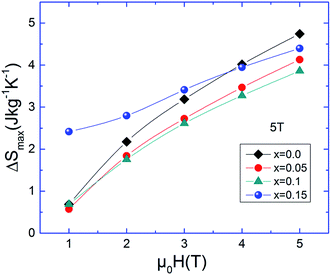
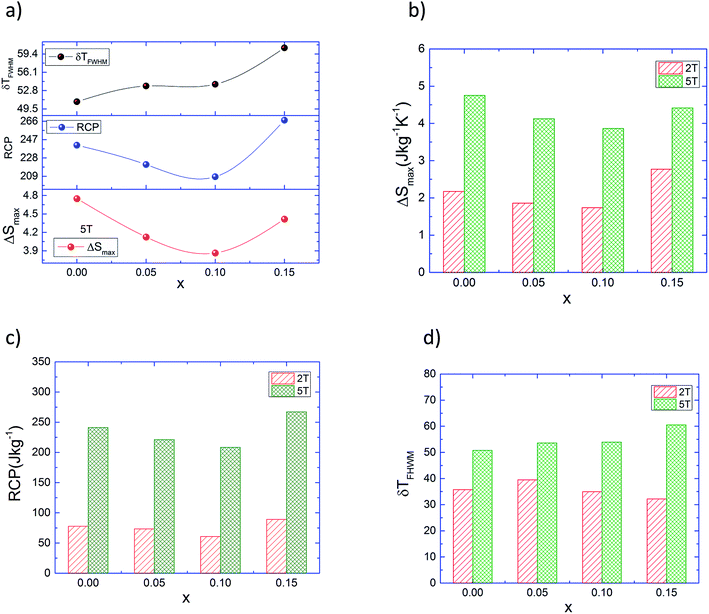
The magnitude of change in the magnetic entropy of the La0.8−x□xNa0.2MnO3 system is significant but lower than that with pure gadolinium (Gd) and ntermetallic Gd5Si2Ge2, which are considered the most conspicuous magnetocaloric materials at room temperature. Unfortunately, the Gd and the Gd5Si2Ge2 materials are more expensive and oxidizable in air. Compared to Gd, our manganites present some advantages including low production cost, chemical stability, high resistivity, and corrosion-free effects. It is clear from Fig. 6 that the magnitude of the peak increases with increasing value of magnetic field for each composition. The main panel of Fig. 7a shows the variations in ΔS, RCP and ΔTFWHM under Δ(μ0H) = 5 T as functions of the La-deficiency amount. While x increases monotonically, ΔTFWHM increases; however, the relative cooling power (RCP) shows a maximum value of ∼268 J kg−1 for x = 0.15 and a minimum value of ∼210 J kg−1 for x = 0.1. The obtained values are comparable to about ∼65.3% for x = 0.15 and ∼51.2% for x = 0.1 of that of pure Gd (RCP = 410 J kg−1 (ref. 40)) which is considered the most conspicuous magnetocaloric material at room temperature. Thus, this indicates that the RCP value is large revealing the applicability of La0.8−x□xNa0.2MnO3 in cooling devices and suggests that these compounds can thus be used in active magnetic refrigeration as suggested by Barclay.41
A comparison of the thermomagnetic properties in the La0.8−x□xNa0.2MnO3 series at 5 T and 2 T magnetic fields is depicted in Fig. 7b, c and d respectively. However, it is interesting to notice that RCP reaches a maximum for the x = 0.15 lanthanum-vacancy level; for this composition, with TC still around room temperature, the sharpness of the ferromagnetic transition makes it a potential candidate competitive with state-of-the art conventional refrigerant materials. Earlier investigations explain that the large MCE in perovskite manganites such as lacunar systems can originate from the spin-lattice coupling related to the magnetic ordering process. Because of this coupling mechanism, the lattice structural change in the Mn–O bond distances and Mn–O–Mn bond angles with temperature which exhibit variation in volume can cause an additional change in the magnetic properties of the magnetic substance.42
For practical MCE use, it is better to have some information about the change in specific heat ΔCP of the compounds, which is desirable for the fulfillment of a magnetic refrigerator. In our case, the ΔCP associated with magnetic field changes from zero to μ0H can be deduced from ΔS induced in the material, by the following expression:
 | (4) |
Through this relation, ΔCP values for several maximum applied magnetic fields ranging from 1 to 5 T of La0.8−x□xNa0.2MnO3 with x = 0, 0.1 and 0.15 are presented in Fig. 8. One can see clear anomalies around TC in all curves, which are due to the magnetic phase transition.43,44 The magnitude of ΔCP varies suddenly from (+) values to (−) values near TC and quickly decreases with decreasing temperature. The sum of the two fractions is the magnetic contribution to the total specific heat which involves the cooling or heating power of the magnetic heat pumps and refrigerators.45 Specific heat presents the advantage of delivering values required for further pump or refrigerator design, should the sample in question be selected.46
 | ||
| Fig. 8 The heat capacity changes −ΔCp as a function of temperature for La0.8Na0.2−x□xMnO3 (a) x = 0.00, (b) x = 0.05, and (c) x = 0.15 compounds. | ||
Fig. 9 presents the temperature dependence of the exponent n for La0.8−x□xNa0.2MnO3 (x = 0 and x = 0.15) describing the field dependence of the maximum magnetic entropy changes calculated locally from 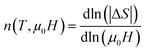 where n is related to the magnetic state of ferromagnetic materials. The value of n depends on the values of the applied field and temperature. For our samples, it can be noted from the obtained curves that the value of n reaches 2 above the Curie temperature and 1 far below the Curie temperature. The n exponent exhibits a moderate decrease with increasing temperature, with a minimum value in the vicinity of the transition temperature, sharply increasing above TC. This exponent takes a minimum value at TC. The obtained values of n are significantly different than 2/3, as predicted by the mean field theory. Thus, this variation of n(T) indicates the inhomogeneous character for all samples, and among the several forms of the inhomogeneous phase is the existence of local inhomogeneities or superparamagnetic clusters in the vicinity of the Curie temperature in the La0.8−x□xNa0.2MnO3 compounds. A similar behavior has been observed in other perovskite materials.47–49
where n is related to the magnetic state of ferromagnetic materials. The value of n depends on the values of the applied field and temperature. For our samples, it can be noted from the obtained curves that the value of n reaches 2 above the Curie temperature and 1 far below the Curie temperature. The n exponent exhibits a moderate decrease with increasing temperature, with a minimum value in the vicinity of the transition temperature, sharply increasing above TC. This exponent takes a minimum value at TC. The obtained values of n are significantly different than 2/3, as predicted by the mean field theory. Thus, this variation of n(T) indicates the inhomogeneous character for all samples, and among the several forms of the inhomogeneous phase is the existence of local inhomogeneities or superparamagnetic clusters in the vicinity of the Curie temperature in the La0.8−x□xNa0.2MnO3 compounds. A similar behavior has been observed in other perovskite materials.47–49
According to the suggestion by Franco and Conde,50 universal scaling analysis helps to determine the nature of the magnetic phase transition (first or second order) in our samples.51 This model can be confirmed by normalizing maximum magnetic entropy changes and temperatures, as defined by the following relation:52
 | (5) |
The temperature axis was rescaled in a different way below and above TC, just by indicating that the position of the reference temperatures Tr1 and Tr2 of each curve corresponds to to θ = ±1:53
 | (6) |
Here, the two additional reference points are chosen such that ΔS(Tr1) = ΔS(Tr2) = ΔSpeak/2. This method should remove the temperature and field dependence of the set of ΔS(μ0H, T) plots so that all curves processed with the same scaling protocol collapse onto a single universal curve. This procedure has been successfully applied to several families of magnetic material.54–58 Fig. 10 shows that the rescaled ΔS data for several applied fields and temperatures can collapse onto a single curve, confirming the master curve behavior in this system and clearly indicating that the magnetic phase transition in La0.8−x□xNa0.2MnO3 is second order. Therefore, materials undergoing second-order transition are preferred for magnetic cooling applications.
 | ||
| Fig. 10 Universal behavior of the scaled entropy change curves at different fields for x = 0.0 and x = 0.15. | ||
5. Conclusion
To summarize, we have investigated the effect of lanthanum vacancy on the structural, magnetic, and magnetocaloric properties of La0.8−x□xNa0.2MnO3 (0 ≤ x ≤ 0.15). The rhombohedral samples are produced from cheap and abundant elements using a simple and straight forward process making them economically attractive for room temperature applications. The increase of lanthanum deficiency induces local distortions in the Mn–O–Mn bonds and consequently causes a random distribution in the magnetic exchange interactions. The competition between magnetic interactions leads to the appearance of an inhomogeneous magnetic state in our lacunar system. Our samples exhibit second order PM–FM transition with decreasing temperature and their Curie temperatures decrease. Consequently, the ability to tune the temperature transition close to room temperature is revealed to be possible by changing the lanthanum-deficiency content as well. The investigation of the magnetocaloric properties reveals a broad peak of −ΔS around TC. As a main result from a practical point of view, for x = 0.15, the relative cooling power shows a maximum value of ∼268 J kg−1 under an applied magnetic field of 5 T, which is sufficient for potential applications in magnetic cooling near room temperature due to the easy synthesis and processing, high chemical stability, very low cost and high chemical stability of these compounds. The ΔS data for different compositions distribute on a single master curve, further confirming the nature of second-order magnetic transition needed for practical applications.Conflicts of interest
There are no conflicts to declare.References
- A. M. Tishin and Y. I. Spichkin, The Magnetocaloric Effect and its Applications, Institute of Physics Publishing, Bristol, 2003 Search PubMed.
- R. Szymczak, M. Czepelak, R. Kolano, A. Kolano-Burian, B. Krzymańska and H. Szymczak, Magnetocaloric effect in La1−xCaxMnO3 for x = 0.3, 0.35, and 0.4, J. Mater. Sci., 2008, 43, 1734 CrossRef CAS.
- V. K. Pecharsky and K. A. Gschneidner Jr, Giant Magnetocaloric Effect in Gd5(Si2Ge2), Phys. Rev. Lett., 1997, 78, 4494 CrossRef CAS.
- V. K. Pecharsky and K. A. Gschneidner Jr, Gd5(SixGe1−x)4: An Extremum Material, Adv. Mater., 2001, 13, 683 CrossRef CAS.
- M. H. Phan and S. C. Yu, Review of the magnetocaloric effect in manganite materials, J. Magn. Magn. Mater., 2007, 308, 325 CrossRef CAS.
- R. M’nassri, Searching the conditions for a table-like shape of the magnetic entropy in the magnetocaloric LBMO2.98/LBMO2.95 composite, Eur. Phys. J. Plus, 2016, 131, 392 CrossRef.
- R. M’nassri, W. Cheikhrouhou-Koubaa, M. Koubaa, N. Boudjada and A. Cheikhrouhou, Magnetic and magnetocaloric properties of Pr0.6-xEuxSr0.4MnO3 manganese oxides, Solid State Commun., 2011, 151, 1579 CrossRef.
- R. M’nassri, W. Cheikhrouhou-Koubaa, M. Kouba and A. Cheikhrouhou, Effect of strontium substitution on the physical properties of Nd0.5Ca0.5−xSrxMnO3 (0.0 ≤ x ≤0.5) manganites, IOP Conf. Ser.: Mater. Sci. Eng., 2012, 28, 012050 CrossRef.
- R. M’nassri, W. Cheikhrouhou-Koubaa, N. Boudjada and A. Cheikhrouhou, Magnetocaloric Effects in Pr0.6−xErxSr0.4MnO3 (0.0≤x≤0.2) Manganese Oxides, J. Supercond. Novel Magn., 2013, 26, 1429 CrossRef.
- R. M’nassri and A. Cheikhrouhou, Magnetocaloric Effect in Different Impurity Doped La0.67Ca0.33MnO3 Composite, J. Supercond. Novel Magn., 2014, 27, 421 CrossRef.
- P. Lampen, A. Puri, M.-H. Phan and H. Srikanth, Structure, magnetic, and magnetocaloric properties of amorphous and crystalline La0.4Ca0.6MnO3+δ nanoparticles, J. Alloys Compd., 2012, 512, 94 CrossRef CAS.
- R. M’nassri and A. Cheikhrouhou, Evolution of Magnetocaloric Behavior in Oxygen Deficient La2/3Ba1/3MnO3−δ Manganites, J. Supercond. Novel Magn., 2014, 27, 1463 CrossRef.
- A. J. Millis, P. B. Littlewood and B. I. Shraiman, Double Exchange Alone Does Not Explain the Resistivity of La1−xSrxMnO3, Phys. Rev. Lett., 1995, 74, 5144 CrossRef CAS PubMed.
- L. Jian-Min, C. H. A. Huan, Y.-W. Du, D. Feng and Z. X. Shen, Magnetic-field-tunable charge carrier localization in sintered polycrystalline La0.75Ca0.25MnO, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 63, 024416 CrossRef.
- P. Alvarez-Alonso, J. L. Sánchez Llamazares, C. F. Sánchez-Valdés, G. J. Cuello, V. Franco, P. Gorria and J. A. Blanco, On the broadening of the magnetic entropy change due to Curie temperature distribution, J. Appl. Phys., 2014, 115, 17A929 CrossRef.
- V. K. Pecharsky, K. A. Gschneidner and A. O. Tsokol, Recent developments in magnetocaloric materials, Rep. Prog. Phys., 2005, 68, 1479 CrossRef.
- B. G. Shen, J. R. Sun and F. X. Hu, et al., Recent progress in exploring magnetocaloric materials, Adv. Mater., 2009, 21, 4545–4564 CrossRef CAS.
- E. Brück, O. Tegus and D. T. Cam Thanh, et al., A review on Mn based materials for magnetic refrigeration: structure and properties, Int. J. Refrig., 2008, 31, 763–770 CrossRef.
- C. R. H. Bahl, D. Velazquez, K. K. Nielsen, K. Engelbrecht, K. B. Andersen, R. Bulatova and N. Pryds, High performance magnetocaloric perovskites for magnetic refrigeration, Appl. Phys. Lett., 2012, 100, 121905 CrossRef.
- R. M’nassri, N. C. Boudjada and A. Cheikhrouhou, Nearly constant magnetic entropy change involving the enhancement of refrigerant capacity in (La0.6Ba0.2Sr0.2MnO3)1−x/(Co2O3)x composite, Ceram. Int., 2016, 42(6), 7447 CrossRef.
- H. Mbarek, R. M’nasri, W. Cheikhrouhou-Koubaa and A. Cheikhrouhou, Magnetocaloric effect near room temperature in (1−y)La0.8Ca0.05K0.15MnO3/y La0.8K0.2MnO3 composites, Status Solidi A, 2014, 211, 975 CrossRef CAS.
- S. Choura Maatar, R. M’nassri, W. Cheikhrouhou-Koubaa, M. Koubaa and A. Cheikhrouhou, Structural, magnetic and magnetocaloric properties of La0.8Ca0.2−xNaxMnO3 manganites (0≤x≤0.2), J. Solid State Chem., 2015, 225, 83 CrossRef CAS.
- W. Boujelben, A. Cheikhrouhou, J. Pierre and J. C. Joubert, Ferromagnetism in the lacunar (Pr, Sr)MnO3 perovskite manganites, Phys. B, 2002, 321, 37 CrossRef CAS.
- S. Choura-Maatar, R. M’nassri, W. Cheikhrouhou-Koubaa, M. Koubaa, A. Cheikhrouhou and E. K. Hlil, Sodium – deficiency effects on the structural, magnetic and magnetocaloric properties of La0.8Na0.2-x□ xMnO3 (0≤ x≤ 0.15), J. Magn. Magn. Mater., 2017, 433, 239 CrossRef CAS.
- M. Ellouze, W. Boujelben, A. Cheikhrouhou, H. Fuess and R. Madar, Solid State Commun., 2002, 124, 125 CrossRef CAS.
- W. Boujelben, A. Cheikhrouhou and J. C. Joubert, Ferromagnetism in lacunar perovskite manganites Pr0.7Sr0.3-x xMnO3 and Pr0.7-x x Sr0.3 MnO3, Eur. Phys. J. B, 2001, 24, 419 CrossRef CAS.
- R. M’nassri, W. Cheikhrouhou-Koubaa, N. ChnibaBoudjada and A. Cheikhrouhou, Effect of barium-deficiency on the structural, magnetic, and magnetocaloric properties of La0.6Sr0.2Ba0.2−x□xMnO3 (0 ≤ x ≤ 0.15), J. Appl. Phys., 2013, 113, 073905 CrossRef.
- H. M. Rietveld, A Profile Refinement Method for Nuclear and Magnetic Structures, J. Appl. Crystallogr., 1965, 2, 65 CrossRef.
- T. Roisnel and J. Rodriguez-Carvajal, Computer Program FULLPROF, LLB-LCSIM, May 2003 Search PubMed.
- R. A. Young, The Rietveld Method, Oxford University Press, New York, 1993 Search PubMed.
- B. Vertruyen, J.-F. Fagnard, P. Vanderbemden, M. Ausloos, A. Rulmont and R. Cloots, Electrical transport and magnetic properties of Mn3O4-La0.7Ca0.3MnO3 ceramic composites prepared by a one-step spray-drying technique, J. Eur. Ceram. Soc., 2007, 27, 3923 CrossRef CAS.
- R. D. Shanon, Structure Reports, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751 CrossRef.
- W. Boujelben, A. Cheikh-Rouhou and J. C. Joubert, Praseodymium Deficiency Effects on the Physical Properties Pr0.7-x□xSr0.3MnO3Perovskite Manganites, J. Solid State Chem., 2001, 156, 68 CrossRef CAS.
- A. H. Morrish, The Physical Principles of Magnetism, IEEE Press, New York, 2001 Search PubMed.
- R. M’nassri, N. Chniba Boudjada and A. Cheikhrouhou, Impact of sintering temperature on the magnetic and magnetocaloric properties in Pr0.5Eu0.1Sr0.4MnO3 manganites, J. Alloys Compd., 2015, 626, 20 CrossRef.
- R. P. Borges, F. Ott, R. M. Thomas, V. Skumryev, J. M. D. Coey, J. I. Arnaudas and L. Ranno, Field-induced transition in the paramagnetic state of (Sm0.65Sr0.35) MnO3 associated with magnetic clusters, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 60, 12847 CrossRef CAS.
- D. T. Morelli, A. M. Mance, J. V. Mantese and A. L. Micheli, Magnetocaloric properties of doped lanthanum manganite films, J. Appl. Phys., 1996, 79, 373 CrossRef CAS.
- J. Mira, J. Rivas, L. E. Hueso, F. Rivadulla and M. A. Lopez Quintela, Drop of magnetocaloric effect related to the change from first- to second-order magnetic phase transition in La2/3(Ca1−xSrx)1/3MnO3, J. Appl. Phys., 2002, 91, 8903 CrossRef CAS.
- V. S. Amaral, J. P. Araújo, Y. G. Pogorelov, J. M. B. Lopes dos Santos, P. B. Tavares, A. A. C. S. Lourenço, J. B. Sousa and J. M. Vieira, Anomalous magnetic behavior in LaCaMnO near the critical point: stable clusters and crossover to uniform ferromagnetism, J. Magn. Magn. Mater., 2001, 226, 837 CrossRef.
- V. K. Pecharsky, K. A. Gschneidner and A. O. Tsokol, Rep. Prog. Phys., 2005, 68, 1479 CrossRef.
- J. A. Barclay, J. Alloys Compd., 1994, 207–208, 355 CrossRef CAS.
- Z. B. Guo, Y. W. Du, J. S. Zhu, H. Huang, W. P. Ding and D. Feng, Large Magnetic Entropy Change in Perovskite-Type Manganese Oxides, Phys. Rev. Lett., 1997, 78, 1142 CrossRef CAS.
- H. Yang, Y. H. Zhu, T. Xian and J. L. Jiang, Synthesis and magnetocaloric properties of La0.7Ca0.3MnO3 nanoparticles with different sizes, J. Alloys Compd., 2013, 555, 150 CrossRef CAS.
- K. Raju, N. Pavan Kumar, P. Venugopal Reddy and D. H. Yoon, Influence of Eu doping on magnetocaloric behavior of La0.67Sr0.33MnO3, Phys. Lett.A, 2015, 379, 1178 CrossRef CAS.
- X. X. Zhang, G. H. Wen, F. W. Wang, W. H. Wang, C. H. Yu and G. H. Wu, Magnetic entropy change in Fe-based compound LaFe10.6Si2.4, Appl. Phys. Lett., 2000, 77, 3072 CrossRef CAS.
- N. Kumar Swamy, N. Pavan Kumar, P. V. Reddy, M. Gupta, S. S. Samatham and D. Venkateshwarulu, et al., Specific heat and magnetocaloric effect studies in multiferroic YMnO3, J. Therm. Anal. Calorim., 2014, 119, 1191 CrossRef.
- T.-L. Phan, P. Zhang, T. D. Thanh and S. C. Yu, Crossover from first-order to second-order phase transitions and magnetocaloric effect in La0.7Ca0.3Mn0.91Ni0.09O3, J. Appl. Phys., 2014, 115, 17 CrossRef.
- A. Selmi, R. M’nassri, W. Cheikhrouhou-Koubaa, N. Chniba Boudjada and A. Cheikhrouhou, Effects of partial Mn-substitution on magnetic and magnetocaloric properties in Pr0.7Ca0.3Mn0.95X0.05O3 (Cr, Ni, Co and Fe) manganites, J. Alloys Compd., 2015, 619, 627 CrossRef CAS.
- F. Saadaoui, R. M’nassri, H. Omrani, M. Koubaa, N. C. Boudjada and A. Cheikhrouhou, Critical behavior and magnetocaloric study in La0.6Sr0.4CoO3 cobaltite prepared by a sol–gel process, RSC Adv., 2016, 6(56), 50968 RSC.
- V. Franco and A. Conde, Scaling laws for the magnetocaloric effect in second order phase transitions: From physics to applications for the characterization of materials, Int. J. Refrig., 2010, 33, 465473 CrossRef.
- C. M. Bonilla, J. Herrero-Albillos, F. Bartolome, L. M. Garcıa, M. Parra-Borderıas and V. Franco, Universal behavior for magnetic entropy change in magnetocaloric materials: An analysis on the nature of phase transitions, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 224424 CrossRef.
- V. Franco, C. F. Conde, J. S. Blázquez, A. Conde, P. Švec, D. Janičkovič and L. F. Kiss, J. Appl. Phys., 2007, 101, 093903 CrossRef.
- V. Franco, A. Conde, V. Provenzano and R. D. Shull, Scaling analysis of the magnetocaloric effect in Gd5Si2Ge1.9X0.1 (X = Al, Cu, Ga, Mn, Fe, Co), J. Magn. Magn. Mater., 2010, 322, 218 CrossRef CAS.
- C. M. Bonilla, J. Herrero-Albillos, F. Bartolome, L. M. Garcıa, M. Parra-Borderıas and V. Franco, Universal behavior for magnetic entropy change in magnetocaloric materials: An analysis on the nature of phase transitions, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 224424 CrossRef.
- R. M’nassri and A. Cheikhrouhou, Magnetocaloric properties in ordered double-perovskite Ba2Fe1−xCrxMoO6 (0 ≤ x ≤ 1), J. Korean Phys. Soc., 2014, 64, 879 CrossRef.
- A. Sakka, R. M’nassri, N. ChnibaBoudjada, M. Ommezzine and A. Cheikhrouhou, Effect of trivalent rare earth doping on magnetic and magnetocaloric properties of Pr0.5(Ce,Eu,Y)0.1Sr0.4MnO3manganites, Appl. Phys. A: Mater. Sci. Process., 2016, 122, 1 CrossRef CAS.
- R. M’nassri, A. Selmi, N. C. Boudjada and A. Cheikhrouhou, Field dependence of magnetocaloric properties of 20% Cr-doped Pr0.7Ca0.3MnO3 perovskite, J. Therm. Anal. Calorim., 2017, 129, 53–64 CrossRef.
- R. M’nassri, M. Khelifi, H. Rahmouni, A. Selmi, K. Khirounic, N. Chniba-Boudjada and A. Cheikhrouhou, Study of physical properties of cobalt substituted Pr0.7Ca0.3MnO3 ceramics, Ceram. Int., 2016, 42, 6145 CrossRef.
| This journal is © The Royal Society of Chemistry 2017 |