Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Spontaneously grown Ni(OH)2 on iron oxide nanoparticles with enhanced energy storage performance for electrodes of asymmetric supercapacitors†
Yue Wangab,
Xianfeng Zhanga,
Xin Li a,
Yang Liua,
Xiaoping Wangc,
Xi Liuc,
Jian Xuc,
Yongwang Lic,
Yongguang Liua,
Hang Weia,
Peng Jiang
a,
Yang Liua,
Xiaoping Wangc,
Xi Liuc,
Jian Xuc,
Yongwang Lic,
Yongguang Liua,
Hang Weia,
Peng Jiang *ab and
Minghui Liang
*ab and
Minghui Liang *a
*a
aCAS Center for Excellence in Nanoscience, Key Laboratory of Nanosystem and Hierarchical Fabrication, National Center for Nanoscience and Technology (NCNST), Beijing 100190, P. R. China. E-mail: pjiang@nanoctr.cn; liangmh@nanoctr.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
cSynfuels China Technology, Co. Ltd., Beijing 101400, P. R. China
First published on 30th October 2017
Abstract
Iron oxide nanoparticles intercalated in Ni(OH)2 nanosheets were prepared in a spontaneous way for the cathode materials of asymmetric supercapacitors. The soluble precursor of Ni(OH)2, Ni(NH3)6(OH)2, was used for growth on α-Fe2O3 nanoparticles. The loading percentages of Ni(OH)2 in the Ni(OH)2–α-Fe2O3 nanocomposites had a great influence on the morphology and size of Ni(OH)2. The composites were characterized by TEM, SEM, ICP-OES, XRD and XPS. The specific capacitance of Ni(OH)2 in the composite can reach 1107 F g−1 at a scan rate of 20 mV s−1, and the specific capacitance of the Ni(OH)2–α-Fe2O3 composite can be up to 376 F g−1 at a scan rate of 5 mV s−1. More importantly, the fabricated Ni(OH)2–α-Fe2O3//AC (activated carbon) asymmetric supercapacitor exhibited energy density and power density of 31.6 W h Kg−1 and 474 W Kg−1, respectively. The cycling performance of the asymmetric supercapacitor was also excellent, and the capacitance retention reached 89.6% after 5000 charge–discharge cycles. Red, blue and yellow LED lights can be lit up with two series of asymmetric supercapacitors, revealing their potential application in energy storage.
1. Introduction
Electrochemical energy is not only an important alternative energy source for fossil fuels but a requisite element in the portable electronic devices, such as cell phones and wearable electronic devices.1–4 Supercapacitors are a kind of energy storage device being able to deliver electric energy in a fast way, but they suffer from relatively low energy densities.5,6 The energy densities of supercapacitors are of positive correlation to their specific capacitances and the square of their operating voltages.7 Therefore, there are two ways to improve the energy densities of supercapacitors. One is to improve the specific capacitance of supercapacitors. And another is to enlarge the potential range of the supercapacitors.Metal oxides/hydroxides, such as MnO2,8,9 NiO/Ni(OH)2,10,11 and RuO2,12,13 have high specific capacitances, thus they are promising choice for high energy density supercapacitors. In particular, Ni(OH)2 not only has high theoretical specific capacitance, but is a cheap and environment-friendly material, thus it has been intensively investigated for the electrode of supercapacitors.14–17 Recently, we reported the preparation and superior energy storage performance of ultra-fine Ni(OH)2 particles supported on carbon materials.18 In that work, soluble precursor of Ni(OH)2, i.e. Ni(NH3)6(OH)2, is used to grow Ni(OH)2 material over carbon materials. However, one drawback for this route is that the superior performance can only be obtained at low loading percentages of Ni(OH)2. The apparent specific capacitance of Ni(OH)2/C materials at low loading percentages of Ni(OH)2 is relatively low. On the other hand, the symmetric supercapacitors based on Ni(OH)2 often have relatively low operating voltages, limiting the energy densities of the supercapacitors.
Asymmetric supercapacitors as one kind of supercapacitors have two dissimilar electrodes, including a battery-type faradic electrode (cathode) as an energy source and a capacitor-type electrode (anode) as a power source.14,19 Asymmetric supercapacitors have wide electrochemical window because of two different electrodes, and thus they have comparable energy densities with batteries and lithium ions batteries.20,21 The cathode materials for asymmetric supercapacitors include metal oxides/hydroxides, and the anode materials are often carbon materials.22 As a result of higher theoretical specific capacitance of cathode materials than that of anode materials, to improve the practical specific capacitance of cathode materials is an important issue for enhancing the performance of asymmetric supercapacitors.23
To apply Ni(OH)2-based materials prepared from soluble Ni(NH3)6(OH)2 in asymmetric supercapacitors, the support materials other than carbon materials are desired to be tried. Iron oxides are not only active for electrochemical energy storage, but stable in alkaline or neutral solution.24–28 On the other hand, iron oxides have higher conductivity than that of Ni(OH)2, leading to better charge transfer than pure Ni(OH)2.29,30 Therefore, iron oxides are the promising candidates for the support of Ni(OH)2 material. The composites of Fe2O3 and Ni(OH)2 for the electrode of supercapacitors have been reported.31–33 However, the composites are prepared with solvothermal methods.29,34,35 With such route, it is difficult to controllably prepare Ni(OH)2 with desired morphology and size, which are important factors influencing the energy storage performance of Ni(OH)2. It is highly desirable to develop a route to controllably prepare Ni(OH)2 based materials with desired morphology and size. The rule of soluble Ni(NH3)6(OH)2 growing on the iron oxide is unknown, being worthy exploring in the viewpoint of nanocomposites preparation. Furthermore, the asymmetric supercapacitors based on Ni(OH)2–α-Fe2O3 composites have not been reported, to the best of our knowledge.
Based on above problems, herein, we report the spontaneous growth of Ni(OH)2 on α-Fe2O3 and the electrochemical energy storage performance of the asymmetric performance based on Ni(OH)2–α-Fe2O3 cathode and active carbon anode. The growth behaviors of Ni(OH)2 on α-Fe2O3 with different loading percentages are also investigated.
2. Experimental
2.1 Reagents
All the chemicals are of analytical grade and used without further purification. NaCl (99.5%), NaOH (96%) were purchased from Sinopharm Chemical Reagents Co. Ltd. FeCl3·6H2O (99%), Acetylene Black, PVDF were supplied by Aladdin Reagents Co. Ltd. NiCl2·6H2O (≥98.0%) was obtained from Tianjin Guangfu Technology Development Co. Ltd. NH3·H2O (25–28%), HNO3 (65.0–68.0%), H2SO4 (95–98%) and DMF were provided by Beijing Chemical Reagents Co. Ltd. The carbon cloth (WOS 1002) was purchased from Ce Tech Co. Ltd.2.2 Material preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 333 K for 2 h and then washed by ethanol, acetone and DI water, respectively before use. The active material (80 wt%), acetylene black (10 wt%) as conductive additive, and PVDF (10 wt%) as binder were mixed with DMF to form slurry. The mixed slurry was coated onto the pretreated CC following drying treatment, and the loading mass of the active material was about 2 mg cm−2.
1) at 333 K for 2 h and then washed by ethanol, acetone and DI water, respectively before use. The active material (80 wt%), acetylene black (10 wt%) as conductive additive, and PVDF (10 wt%) as binder were mixed with DMF to form slurry. The mixed slurry was coated onto the pretreated CC following drying treatment, and the loading mass of the active material was about 2 mg cm−2.2.3 Material characterizations
Scanning electron microscopy (SEM) images were collected on NOVA Nano SEM 430; transmission electron microscopy (TEM) images and HRTEM images were obtained on Tecnai G2 F20 U-TWIN. The X-ray diffraction (XRD) measurements were performed on an X-ray diffractometer (XRD, Rigaku, D/MAX-TTRIII, 200 kV, 40 mA) with Cu Kα radiation (λ = 0.154178 nm). The loading percentages of Ni(OH)2 on the Ni(OH)2–α-Fe2O3 were obtained by an inductively coupled plasma atomic emission spectroscopy (ICP-OES, Optima 8300). The chemical valence state and composition of the samples were measured on the X-ray photoelectron spectroscopy spectra (XPS, ESCALAB250Xi). The BET data were obtained on ASAP 2020 (Micrometrics). The elemental mapping images were collected on Talos™ 200A.2.4 Electrochemical measurements
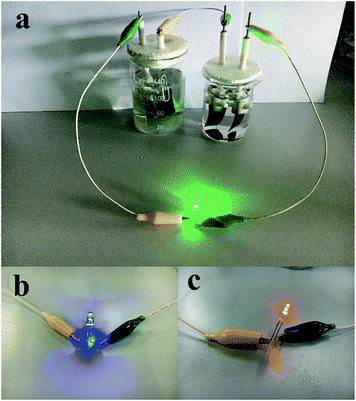
The electrochemical measurements of the Ni(OH)2–α-Fe2O3 composite electrodes were performed on an electrochemical workstation (CHI 660D) in a standard three-electrode cell. The Ni(OH)2–α-Fe2O3 on the CC substrate (1 cm × 2 cm), a Ag/AgCl electrode (with saturated KCl solution), and a platinum foil (2 cm × 4 cm) were used as the working, reference and counter electrode, respectively. All the electrochemical measurements were executed in 1 M KOH aqueous electrolyte. The distance between working electrode and counter electrode was about 2.0 cm. The cyclic voltammograms (CVs) were measured between 0 and 0.6 V at the scan rates from 5 to 80 mV s−1. The galvanostatic charging/discharging (GCD) curves were collected between 0 and 0.45 V at the current densities from 2 to 20 A g−1. The electrochemical impedance spectroscopy (EIS) was obtained by applying an AC voltage of 5 mV amplitude in the frequency from 0.01 Hz to 10 kHz. The specific capacitances of the composite and α-Fe2O3 were calculated from CV and galvanostatic charge/discharge curves according to the following two equations,40 respectively:
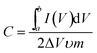 | (1) |
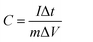 | (2) |
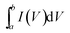 is the integrated area of CV curve in one cycle, ΔV is the voltage window, ν is the scan rate, I is the constant discharge current, Δt is the discharging time, and m is the mass of the Ni(OH)2 or Ni(OH)2–α-Fe2O3.
is the integrated area of CV curve in one cycle, ΔV is the voltage window, ν is the scan rate, I is the constant discharge current, Δt is the discharging time, and m is the mass of the Ni(OH)2 or Ni(OH)2–α-Fe2O3.
The asymmetric supercapacitors based on AC/CC and Ni(OH)2–α-Fe2O3/CC were fabricated in 1 M KOH aqueous solution. The specific capacitances of the symmetric supercapacitors were calculated from CV and galvanostatic charge/discharge curves according to the following two equations, respectively:
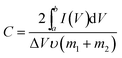 | (3) |
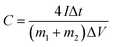 | (4) |
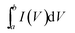 is the integrated area of CV curve in one cycle, ΔV is the voltage window, ν is the scan rate, I is the constant discharge current, Δt is the discharging time, and m1 and m2 are the masses of double electrodes, i.e. Ni(OH)2–α-Fe2O3 or AC.
is the integrated area of CV curve in one cycle, ΔV is the voltage window, ν is the scan rate, I is the constant discharge current, Δt is the discharging time, and m1 and m2 are the masses of double electrodes, i.e. Ni(OH)2–α-Fe2O3 or AC.
3. Results and discussion
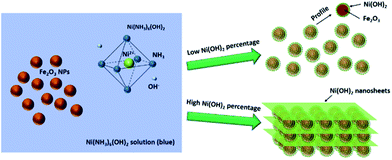
Ni(NH3)6(OH)2 is a soluble precursor for Ni(OH)2.41,42 After the evaporation of NH3 and water, Ni(OH)2 can be spontaneously produced. Based on this mechanism, the Ni(OH)2–α-Fe2O3 nanocomposites with different loading percentages of Ni(OH)2 can be prepared according to Scheme 1. With low loading percentages of Ni(OH)2, the ultra-small Ni(OH)2 nanoparticles on the surface of α-Fe2O3 particles can be obtained as we have proved in our previous work; with high loading percentages of Ni(OH)2, the α-Fe2O3 nanoparticles were intercalated in the Ni(OH)2 nanosheets. The percentages of Ni(OH)2 in the Ni(OH)2–α-Fe2O3 nanocomposites measured by ICP-OES were listed in Table 1. According to the desired percentages of Ni(OH)2, the samples were denoted as X-NF (X is the desired percentage plus 100; for example, 20-NF for 20 wt% Ni(OH)2–α-Fe2O3 composite).| Sample | Fe2O3 | 5-NF | 10-NF | 20-NF | 50-NF |
|---|---|---|---|---|---|
| Desired percentage | 0 | 5% | 10% | 20% | 50% |
| Measured percentage | 0.01% | 5.35% | 11.57% | 22.88% | 48.97% |
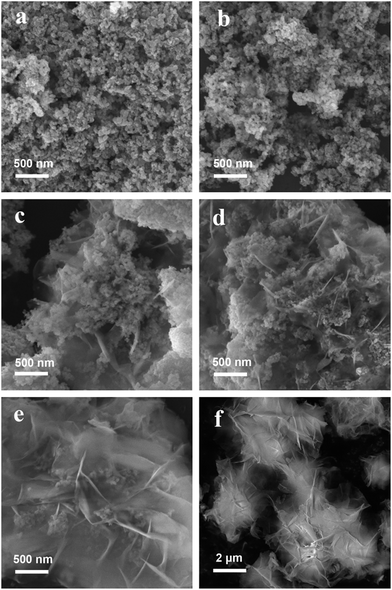
Fig. 1 shows the scanning electron microscopy (SEM) images of the pure α-Fe2O3 sample and Ni(OH)2–α-Fe2O3 nanocomposites with different loading amounts of Ni(OH)2. When the loading percentages of Ni(OH)2 were lower than 10%, the Ni(OH)2 aggregates cannot be observed in the SEM images. As the loading percentage reached 10%, the Ni(OH)2 nanosheets can be observed. The loading percentages of Ni(OH)2 further increased, the thickness and the amount of Ni(OH)2 nanosheets increased. α-Fe2O3 nanoparticles were intercalated in the Ni(OH)2 nanosheets for 20-NF and 50-NF as Scheme 1 depicted. The same phenomena can also be seen in the TEM images of these samples (Fig. S1†). Pure Ni(OH)2 nanosheets can be obtained by evaporating all of the liquid of Ni(NH3)6(OH)2 aqueous solution (Fig. 1f).
The TEM and HRTEM images of α-Fe2O3 nanoparticles, 20-NF and Ni(OH)2 are shown in Fig. 2. The size of prepared α-Fe2O3 nanoparticles is about 10–30 nm. The crystalline lattice of α-Fe2O3 with a d-spacing distance of 0.369 nm can be clearly observed in the Fig. 2b, which can be attributed to the crystalline information of α-Fe2O3 (012).43 The Ni(OH)2 nanosheets in 20-NF and Ni(OH)2 can be found in Fig. 2c and e, respectively. As seen from Fig. 2d and f, the crystalline lattice (0.336 nm) being able to attribute to Ni(OH)2 (101) can be clearly observed for the sample 20-NF and Ni(OH)2.
 | ||
| Fig. 2 (a) The TEM images and (b) the high resolution image of α-Fe2O3; (c) the TEM images and (d) the high resolution image of 20-NF; (e) the TEM images and (f) the high resolution image of Ni(OH)2. | ||
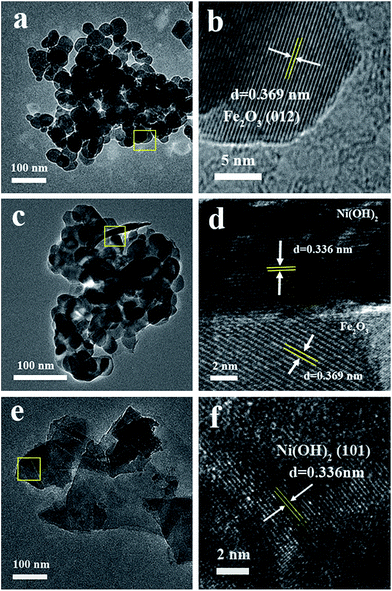
In our previous report, ultrafine Ni(OH)2 particles grown on activated carbon can be observed with elemental mapping technique. To reveal the structure of Ni(OH)2 grown on iron oxide with low loading percentages, the elemental mapping images of 10-NF were obtained (Fig. 3). Fig. 3a and b show the TEM image and STEM image of 10-NF for the same sample, respectively. The Ni(OH)2 nanosheets cannot be observed in this sample, even if the sample was observed at high resolution TEM images (Fig. S2†). However, the signals of Ni and Fe can be found in the EDS pattern (Fig. 3c), and the signal of Ni is weak. These results reveal the existence of nickel in the selected area. The elemental mapping results for Ni and Fe further prove the existence of Ni in the composite (Fig. 3d–f). The typical size of Ni in the composite is in the range of 1.5 to 3.9 nm, and Ni was evenly distributed on the surface of iron oxide according to the crystalline structure of iron oxide and Fig. 3f. It should be stated that the observed signal of Ni is the sum of the signal in the vertical way, so the actual size of Ni on the surface should be smaller than that we observed.
 | ||
| Fig. 3 (a) The TEM and (b) STEM images of 10-NF; (c) the EDS spectrum of 10-NF; the element mapping of (d) Ni, (e) Fe and (f) combined signals of Ni and Fe. | ||
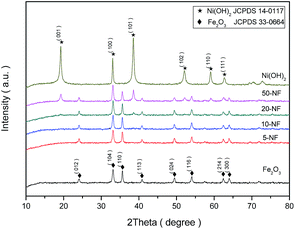
The XRD patterns of the prepared α-Fe2O3, 5-NF, 10-NF, 20-NF, 50-NF, and Ni(OH)2 are shown in Fig. 4. The diffraction peaks for α-Fe2O3 (012), (104), (110) and Ni(OH)2 (001), (100), (101) etc. can be indexed as JCPDS no. 33-0664 and no. 14-0117, respectively.44–46 The loading percentage of Ni(OH)2 being lower than 10 wt%, the diffraction signals of Ni(OH)2 cannot be observed in the XRD patterns, which is consistent with the results of TEM, elemental mapping results and SEM. The signals of Ni(OH)2 and α-Fe2O3 can be found in the XRD patterns for 20-NF and 50-NF, revealing Ni(OH)2–α-Fe2O3 nanocomposites with high Ni(OH)2 loading were composed of the crystalline Ni(OH)2 nanosheets and α-Fe2O3 materials.
To know the specific surface area and porous structure of Ni(OH)2–α-Fe2O3 nanocomposite, the adsorption and desorption isotherm curves of Ni(OH)2, α-Fe2O3 and 50-NF were obtained as shown in Fig. S3.† The specific surface areas of Ni(OH)2, α-Fe2O3 and 50-NF were measured as 29.6, 27.6 and 32.8 m2 g−1, respectively. The porous volumes of Ni(OH)2, α-Fe2O3 and 50-NF were 0.036, 0.186 and 0.139 cm3 g−1, respectively. The pore distribution of Ni(OH)2, α-Fe2O3 and 50-NF are shown in the insets of Fig. S3.† There are little difference in their specific surface areas, and the pore volume of 50-NF is close to that of α-Fe2O3, so the synergistic effect between Ni(OH)2 and α-Fe2O3 in electrochemical energy storage would be easily judged.
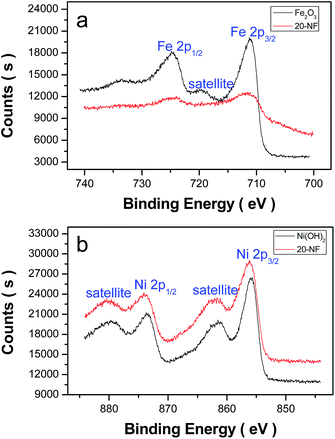
To reveal the chemical state of Ni and Fe in the Ni(OH)2–α-Fe2O3 nanocomposite, the XPS (X-ray photoelectron spectroscopy) spectra of 20-NF were collected as shown in Fig. 5. The XPS survey spectrum of Ni(OH)2–α-Fe2O3 reveals the presence of Ni, Fe, O (Fig. S4†). Two peaks in the Fe 2p XPS spectrum at about 711.07 and 724.67 eV (Fig. 5b) can be ascribed to Fe 2p3/2 and Fe 2p1/2 for α-Fe2O3, respectively.47,48 The satellite peak centered at 719.62 eV is the characteristic signal of α-Fe2O3. The signals of iron in 20-NF are weaker than that in α-Fe2O3, indicating that the deposited Ni(OH)2 on the surface of α-Fe2O3 reduced the exposed surface of α-Fe2O3. The binding energy of Ni in 20-NF was higher than that in Ni(OH)2 for about 0.2 eV, indicating an electron transfer from Ni(OH)2 to α-Fe2O3.
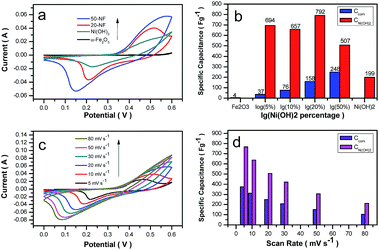
As the active materials in the working electrode, the energy storage performance of Ni(OH)2/α-Fe2O3 nanocomposites was evaluated in a three-electrode system. Fig. 6a shows the CV curves of α-Fe2O3, 20-NF, 50-NF and Ni(OH)2 at a scan rate of 20 mV s−1 under the voltage range of 0–0.6 V (vs. Ag/AgCl), and the CV curves of 5-NF and 10-NF are shown in Fig. S5.† A pair of redox peaks can be clearly observed over these samples, suggesting that the measured capacitance is mainly based on the pseudocapacitance derived from faradic processes.17,49,50 The reaction process can be expressed as follows:
| Ni(OH)2 + OH− → NiOOH + H2O + e− |
The influence of loading percentages for Ni(OH)2 on the capacitance of the composites and Ni(OH)2 is shown in Fig. 6b. We calculated the capacitive performance of the composites and Ni(OH)2 according to the eqn (5) and (6):
| Ccom = CFe2O3 × PFe2O3 + CNi(OH)2 × PNi(OH)2 | (5) |
| CNi(OH)2 = (Ccom − CFe2O3 × PFe2O3)/PNi(OH)2 | (6) |
The calculated specific capacitances of Ni(OH)2 in 20-NF can be up to 1107 F g−1 at a scan rate of 5 mV s−1. However, as the loading percentages of Ni(OH)2 reached 50 wt%, the utilized efficiency of Ni(OH)2 for energy storage decreased to 507 F g−1, which may be caused by the produced thick Ni(OH)2 nanosheets in the composite. Interestingly, the specific capacitance of 50-NF was higher than that of Ni(OH)2 and α-Fe2O3. The BET results revealed the similar specific surface areas for Ni(OH)2, α-Fe2O3 and 50-NF, so a strong synergistic effect between α-Fe2O3 and Ni(OH)2 nanosheets in NF composites can be concluded. The cyclic voltammetry (CV) curves of 50-NF and the specific capacitances for 50-NF and Ni(OH)2 at the scanning rates from 5 to 80 mV s−1 are shown in Fig. 6c and d, respectively. The specific capacitance of 50-NF at the scan rate of 5 mV s−1 can reach 376 F g−1. The calculated specific capacitances of Ni(OH)2 in 50-NF were 768, 637, 507, 422, 306, and 213 F g−1 at scan rates of 5, 10, 20, 30, 50, and 80 mV s−1, respectively. As the scan rate increased for 16 times (5 mV s−1 to 80 mV s−1), the calculated specific capacitance of Ni(OH)2 had a retention of 28%.
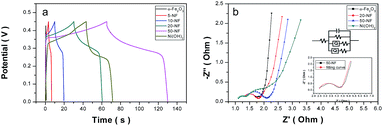
Furthermore, the GCD curves of the composites were collected to observe the charge–discharge behavior of the composites (Fig. 7a and S6†). Two steps involved for the charge–discharge process.51 A fast charge step happened below 0.35 V and a slow charge step took place in the range of 0.35 to 0.45 V. The fast charge step maybe caused by capacitive charge, and during the slow step the faradic process happened. As the percentages of Ni(OH)2 increased, the slow charge step experienced longer time. One possible reason is that increased amount of Ni(OH)2 demanded longer time to complete the charge–discharge process. Another reason is that Ni(OH)2 has worse conductivity than α-Fe2O3 and the composite with higher Ni(OH)2 loading has lower conductivity.
To prove this point, EIS (electrochemical impedance spectroscopy) curves of the composites were obtained as shown in Fig. 7b. The EIS plots consist of a quasi-semicircle corresponding to the faradic reactions with the diameter representing the interfacial charge-transfer impedance (Rct) at low frequency region, and a linear part representing the value of the internal resistance (Rs) at high frequency region.52–54 The Rct of α-Fe2O3, 20-NF, 50-NF and Ni(OH)2 were calculated as 0.2 Ω, 0.43 Ω, 0.67 Ω, and 1.0 Ω, respectively. These results revealed that the increased loading percentages of Ni(OH)2 led to higher resistance for charge transfer. This phenomenon may be caused by the lower conductivity of Ni(OH)2 than α-Fe2O3. In the other viewpoint, the added α-Fe2O3 plays double roles: improving conductivity of the composite and acting as the intercalated substance in between Ni(OH)2 nanosheets.
To investigate the potential for practical application of Ni(OH)2–α-Fe2O3 materials, an asymmetric supercapacitor (ASC) was fabricated using the Ni(OH)2–α-Fe2O3 composite as the positive electrode and AC as the negative electrode. The mass of the negative electrode (AC) was determined according to the charge balance theory.40,55 The charge stored on each electrode was obtained from eqn (1) and (2).56 Where C is specific capacitance, ΔV is the voltage range during charge–discharge test, and m is the mass of active material.
| C = I × t/ΔV × m | (7) |
| q = C × ΔV × m | (8) |
The charge balance follows the equation q+ = q−, we can get eqn (9):
| m+/m− = C− × ΔV−/C+ × ΔV+ | (9) |
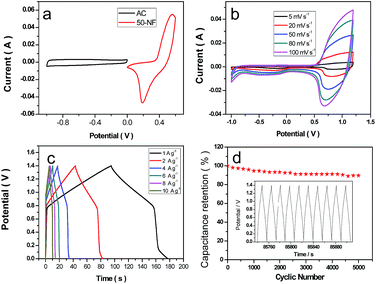
Fig. 8b shows a series of CV curves of the asymmetric supercapacitor at various scan rates from 5 to 100 mV s−1 in a 1 M KOH aqueous electrolyte under the electrochemical window of −1.0–1.4 V. Furthermore, Fig. 8c shows the GCD curves of the asymmetric supercapacitor at different current densities under a potential window of 0–1.6 V. The energy density and power density of the symmetric supercapacitors were calculated from CV curves according to the following two equations, respectively:
| E = 1/7.2CV2 | (10) |
| P = 3600E/t | (11) |
The Ni(OH)2/Fe2O3//AC asymmetric supercapacitor cell exhibited high energy density and excellent power density of 31.6 W h Kg−1 and 474 W Kg−1 at a scan rate of 5 mV s−1, 26.1 W h Kg−1 and 1567 W Kg−1 at 20 mV s−1, 22.1 W h Kg−1 and 3308 W Kg−1 at 50 mV s−1, 19.8 W h Kg−1 and 4748 W Kg−1 at 80 mV s−1, 18.6 W h Kg−1 and 5578 W Kg−1 at 100 mV s−1, respectively. When the power density of the asymmetric supercapacitor increased for more than 10 times, the retention of energy density can reach 59%. The cycling performance of Ni(OH)2–α-Fe2O3//AC asymmetric supercapacitors is shown in Fig. 8d. After 5000 cycles, the capacitance retention rate was still 89.6%. To reveal the change of Ni(OH)2–α-Fe2O3 after charge–discharge process, the TEM and SEM images for the cycled sample were obtained in Fig. S7.† However, there were not remarkable changes being able to be observed. The high capacitance retention maybe cause by the stable structure of Ni(OH)2–α-Fe2O3 during charge–discharge process. The performance of our sample was compared with the reported concerning samples as listed in Table S1.† Our Ni(OH)2–α-Fe2O3 sample has comparable performance with the reported electrodes. More importantly, the asymmetric capacitors based on Ni(OH)2–α-Fe2O3 composite are reported by us for the first time.
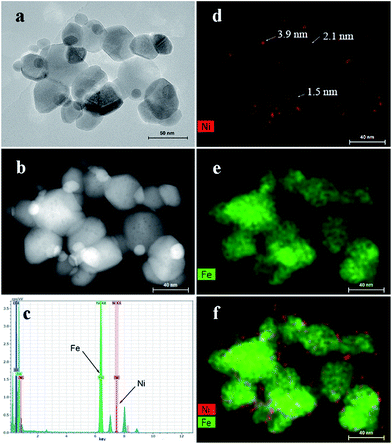
Two series of Ni(OH)2–α-Fe2O3//AC ASC devices charged by galvanostatic charging/discharging (GCD) techniques to 1.6 V can be used to drive different types of LED lights. Red LED light with a threshold voltage of 1.8 V, blue LED light with 2.6 V, and green LED with 2.9 V can be lighted up by the series devices, as shown in Fig. 9. More importantly, the charged ASC devices with 5 s charging time at a current density of 40 A g−1 can keep the red LED light lighting for 10 min.
 | ||
| Fig. 9 The photographs of green LED (a), blue LED (b) and red LED (c) lights being lighted up by the series asymmetric supercapacitors. | ||
4. Conclusions
In summary, we have developed a way to spontaneously grow Ni(OH)2-based composites and investigate their electrochemical energy storage performance for asymmetric supercapacitors. Under low loading percentages of Ni(OH)2 on α-Fe2O3, the ultrasmall Ni(OH)2 particles form over α-Fe2O3 nanoparticles. The higher loading percentages of Ni(OH)2 on α-Fe2O3 lead to the formation of the intercalated composites. Various characterization results including TEM, SEM, ICP-OES, XRD and XPS reveal the structure and composition of our composites. The energy storage performance of Ni(OH)2–α-Fe2O3 composites is measured with CV method, and the specific capacitance of Ni(OH)2–α-Fe2O3 composites increased with the increased Ni(OH)2 loading. More interestingly, Ni(OH)2–α-Fe2O3 composite with 50% Ni(OH)2 loading exhibited higher specific capacitance that of Ni(OH)2 and α-Fe2O3, revealing the strong synergistic effect between Ni(OH)2 nanosheets and α-Fe2O3 nanoparticles. The fabricated asymmetric supercapacitor based on Ni(OH)2–α-Fe2O3 and activated carbon exhibits the energy density and power density of 31.6 W h Kg−1 and 474 W Kg−1. More importantly, even if the power density increased to 5578 W Kg−1, the energy density still has 18.6 W h Kg−1, implying their important application in high power devices. For practical application of the asymmetric supercapacitors, all kinds of LED lights can be lighted up by two series asymmetric supercapacitors. With 5 second charging time, the charged asymmetric supercapacitors can supply the energy source for LED light with 10 minutes.Contribution statement
Ms Y. Wang prepared most samples, executed the electrochemical measurements and analysed all data, Dr X. Zhang and Ms H. Wei obtained the SEM images, Mr X. Li obtained TEM images, Ms Y. Liu measured the XRD patterns, Ms X. Wang and Mr X. Liu measured the elemental mapping images and HRTEM images, Dr J. Xu and Dr W. Li measured the BET data, Mr Y. Liu prepared iron oxide sample, Ms Y. Wang, Dr M. Liang and Dr P. Jiang co-wrote the manuscript, Dr Liang and Dr Jiang co-designed the experiments.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by Beijing Municipal Science and Technology Project (No. Z161100001116080), National Key Scientific Instrument and Equipment Development Projects (No. 2014YQ090709), NSFC (21673273, 21103030), and Major Project of the Ministry of Science and Technology (No. 2016YFA0200904).Notes and references
- L. Dong, C. Xu, Y. Li, Z.-H. Huang, F. Kang, Q.-H. Yang and X. Zhao, J. Mater. Chem. A, 2016, 4, 4659–4685 CAS.
- X. Liu, Z. Wu and Y. Yin, Chem. Eng. J., 2017, 323, 330–339 CrossRef CAS.
- D. P. Dubal, O. Ayyad, V. Ruiz and P. Gomez-Romero, Chem. Soc. Rev., 2015, 44, 1777–1790 RSC.
- M. Gao, L. Li and Y. Song, J. Mater. Chem. C, 2017, 5, 2971–2993 RSC.
- L. Kou, T. Q. Huang, B. N. Zheng, Y. Han, X. L. Zhao, K. Gopalsamy, H. Y. Sun and C. Gao, Nat. Commun., 2014, 5, 10 Search PubMed.
- Y. Zhang, Y. Zhao, X. L. Cheng, W. Weng, J. Ren, X. Fang, Y. S. Jiang, P. N. Chen, Z. T. Zhang, Y. G. Wang and H. S. Peng, Angew. Chem., Int. Ed., 2015, 54, 11177–11182 CrossRef CAS PubMed.
- J. Wang, S. Y. Dong, B. Ding, Y. Wang, X. D. Hao, H. Dou, Y. Y. Xia and X. G. Zhang, Natl. Sci. Rev., 2017, 4, 71–90 Search PubMed.
- Q. Zhu, K. Liu, J. Zhou, H. Hu, W. Chen and Y. Yu, Chem. Eng. J., 2017, 321, 554–563 CrossRef CAS.
- Y. Huang, H. Cheng, D. Shu, J. Zhong, X. Song, Z. Guo, A. Gao, J. Hao, C. He and F. Yi, Chem. Eng. J., 2017, 320, 634–643 CrossRef CAS.
- Q. Ke, M. Zheng, H. Liu, C. Guan, L. Mao and J. Wang, Sci. Rep., 2015, 5, 13940 CrossRef PubMed.
- Y. Zhao, L. Hu, S. Zhao and L. Wu, Adv. Funct. Mater., 2016, 26, 4085–4093 CrossRef CAS.
- B. Shen, X. Zhang, R. Guo, J. Lang, J. Chen and X. Yan, J. Mater. Chem. A, 2016, 4, 8180–8189 CAS.
- C. Zhang, T. M. Higgins, S.-H. Park, S. E. O'Brien, D. Long, J. Coleman and V. Nicolosi, Nano Energy, 2016, 28, 495–505 CrossRef CAS.
- F. Wang, S. Xiao, Y. Hou, C. Hu, L. Liu and Y. Wu, RSC Adv., 2013, 3, 13059–13084 RSC.
- L. Aguilera, Y. Leyet, R. Peña-Garcia, E. Padrón-Hernández, R. R. Passos and L. A. Pocrifka, Chem. Phys. Lett., 2017, 677, 75–79 CrossRef CAS.
- B. Dong, M. Li, S. Chen, D. Ding, W. Wei, G. Gao and S. Ding, ACS Appl. Mater. Interfaces, 2017, 9, 17890–17896 CAS.
- D. S. Hall, D. J. Lockwood, C. Bock and B. R. MacDougall, Proc. R. Soc. A, 2015, 471, 20140792 CrossRef PubMed.
- Y. Wang, X. F. Zhang, X. Li, X. J. Li, Y. Zhao, H. Wei, Y. G. Liu, P. Jiang and M. H. Liang, J. Colloid Interface Sci., 2017, 490, 252–258 CrossRef CAS PubMed.
- N. Choudhary, C. Li, J. Moore, N. Nagaiah, L. Zhai, Y. Jung and J. Thomas, Adv. Mater., 2017, 29, 1605336 CrossRef PubMed.
- N. Jabeen, A. Hussain, Q. Xia, S. Sun, J. Zhu and H. Xia, Adv. Mater., 2017, 29, 1700804 CrossRef PubMed.
- A. Singh and A. Chandra, Sci. Rep., 2015, 5, 12 Search PubMed.
- Z. Zhang, H. Wang, Y. Zhang, X. Mu, B. Huang, J. Du, J. Zhou, X. Pan and E. Xie, Chem. Eng. J., 2017, 325, 221–228 CrossRef CAS.
- J. Sun, C. Wu, X. Sun, H. Hu, C. Zhi, L. Hou and C. Yuan, J. Mater. Chem. A, 2017, 5, 9443–9464 CAS.
- Z. H. Liu, X. C. Tian, X. Xu, L. He, M. Y. Yan, C. H. Han, Y. Li, W. Yang and L. Q. Mai, Nano Res., 2017, 10, 2471–2481 CrossRef CAS.
- L. B. Wang, H. L. Yang, X. X. Liu, R. Zeng, M. Li, Y. H. Huang and X. L. Hu, Angew. Chem., Int. Ed., 2017, 56, 1105–1110 CrossRef CAS PubMed.
- Y. Li, J. Xu, T. Feng, Q. F. Yao, J. P. Xie and H. Xia, Adv. Funct. Mater., 2017, 27, 10 Search PubMed.
- S. Zhang, B. Yin, Z. Wang and F. Peter, Chem. Eng. J., 2016, 306, 193–203 CrossRef CAS.
- X. Zheng, X. Yan, Y. Sun, Y. Yu, G. Zhang, Y. Shen, Q. Liang, Q. Liao and Y. Zhang, J. Colloid Interface Sci., 2016, 466, 291–296 CrossRef CAS PubMed.
- H. Quan, B. Cheng, Y. Xiao and S. Lei, Chem. Eng. J., 2016, 286, 165–173 CrossRef CAS.
- S. Deabate, F. Henn, S. Devautour and J. C. Giuntini, J. Electrochem. Soc., 2003, 150, J23–J31 CrossRef CAS.
- Y. Jiao, Y. Liu, B. Yin, S. Zhang, F. Qu and X. Wu, Nano Energy, 2014, 10, 90–98 CrossRef CAS.
- W. Tian, X. Wang, C. Zhi, T. Zhai, D. Liu, C. Zhang, D. Golberg and Y. Bando, Nano Energy, 2013, 2, 754–763 CrossRef CAS.
- Z. Li, W. Zhang, Y. Su, Z. Li and J. Groeper, Nanotechnology, 2017, 28, 045603 CrossRef PubMed.
- Y. Gao, D. L. Wu, T. Wang, D. Z. Jia, W. Xia, Y. Lv, Y. L. Cao, Y. Y. Tan and P. G. Liu, Electrochim. Acta, 2016, 191, 275–283 CrossRef CAS.
- H. Zhang, Q. M. Gao, K. Yang, Y. L. Tan, W. Q. Tian, L. H. Zhu, Z. Y. Li and C. X. Yang, J. Mater. Chem. A, 2015, 3, 22005–22011 CAS.
- M. H. Liang, X. D. Wang, H. Q. Liu, H. C. Liu and Y. Wang, J. Catal., 2008, 255, 335–342 CrossRef CAS.
- C. Xiao, M. H. Liang, A. Gao, J. L. Xie, Y. Wang and H. C. Liu, J. Nanopart. Res., 2013, 15, 11 Search PubMed.
- H. Q. Liu, M. H. Liang, C. Xiao, N. Zheng, X. H. Feng, Y. Liu, J. L. Xie and Y. Wang, J. Mol. Catal. A: Chem., 2009, 308, 79–86 CrossRef CAS.
- X. Li, Y. Wang, L. Q. Li, W. Q. Huang, Z. C. Xiao, P. F. Wu, W. B. Zhao, W. Guo, P. Jiang and M. H. Liang, J. Mater. Chem. A, 2017, 5, 11294–11300 CAS.
- Y. Zhao, Y. N. Meng, H. P. Wu, Y. Wang, Z. X. Wei, X. J. Li and P. Jiang, RSC Adv., 2015, 5, 90307–90312 RSC.
- M. Freitas, R. Silva, D. M. Anjos, A. Rozario and P. G. Manoel, J. Power Sources, 2007, 165, 916–921 CrossRef CAS.
- L. Badr and R. Sultan, Chem. Phys. Lett., 2008, 453, 40–44 CrossRef CAS.
- X. Hu, S. Liu, C. Li, J. Huang, J. Luv, P. Xu, J. Liu and X.-Z. You, Nanoscale, 2016, 8, 11797–11802 RSC.
- X. F. Lu, X. Y. Chen, W. Zhou, Y. X. Tong and G. R. Li, ACS Appl. Mater. Interfaces, 2015, 7, 14843–14850 CAS.
- H. Jiang, H. Ma, Y. Jin, L. Wang, F. Gao and Q. Lu, Sci. Rep., 2016, 6, 31751 CrossRef CAS PubMed.
- R. Yang and L. Gao, J. Colloid Interface Sci., 2006, 297, 134–137 CrossRef CAS PubMed.
- L. Liu, J. Wang, C. Wang and G. Wang, Appl. Surf. Sci., 2016, 390, 303–310 CrossRef CAS.
- C. Zhao, X. Shao, Y. Zhang and X. Qian, ACS Appl. Mater. Interfaces, 2016, 8, 30133–30142 CAS.
- H. T. Zhang, X. Zhang, D. C. Zhang, X. Z. Sun, H. Lin, C. H. Wang and Y. W. Ma, J. Phys. Chem. B, 2013, 117, 1616–1627 CrossRef CAS PubMed.
- Y. Miao, L. Ouyang, S. Zhou, L. Xu, Z. Yang, M. Xiao and R. Ouyang, Biosens. Bioelectron., 2014, 53, 428–439 CrossRef CAS PubMed.
- H. Yi, H. Wang, Y. Jing, T. Peng, Y. Wang, J. Guo, Q. He, Z. Guo and X. Wang, J. Mater. Chem. A, 2015, 3, 19545–19555 CAS.
- J. Li, F. L. Luo, X. Q. Tian, Y. Lei, H. Y. Yuan and D. Xiao, J. Power Sources, 2013, 243, 721–727 CrossRef CAS.
- K. X. Li, Y. H. Luo, Z. X. Yu, M. H. Deng, D. M. Li and Q. B. Meng, Electrochem. Commun., 2009, 11, 1346–1349 CrossRef CAS.
- T. N. Murakami, S. Ito, Q. Wang, M. K. Nazeeruddin, T. Bessho, I. Cesar, P. Liska, R. Humphry-Baker, P. Comte, P. Pechy and M. Gratzel, J. Electrochem. Soc., 2006, 153, A2255–A2261 CrossRef CAS.
- X. Bai, Q. Liu, J. Liu, H. Zhang, Z. Li, X. Jing, P. Liu, J. Wang and R. Li, Chem. Eng. J., 2017, 315, 35–45 CrossRef CAS.
- J. J. Shen, X. C. Li, L. Wan, K. Liang, B. K. Tay, L. B. Kong and X. B. Yan, ACS Appl. Mater. Interfaces, 2017, 9, 668–676 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: TEM images, XPS spectra, CV curves, BET data, and GCD curves. See DOI: 10.1039/c7ra09119b |
| This journal is © The Royal Society of Chemistry 2017 |