Open Access Article
Open Access ArticleSynthesis of four-angle star-like CoAl-MMO/BiVO4 p–n heterojunction and its application in photocatalytic desulfurization
Limin Yuna,
Zhanxu Yang *a,
Zong-Bao Yu
*a,
Zong-Bao Yu a,
Tianfeng Caia,
Yue Lib,
Changyou Guoc,
Chengyuan Qia and
Tieqiang Rena
a,
Tianfeng Caia,
Yue Lib,
Changyou Guoc,
Chengyuan Qia and
Tieqiang Rena
aCollege of Chemistry, Chemical Engineering and Environment Engineering, Liaoning Shihua University, Fushun, Liaoning 113001, P. R. China. E-mail: zhanxuy@126.com
bSchool of Foreign Languages, Liaoning Shihua University, Fushun, Liaoning 113001, P. R. China
cSINOPEC Fushun Research Institute of Petroleum and Petrochemicals, Fushun 113001, China
First published on 11th May 2017
Abstract
A four-angle star-like Co–Al mixed metal oxide (CoAl-MMO)/BiVO4 heterojunction has been synthesized via a hydrothermal method and following sintering. The CoAl-MMO/BiVO4 is derived from CoAl-LDHs/BiVO4, in which CoAl-LDHs leads to a distribution of amorphous CoAl-MMO. The CoAl-MMO loading on BiVO4 greatly enhances visible light absorption, improves charge separation by band offset charge transfer, and makes flat band potential more negative. The three effects together result in excellent photocatalytic activity. Under visible light irradiation, desulfurization efficiency of thiophene has achieved up to 98.58% on CoAl-MMO/BiVO4 with molar ratio of 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.
5.
Introduction
Organic sulfur from petroleum is a major contributor to environmental pollution. Nowadays, the most widely employed method to remove thiophenic compounds in fuel are hydrodesulfurization (HDS)1 and adsorption desulfurization.2 Both of the processes need hydrogen, relatively high-pressure conditions and high energy consumption for deep desulfurization. Therefore, the development of desulfurization process with low energy consumption, mild operating conditions and environmental friendliness has become the research focus.3Photocatalytic desulfurization technology has attracted much attention, because it can provide a cleaner and more environmental friendly way to realize desulfurization. TiO2-based photocatalyst has been widely studied and used in photocatalytic oxidative desulfurization.4 But since its band gap is 3.2 eV, it can only absorb UV light, which limits its application.5 Therefore, the development of photocatalysts with visible light response has become a research hotspot. Bismuth vanadate (BiVO4, n-type semiconductor), with a band gap of 2.4 eV, has attracted much attention because it shows activation under visible light irradiation.6–10 However, the photocatalytic activity of pure BiVO4 is always low because of the rapid recombination of carriers (electrons and holes). To overcome these drawbacks, strategy of heterogeneous structure construction has been developed,11–14 to spatially separate photogenerated carriers by band offset. Besides, one widely common strategy is to combine BiVO4 with other noble metal or noble metal oxides,15,16 such as Pt, Ag, RuO2. For example, Lin et al.17 reported a visible-light responsive photocatalyst BiVO4 co-loaded with Pt and RuO2 co-catalysts, which photocatalytically oxidized thiophene to SO3 and achieved over 99% of thiophene conversion. Gao et al.18 synthesized Ag–BiVO4 photocatalysts via hydrothemal method and photocatalytic desulfurization efficiency under visible light irradiation at pH = 7 could be up to 95%. Although the desulfurization efficiency of the above photocatalyst is high, the cost of cocatalysts using noble metal or noble metal oxides is expensive. It is necessary to develop low-cost cocatalysts to combine with BiVO4 to achieve efficient desulfurization.
Herein we report the photocatalytic oxidation of thiophene by Co–Al mixed metal oxide (CoAl-MMO) loaded BiVO4. However, investigation indicates that CoAl-MMO does not act as cocatalyst of BiVO4, but gets three unprecedented effects. The CoAl-MMO loading enhances visible light absorption, improves charge separation by band offset charge transfer, and makes flat band potential more negative. Combination of these effects largely enhances photocatalytic efficiency of thiophene oxidation. The CoAl-MMO derives from a Co–Al-layered double hydroxide (CoAl-LDH) precursor.19,20 Since LDH has a uniform distribution of metal cations on the atomic level, sintering leads to CoAl-MMO with a uniform distribution of cobalt and aluminum.21 It notes that band gap of CoAl2O4 (ref. 22) is narrow while that of Al2O3 is wide,23 but neither has been found from our sample by X-ray diffraction (XRD). Besides XRD, samples were characterized by scanning electron microscopy (SEM) and transmission electron microscopy (TEM), ultraviolet-visible diffusive reflectance spectroscopy (UV-vis DRS) and photoluminescence (PL) spectra. The desulfurization activity has been explored under visible-light.
Experiment
Chemicals
All the reagents were analytical grade and used without any further purification.Preparation of BiVO4 and CoAl-MMO/BiVO4 samples
In a typical synthesis, under stirring conditions, 0.17 mmol P123 (analysis) and 5 mmol Bi(NO3)3·5H2O were dissolved in a mixed solution of 5 mL HNO3 solution (3 mol L−1) and 20 mL ethylene glycol solution to obtain solution A. 5 mmol NH4VO3 was dissolved in 20 mL deionized water with 70 °C to obtain solution B. Solution A and B at room temperature were stirred for 30 min each, then solution A was added to B dropwise. Then pH value was adjusted with NH3 H2O (14 wt%) and was stirred for 60 min to obtain BiVO4 precursor. A certain amount of Co(NO3)2·6H2O and Al(NO3)3·9H2O was dissolved in deionized water (Co2+/Al3+ molar ratio = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), then a solution containing 1.0 mol L−1 NaOH and 0.30 mol L−1 Na2CO3 was added into it. Stirring for 60 min, Co–Al layered double hydroxide (CoAl-LDHs) precursor had been obtained. CoAl-LDHs precursor was added to BiVO4 precursor, pH value was adjusted to 10 with NaOH (1.0 mol L−1) solution and stirred for 60 another min. The mixed solution was transferred to a 100 mL Teflon lined stainless steel autoclave and hydrothermal treatment was conducted at 180 °C for 12 h. After that, the resulting CoAl-LDHs/BiVO4 catalysts were washed with deionized water and absolute ethanol, dried at 80 °C for 3 h. Molar ratio of CoAl-LDHs/BiVO4 for 0
1), then a solution containing 1.0 mol L−1 NaOH and 0.30 mol L−1 Na2CO3 was added into it. Stirring for 60 min, Co–Al layered double hydroxide (CoAl-LDHs) precursor had been obtained. CoAl-LDHs precursor was added to BiVO4 precursor, pH value was adjusted to 10 with NaOH (1.0 mol L−1) solution and stirred for 60 another min. The mixed solution was transferred to a 100 mL Teflon lined stainless steel autoclave and hydrothermal treatment was conducted at 180 °C for 12 h. After that, the resulting CoAl-LDHs/BiVO4 catalysts were washed with deionized water and absolute ethanol, dried at 80 °C for 3 h. Molar ratio of CoAl-LDHs/BiVO4 for 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (BiVO4), 0.1
5 (BiVO4), 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 0.3
5, 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 0.5
5, 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 were controlled. Final products were obtained by sintering in a muffle furnace at a ramp of 1 °C min−1 from 30 °C to 400 °C and maintaining for 4 h. The final products were noted as CoAl-MMO/BiVO4.
5 were controlled. Final products were obtained by sintering in a muffle furnace at a ramp of 1 °C min−1 from 30 °C to 400 °C and maintaining for 4 h. The final products were noted as CoAl-MMO/BiVO4.
Characterizations
X-ray powder diffraction (XRD) measurements were performed on a Bruker D8 Advance diffractometer operated at 40 kV and 40 mA at the scanning range from 2 to 70 degree with Cu-Kα radiation (λ = 0.15406 nm). The particle morphologies of the products were observed by scanning electron microscopy (SEM) (Hitachi SU 8010) and by transmission electron microscopy (TEM) (JEM 2200FS). A Cary 5000 UV-vis spectrometer (Agilent Technologies) was used to obtain the reflectance spectra of the samples over a range of 400–800 nm. Electrochemical analysis was carried out with a standard three-electrode system with a Pt plate as the counter electrode, Hg/Hg2Cl2 (saturated with KCl) as a reference electrode, and ITO glass coated with the sample was used as the working electrode. A 0.5 M Na2SO4 solution was used as electrolyte. A 500 W Xe arc lamp (CHF-XM35-500W) was utilized as light source. Transient short-circuit photocurrent measurements and Mott–Schottky experiments with amplitude of 50 mV and a frequency of 1000 Hz were taken on a CHI660E workstation.Photocatalytic oxidative desulfurization tests
The photocatalytic oxidative desulfurization tests were carried out in a quartz tube reactor with a water condenser at atmospheric pressure and room temperature, using air and H2O2 (30%) as oxidants. The model oil, with sulfur content of 200 ppm is prepared by dissolving thiophene into n-octane. 50 mg photocatalyst and 50 mL model oil were added to the reactor. The suspension was stirred in dark for 30 min to obtain an adsorption–desorption equilibrium between the CoAl/BiVO4 photocatalyst and model oil. Then, 0.128 mL H2O2 was added and the suspension was irradiated by a 500 W Xe arc lamp (CHF-XM35-500W), the airflow velocity was of 5 mL min−1. Desulfurized oil was collected periodically and extracted with acetonitrile. Sulfur content was determined by a TSN-5000 series fluorescence nitrogen/sulfur analyzer (Jiangfen Electroanalytical Instrument Co., Ltd., China).Results and discussion
Fig. 1 shows XRD patterns of BiVO4 and CoAl-MMO/BiVO4 samples. The diffraction peaks at 2θ of 18.6°, 28.9°, 30.5°, 34.4°, 35.3°, 39.4°, 42.3°, 46.1°, 46.6°, 47.3°, 53.3°, 58.3°, and 59.9° can be observed, which index to monoclinic BiVO4 (JCPDS no. 14-0688) and correspond to the crystalline planes of (101), (013), (112), (200), (020), (211), (105), (123), (204), (024), (301), (303) and (224).24 It is clearly observed from the graph that as CoAl-MMO loading ratio increases, the intensity of CoAl-MMO/BiVO4 samples becomes weaker; and no other peaks can be found in XRD patterns of CoAl-MMO/BiVO4 catalysts, which may due to the amorphous characteristics of CoAl-MMO. | ||
Fig. 1 XRD patterns of samples (a) BiVO4 and CoAl-MMO/BiVO4 with molar ratio (b) 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, (c) 0.3 5, (c) 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, (d) 0.5 5, (d) 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. 5. | ||
The morphology of samples has been characterized by SEM and TEM (Fig. 2). The morphology of BiVO4 shows four angles with star-like and the particle size is uniform. After loaded with CoAl-MMO, the morphology becomes more regular. Low molar loading ratio makes the surface of CoAl-MMO/BiVO4 particles look smooth, as molar loading ratio reaches 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, the surface become rough. And under magnified TEM many small particles on the surface can be observed (Fig. 2(d)). As red dashed line and red arrows point, CoAl-MMO nanoparticles are amorphous and dispersed on BiVO4 surface. As shown in inset graph of Fig. 2(b) and (c), the thickness of CoAl-MMO/BiVO4 with molar ratio 0.1
5, the surface become rough. And under magnified TEM many small particles on the surface can be observed (Fig. 2(d)). As red dashed line and red arrows point, CoAl-MMO nanoparticles are amorphous and dispersed on BiVO4 surface. As shown in inset graph of Fig. 2(b) and (c), the thickness of CoAl-MMO/BiVO4 with molar ratio 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 0.3
5 and 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 are about 750 nm and 1 μm, respectively, meaning that with the loading ratio increasing, more CoAl-MMO formed on BiVO4 surface and the particles become thicker. The particle size rises also, which may be due to CoAl-LDH playing a role of template.
5 are about 750 nm and 1 μm, respectively, meaning that with the loading ratio increasing, more CoAl-MMO formed on BiVO4 surface and the particles become thicker. The particle size rises also, which may be due to CoAl-LDH playing a role of template.
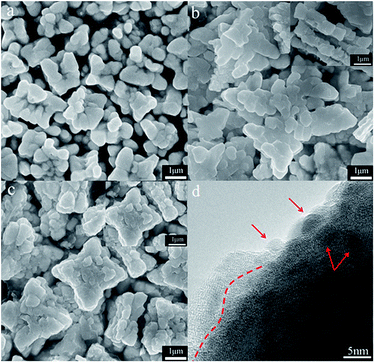 | ||
Fig. 2 SEM images of samples (a) BiVO4 and CoAl-MMO/BiVO4 with molar ratio (b) 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, (c) 0.3 5, (c) 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and (d) TEM image of that with ratio of 0.3 5 and (d) TEM image of that with ratio of 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. 5. | ||
Fig. 3(A) shows UV-vis diffuse reflectance spectra of BiVO4 and CoAl-MMO/BiVO4. BiVO4 shows an absorption region between 200–530 nm, which covers both UV and partial visible light region. Interestingly, after loaded with CoAl-MMO the absorption region extends to 700 nm, which is throughout the UV to visible light region. For crystalline semiconductor, the optical absorption near the band edge follows the formula:25
| αhν = A(hν − Eg)n/2 |
α, ν, Eg and A refer to coefficient, light frequency, band gap and a constant (A = 1), respectively. n depends on the characteristics of the transition in a semiconductor, for direct transition, n = 1, for indirect transition n = 4. For BiVO4, n = 1.26 The plots of (αhν)2 versus photon energy (hν) is shown in the inset graph of Fig. 3(A), and the band gap of BiVO4 and CoAl-MMO/BiVO4 can be obtained by extroplating the curve to α = 0. As a result, the band gaps CoAl-MMO/BiVO4 with loading ratio of 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 0.1
5, 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 0.3
5, 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 0.5
5 and 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 obtained are 2.4 eV, 2.35 eV, 2.06 eV and 2.08 eV, respectively. Compared to BiVO4, CoAl-MMO/BiVO4 samples exhibit stronger absorption in the visible light range and narrower band gap. BiVO4 is a n-type semiconductor27 while CoAl2O4 p-type,28 (although not detected by XRD but may exist as amorphous), a p–n heterojunction should have been formed at the interface of CoAl2O4 and BiVO4 particles, which extends the absorption range and makes the band gap narrower. For sample with molar loading ratio of 0.3
5 obtained are 2.4 eV, 2.35 eV, 2.06 eV and 2.08 eV, respectively. Compared to BiVO4, CoAl-MMO/BiVO4 samples exhibit stronger absorption in the visible light range and narrower band gap. BiVO4 is a n-type semiconductor27 while CoAl2O4 p-type,28 (although not detected by XRD but may exist as amorphous), a p–n heterojunction should have been formed at the interface of CoAl2O4 and BiVO4 particles, which extends the absorption range and makes the band gap narrower. For sample with molar loading ratio of 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, these small particles on the surface leads to expanding of contact area and stable heterojunction structure. However, for sample 0.5
5, these small particles on the surface leads to expanding of contact area and stable heterojunction structure. However, for sample 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, the absorption capacity become lower and the band gap slightly increases. It may be due to that some growing amount of CoAl-MMO particles are not well-loaded on the surface of BiVO4, but just scatter around, which makes the heterojunction structure unstable and restrain the interface interaction between CoAl-MMO and BiVO4.
5, the absorption capacity become lower and the band gap slightly increases. It may be due to that some growing amount of CoAl-MMO particles are not well-loaded on the surface of BiVO4, but just scatter around, which makes the heterojunction structure unstable and restrain the interface interaction between CoAl-MMO and BiVO4.
Photoluminescence (PL) spectra of semiconductor materials derive from recombination of photo-induced charge carriers. Higher PL intensity means higher recombination rate of carriers (electrons and holes) and photocatalytic activity becomes correspondingly lower.29–31 Fig. 3(B) shows PL spectra of BiVO4 and CoAl-MMO/BiVO4 samples with excitation wavelength of 320 nm. The PL emission wavelength of all samples is centered at 423 nm, different from 590 nm reported by R. Tang et al. on BiVO4 nanosheets and its complex with graphene.8 BiVO4 alone has the highest PL intensity, and the PL intensity decreases by different extent with CoAl-MMO loading amount, the least one obtained on sample ratio of 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. It's caused by the electron transfer from CoAl2O4 conduction band (CB) to BiVO4 CB, and meanwhile holes transfer from BiVO4 valence band (VB) to CoAl2O4 VB under the potential of band energy difference, namely, band offset.28,32 The above migration of photogenerated carriers makes electrons and holes spatial separation, and tremendous reduces their recombination probability. Accordingly, photogenerated carriers have longer life time to take part in photocatalytic reactions, and the photocatalytic activity of CoAl-MMO/BiVO4 would be improved upon BiVO4. As a photocatalytic activity test, transient photocurrent responses of the composite electrodes with on–off cycles of intermittent visible-light irradiation are studied, as shown in Fig. 3(C). The anodic photocurrent in Na2SO4 solution represents photocatalytic water oxidation efficiency of generated holes on surface of sample electrode. Among the catalysts, BiVO4 shows the lowest photocurrent, meaning that the least amount of holes participates in water oxidation, caused by the worst separation efficiency between carriers.33 With the molar ratio being 0.3
5. It's caused by the electron transfer from CoAl2O4 conduction band (CB) to BiVO4 CB, and meanwhile holes transfer from BiVO4 valence band (VB) to CoAl2O4 VB under the potential of band energy difference, namely, band offset.28,32 The above migration of photogenerated carriers makes electrons and holes spatial separation, and tremendous reduces their recombination probability. Accordingly, photogenerated carriers have longer life time to take part in photocatalytic reactions, and the photocatalytic activity of CoAl-MMO/BiVO4 would be improved upon BiVO4. As a photocatalytic activity test, transient photocurrent responses of the composite electrodes with on–off cycles of intermittent visible-light irradiation are studied, as shown in Fig. 3(C). The anodic photocurrent in Na2SO4 solution represents photocatalytic water oxidation efficiency of generated holes on surface of sample electrode. Among the catalysts, BiVO4 shows the lowest photocurrent, meaning that the least amount of holes participates in water oxidation, caused by the worst separation efficiency between carriers.33 With the molar ratio being 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, CoAl-MMO/BiVO4 generates the highest photocurrent density.
5, CoAl-MMO/BiVO4 generates the highest photocurrent density.
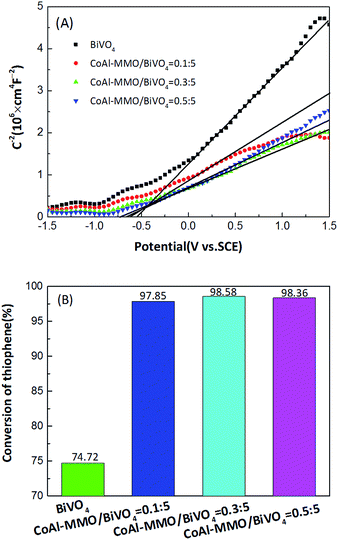
To investigate the electronic effect, Mott–Schottky (MS) measurement34,35 has been performed on BiVO4 and CoAl-MMO/BiVO4 series, as shown in Fig. 4(A). For CoAl-MMO/BiVO4 with molar loading ratio of 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 0.1
5, 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 0.3
5, 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 0.5
5 and 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, the Vfb are −0.54 V, −0.61 V, −0.72 V and −0.64 V vs. SCE (equivalent to −0.06 V, −0.13 V, −0.24 V and −0.16 V vs. NHE at PH = 0), respectively. It is well known that CB potential (ECB) of a n-type semiconductor is 0–0.2 V more negative than Vfb and is dependent on carrier concentration and electron effective mass. It can be seen that ECB of the samples are more negative than the standard redox potential of O2/˙O2− (0.28 V vs. NHE). It indicates the photogenerated electrons could react readily with adsorbed O2 to produce ˙O2− (ref. 36), and ˙O2− is active free radical in desulfurization reactions. Photocatalytic desulfurization activity has been investigated via thiophene decomposition, the result shown in Fig. 4(B). The most negative Vfb of −0.24 V corresponds to the highest desulfurization efficiency, is obtained with CoAl-MMO/BiVO4 ratio of 0.3
5, the Vfb are −0.54 V, −0.61 V, −0.72 V and −0.64 V vs. SCE (equivalent to −0.06 V, −0.13 V, −0.24 V and −0.16 V vs. NHE at PH = 0), respectively. It is well known that CB potential (ECB) of a n-type semiconductor is 0–0.2 V more negative than Vfb and is dependent on carrier concentration and electron effective mass. It can be seen that ECB of the samples are more negative than the standard redox potential of O2/˙O2− (0.28 V vs. NHE). It indicates the photogenerated electrons could react readily with adsorbed O2 to produce ˙O2− (ref. 36), and ˙O2− is active free radical in desulfurization reactions. Photocatalytic desulfurization activity has been investigated via thiophene decomposition, the result shown in Fig. 4(B). The most negative Vfb of −0.24 V corresponds to the highest desulfurization efficiency, is obtained with CoAl-MMO/BiVO4 ratio of 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.
5.
In summary, there are three significant merits of CoAl-MMO loading on BiVO4 via sintering mixture of CoAl-LDH and BiVO4. Firstly, it exhibits red shift of the whole band edge upon CoAl-MMO loading. That widens and enhances visible light absorption and utilization. Secondly, nanosized n–p heterojunction has been formed between CoAl-MMO particles and BiVO4. It endows carriers' spatial separation, which has been proved by PL intensity decrease. Thirdly, more negative Vfb has been obtained (dashed lines in Scheme 1), which benefits formation of ˙O2−. Interestingly, the above three effects changes along with CoAl-MMO loading amount, and 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 is the best in each aspect. Thus, based on the three merits, highly efficient thiophene desulfurization has been realized on CoAl-MMO/BiVO4. Schematic description of the mechanism for thiophene desulfurization on CoAl-MMO/BiVO4 has been shown in Scheme 1. Along with CoAl-MMO loading amount modifying, band gap energy of CoAl-MMO/BiVO4 becomes smaller and more visible light has been absorbed to excite carriers. 3 colored lines above EVB in Scheme 1 indicate that EVB moves upward, corresponding to band gap shortening, caused by changing loading amount of CoAl-MMO. Due to the band offset of heterojunction, photogenerated holes and electrons transfer to CoAl-MMO and BiVO4 respectively (black curved arrows in Scheme 1). Electron on BiVO4 reacts with absorbed O2 to ˙O2−. On the other hand, hole on CoAl-MMO reacts with OH− forming ˙OH, meanwhile some hole is readily captured by thiophene to forming radical cation C4H4S˙+ (blue curved arrows in Scheme 1). Interaction of the active oxygen species (˙O2− and ˙OH) with the sulfur radical cations (C4H4S˙+) induces a series of oxidation reactions, at last, thiophene has been almost completely oxidized to SO3, CO2, and H2O.36–38 As a result of the profound effects of CoAl-MMO loading and optimization, the desulfurization efficiency reaches up to 98.58% on 0.3
5 is the best in each aspect. Thus, based on the three merits, highly efficient thiophene desulfurization has been realized on CoAl-MMO/BiVO4. Schematic description of the mechanism for thiophene desulfurization on CoAl-MMO/BiVO4 has been shown in Scheme 1. Along with CoAl-MMO loading amount modifying, band gap energy of CoAl-MMO/BiVO4 becomes smaller and more visible light has been absorbed to excite carriers. 3 colored lines above EVB in Scheme 1 indicate that EVB moves upward, corresponding to band gap shortening, caused by changing loading amount of CoAl-MMO. Due to the band offset of heterojunction, photogenerated holes and electrons transfer to CoAl-MMO and BiVO4 respectively (black curved arrows in Scheme 1). Electron on BiVO4 reacts with absorbed O2 to ˙O2−. On the other hand, hole on CoAl-MMO reacts with OH− forming ˙OH, meanwhile some hole is readily captured by thiophene to forming radical cation C4H4S˙+ (blue curved arrows in Scheme 1). Interaction of the active oxygen species (˙O2− and ˙OH) with the sulfur radical cations (C4H4S˙+) induces a series of oxidation reactions, at last, thiophene has been almost completely oxidized to SO3, CO2, and H2O.36–38 As a result of the profound effects of CoAl-MMO loading and optimization, the desulfurization efficiency reaches up to 98.58% on 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 CoAl-MMO/BiVO4.
5 CoAl-MMO/BiVO4.
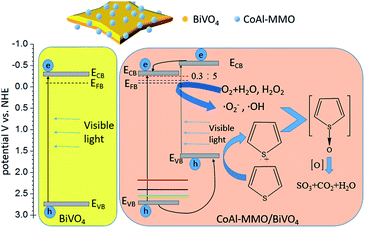 | ||
| Scheme 1 Schematic description of the mechanism for the photocatalytic oxidation of thiophene on CoAl-MMO/BiVO4. | ||
Conclusions
Four-angle star-like CoAl-MMO/BiVO4 photocatalysts has been synthesized by a hydrothermal method and later sintering. CoAl-MMO loading on BiVO4, dispersed as amorphous particles on its surface, brings advantages to photocatalytic performance from three aspects, such as visible light enhancement, heterojunctions for carrier spatial separation, and making Vfb more negative as well. Therefore, photocatalytic desulfurization efficiency by CoAl-MMO/BiVO4 has been improved largely, compared to BiVO4 under visible light irradiation, from under 75% to over 97%. After optimizing the loading amount, 98.58% conversion of thiophene has been achieved with molar loading ratio of 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. This work has demonstrated that CoAl-MMO is not only cost-effective, but also plays a significant role in enhancing the photocatalytic activity of BiVO4. It points that low-cost and effective mixed metal oxide loading on other photocatalysts may be a promising choice in photocatalytic desulfurization.
5. This work has demonstrated that CoAl-MMO is not only cost-effective, but also plays a significant role in enhancing the photocatalytic activity of BiVO4. It points that low-cost and effective mixed metal oxide loading on other photocatalysts may be a promising choice in photocatalytic desulfurization.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (21401093), Program for Liaoning Excellent Talents in University (LNET LR2015036), the Opening Funds of State Key Lab of Chemical Resource Engineering.Notes and references
- V. L. Vopa and C. N. Satterfield, J. Catal., 1988, 110, 375 CrossRef.
- A. J. Hernández-Maldonado and R. T. Yang, J. Am. Chem. Soc., 2004, 126, 992 CrossRef PubMed.
- G. Palmisano, V. Augugliaro, M. Pagliaro and L. Palmisano, Chem. Commun., 2007, 33, 3425 RSC.
- T. H. T. Vu, T. T. T. Nguyen, P. H. T. Nguyen, M. H. Do, H. T. Au, T. B. Nguyen, D. L. Nguyen and J. S. Park, Mater. Res. Bull., 2012, 47, 308 CrossRef CAS.
- A. L. Linsebigler, G. Lu and J. T. Yates, Chem. Rev., 1995, 95, 735 CrossRef CAS.
- A. Kudo, K. Omori and H. Kato, J. Am. Chem. Soc., 1999, 121, 11459 CrossRef CAS.
- Y. Chen, X. Ma, D. Li, H. Wang and C. Huang, RSC Adv., 2017, 7, 4395 RSC.
- Z.-R. Tang, Q. Yu and Y.-J. Xu, RSC Adv., 2014, 4, 58448 RSC.
- S. Liu, Z.-R. Tang, Y. Sun, J. C. Colmenares and Y.-J. Xu, Chem. Soc. Rev., 2015, 44, 5053 RSC.
- C. Han, N. Zhang and Y.-J. Xu, Nano Today, 2016, 11(3), 351 CrossRef CAS.
- X. Lin, B. Wei, X. Zhang, M. Song, S. Yu, Z. Gao, H. Zhai, L. Zhao and G. Che, Sep. Purif. Technol., 2016, 169, 9 CrossRef CAS.
- X. Lin, D. Xu, J. Zheng, M. Song, G. Che, Y. Wang, Y. Yang, C. Liu, L. Zhao and L. Chang, J. Alloys Compd., 2016, 688, 891–898 CrossRef CAS.
- X. Lin, D. Xu, S. Jiang, F. Xie, M. Song, H. Zhai, L. Zhao, G. Che and L. Chang, Catal. Commun., 2017, 89, 96 CrossRef CAS.
- X. Lin, D. Xu, Y. Xi, R. Zhao, L. Zhao, M. Song, H. Zhai, G. Che and L. Chang, Colloids Surf., A, 2017, 513, 117 CrossRef CAS.
- B. Zhang, J. Li, B. Zhang, R. Chong, R. Li, B. Yuan, S. M. Lu and C. Li, J. Catal., 2015, 332, 95 CrossRef CAS.
- Q. Yang, F. Chen, X. Li, D. Wang, Y. Zhong and G. Zeng, RSC Adv., 2016, 6, 60291 RSC.
- F. Lin, D. Wang, Z. Jiang, Y. Ma, J. Li, R. Li and C. Li, Energy Environ. Sci., 2012, 5, 6400 CAS.
- X. Gao, F. Fu, L. P. Zhang and W. Li, Phys. B, 2013, 419, 80 CrossRef CAS.
- Y. Tang, R. Wang, Y. Yang, D. Yan and X. Xiang, ACS Appl. Mater. Interfaces, 2016, 8, 19446 CAS.
- J. Zhao, Z. Lu, M. Shao, D. Yan, M. Wei, D. G. Evans and X. Duan, RSC Adv., 2013, 3, 1045 RSC.
- Z. Yang, Z. Song, G. Chu, X. Kang, T. Ren, W. Yang and Q. Qiao, J. Mater. Sci., 2012, 47, 4205 CrossRef CAS.
- A. Walsh, S. H. Wei, Y. Yan, M. M. Al-Jassim, J. A. Turner, M. Woodhouse and B. A. Parkinson, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 165119 CrossRef.
- S. Miyazaki, J. Vac. Sci. Technol., B, 2001, 19, 2212 CAS.
- H. Xu, C. Wu, H. Li, J. Chu, G. Sun, Y. Xu and Y. Yan, Appl. Surf. Sci., 2009, 256, 597 CrossRef CAS.
- M. A. Butler, J. Appl. Phys., 1997, 48, 1914 CrossRef.
- L. Zhou, W. Wang, S. Liu, L. Zhang, H. Xu and W. Zhu, J. Mol. Catal. A: Chem., 2006, 252, 120 CrossRef CAS.
- X. Dang, X. Zhang, X. Dong, W. Ruan, H. Ma and M. Xue, RSC Adv., 2014, 4, 54655 RSC.
- N. G. Matveeva and A. I. Shelykh, Phys. Status Solidi B, 1972, 50, 83 CrossRef CAS.
- F. Wang, L. Liang, L. Shi, K. Chen and J. Sun, Appl. Catal., A, 2016, 521, 104 CrossRef CAS.
- L. Shi, L. Lin, J. Ma and J. Sun, Superlattices Microstruct., 2013, 62, 128 CrossRef CAS.
- L. Jing, Y. Qu, B. Wang, S. Li, B. Jiang, L. Yang, W. Fu, H. Fu and J. Sun, Sol. Energy Mater. Sol. Cells, 2006, 90, 1773 CrossRef CAS.
- T. Fan, C. Chen and Z. Tang, RSC Adv., 2016, 6, 9994 RSC.
- Z. Zhang, M. Wang, W. Cui and H. Sui, RSC Adv., 2017, 7, 8167 RSC.
- E. Gao, W. Wang, M. Shang and J. Xu, Phys. Chem. Chem. Phys., 2011, 13, 2887 RSC.
- F. Cardon and W. P. Gomes, J. Phys. D: Appl. Phys., 1978, 11, L63 CrossRef CAS.
- F. Lin, Z. Jiang, N. Tang, C. Zhang, Z. Chen, T. Liu and B. Dong, Appl. Catal., B, 2016, 188, 253 CrossRef CAS.
- R. M. Mohamed and E. S. Aazam, Appl. Catal., A, 2014, 480, 100 CrossRef CAS.
- F. Lin, Y. Zhang, L. Wang, Y. Zhang, D. Wang, M. Yang, J. Yang, B. Zhang, Z. Jiang and C. Li, Appl. Catal., B, 2012, 127, 363 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |