Open Access Article
Open Access ArticleInteresting Ag3PO4 concave rhombic dodecahedra: the same face with different morphologies and photocatalytic properties
Ruirui Guoa,
Yaoting Fanb and
Yu Tang *a
*a
aKey Laboratory of Nonferrous Metals Chemistry, Resources Utilization of Gansu Province, State Key Laboratory of Applied and Organic Chemistry, College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000, P. R. China. E-mail: tangyu@lzu.edu.cn; Fax: +86-931-8912582; Tel: +86-931-8912552
bCollege of Chemistry and Molecular Engineering, Zhengzhou University, Zhengzhou 450001, P. R. China
First published on 3rd May 2017
Abstract
Herein, a novel and interesting Ag3PO4 crystal with concave rhombic dodecahedra morphology (Ag3PO4 CRD) via facile wet chemical method is presented. Compared to Ag3PO4 with rhombic dodecahedra structure (Ag3PO4 RD), the Ag3PO4 CRD was also enclosed by {110} facets but with different geometrical shapes, formed through carving out four parallelepipeds at special junctions. The photocatalytic activity of Ag3PO4 CRD dramatically decreased, this was attributed to the concave nature inducing easier recombination of photogenerated holes and electrons and the reaction with organic molecules being more difficult due to space limitation and the shadow effect. The results indicated that the geometrical distribution of crystal facets could play an important role in determining photocatalytic properties.
Introduction
Photocatalysts have received great attention as environmentally friendly and efficient materials in the field of environmental protection.1–5 Developing visible-light-driven photocatalysts has become a very active topic, such as chalcogenides, silver halide, and so on.6–9 With a band gap of 2.36 eV, Ag3PO4 shows excellent, high photocatalytic activity in the degradation of organic dyes under visible light irradiation.10 Morphology control of Ag3PO4 has been considered to be one of the most promising methods to improve its photocatalytic activity.11–14 Accordingly, it is of great value to further investigate Ag3PO4 crystals with new morphologies and structures.Recently, some morphologies of Ag3PO4 have been reported. Ye et al. fabricated Ag3PO4 rhombic dodecahedrons (RD) with only {110} facets exposed, which exhibited much higher photocatalytic activities than cubes bound by {100} facets for the degradation of organic contaminants.10 Zheng et al. reported the tetrahedral Ag3PO4 microcrystals with exposed {111} facets showed the highest photocatalytic activity in visible light irradiation among the {111}, {110} and {100} facets.11 It was elucidated that the excellent performance of tetrahedral Ag3PO4 crystals composed of {111} facets, was attributed to a high surface energy, leading to high charge carrier mobility and active surface reaction sites. More importantly, the reported Ag3PO4 crystals with various morphologies were obtained by adjusting internal experimental conditions such as raw materials, solvents, pH values, and additives,15–19 which also could result in the difference of photocatalytic activity. Thus, it is valuable and necessary to investigate the Ag3PO4 crystals with various morphologies obtained by almost the same condition.
In our work, Ag3PO4 CRD and Ag3PO4 RD were obtained via a facile wet chemical method only by tuning the concentration of NH3·H2O and H3PO4 (keep pH ≈ 7). With the same exposed {110} facets but different geometrical shapes, some outside faces of Ag3PO4 CRD were replaced by the corresponding inner faces at four special junctions. Thus, the relationships between morphologies and photocatalytic activities can be singly investigated in this situation.
Experimental
Sample preparation
Sample characterization
X-ray diffraction (XRD) experiments were carried out with a D/max-2400 diffractometer (Rigaku, Japan) using Cu-Kα radiation. The morphologies were examined by scanning electron microscopy (SEM, Hitachi S-4800). Diffuse reflectance ultraviolet-visible (UV-Vis) absorption spectra were measured by a PerkinElmer Lambda 950 spectrometer in the region of 200–700 nm, while BaSO4 was used as a reference. Electrochemical and photoelectrochemical measurements were performed in 0.5 M Na2SO4 electrolyte solution in a three-electrode quartz cell, platinum wire and Ag/AgCl electrode were served as the counter electrode and reference electrode respectively. The measurements were performed on a CHI-660C electrochemical workstation. Specific surface areas were computed from the isotherms by applying the Brunauer–Emmett–Teller (BET) method using a Micromeritics ASAP 2000 system.Evaluation of photocatalytic activity
The photocatalytic activity of samples was measured by the decomposition of Rhodamine B (RhB) solution in a reactor at room temperature. In a typical process for degradation, 200 mg of photocatalyst was suspended in the RhB solution (10 mg L−1, 80 mL). Before irradiation, the suspensions were stirred for 30 min in the dark to ensure the establishment of adsorption–desorption equilibrium. A 350 W Xenon lamp equipped with an ultraviolet cutoff filter (λ ≧ 420 nm) was employed for the visible light irradiation source which was positioned 20 cm away from the reactor to trigger the photocatalytic reaction. A certain volume of suspension was withdrawn at selected times for analysis. After recovering the photocatalyst by centrifugation, the concentration of dye solution was analyzed by measuring the light absorption of the clear solution at 554 nm (λmax for RhB solution) using a spectrophotometer (WFJ-7200, Unico, USA). The percentage of degradation was calculated by C/C0. Here, C is the concentration of remaining dye solution at each irradiated time interval, while C0 is the initial concentration.Results and discussion
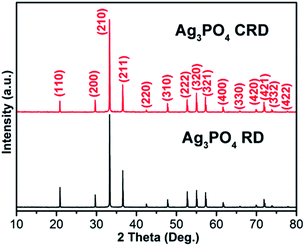
XRD patterns of Ag3PO4 CRD and Ag3PO4 RD shown in Fig. 1 indicate that all peaks of the Ag3PO4 can be indexed very well with JCPDS No. 06-0505, and no peaks of other impurities can be detected. Compared with Ag3PO4 RD, the relative intensity of the (110) peak decreases as shown in Table 1, which could be attributed to the change of the geometrical shape of two kinds of Ag3PO4.20| Intensity ratio | Ag3PO4 RD | Ag3PO4 CRD |
|---|---|---|
| (110)/(222) | 1.5 | 1.1 |
| (211)/(222) | 2.9 | 2.5 |
Fig. 2 shows the SEM images of Ag3PO4 CRD and Ag3PO4 RD. Compared with Ag3PO4 RD, there are four pits at the special corners on Ag3PO4 CRD. Fig. 3(a1–a3) presents the geometrical shapes of the Ag3PO4 RD from different view. It can be seen that the Ag3PO4 RD consists of 12 rhombus facets and 14 junctions. Fig. 3(b1–b3) and (c1–c3) present the diamond visualization of Ag3PO4 RD and Ag3PO4 CRD from different view, respectively, and the atoms of Ag and O were hided and only P displayed in order to identify easily. Compared with Ag3PO4 RD, every three face at special corners on Ag3PO4 CRD, such as the blue site pointed in Fig. 3(a1–a3), were carving out a parallelepiped. Then, four concaves with four new inner junctions were formed and corresponding four initial outside junctions disappear, as shown in Fig. 3(c1–c3). Thus, the spacial facets of Ag3PO4 RD were replaced by the new inner facets in Ag3PO4 CRD with the same area and the same {110} facet, which is firstly reported.
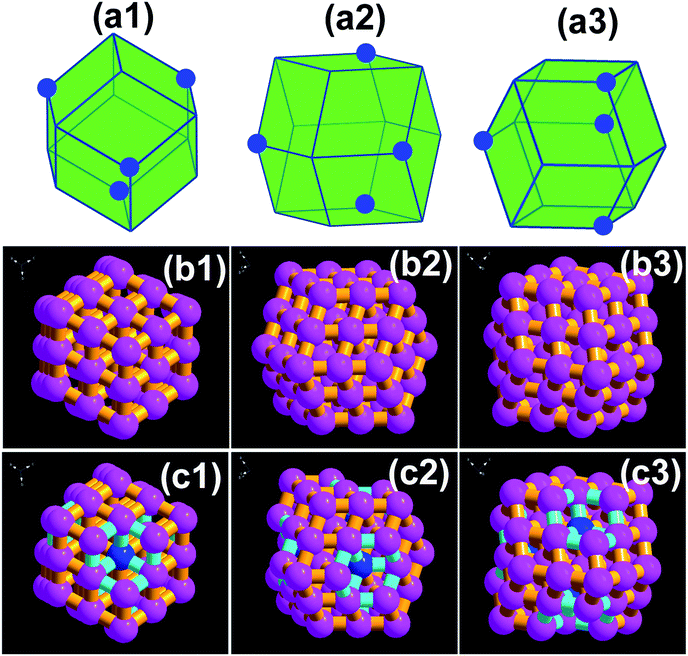 | ||
| Fig. 3 (a1–a3) Geometrical shapes of the Ag3PO4 RD from different view; diamond visualization of (b1–b3) Ag3PO4 RD and (c1–c3) Ag3PO4 CRD from different view. | ||
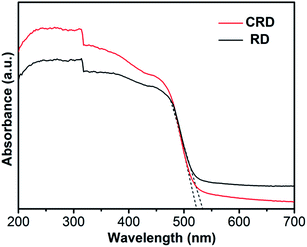
Before testing the photocatalytic activity for RhB solution, the UV-Vis absorption spectra of Ag3PO4 CRD and Ag3PO4 RD were measured. As shown in Fig. 4, there is a slight difference between the samples. Ag3PO4 RD is able to absorb light up to approximately 535 nm, while Ag3PO4 CRD has an absorption edge around 520 nm. Since the size of the crystals is very similar (2 μm) and there are no signs of foreign elemental doping in the sample, it is thought that the absorption edge shift is likely to be due to the morphology transformation, which may result in the XRD peaks ratios change.10,21 According to the previous report,11 the band gaps of the Ag3PO4 {111}, {110} and {100} surfaces were calculated to be 2.736 eV, 2.613 eV and 2.032 eV, respectively.
This indicates that Ag3PO4 CRD with lower intensity ratios of XRD peaks (110)/(222) can be attributed to the morphology transformation, thus possesses larger band gap value, which is consisted with the blue shift of absorption edge.
Using the BET method, it is turned out to be that the Ag3PO4 RD has a specific surface of 1.6 m2 g−1 and the Ag3PO4 CRD is 5.91 m2 g−1, respectively. According to the different morphologies of Ag3PO4 RD and Ag3PO4 CRD, it can be seen that every three face at special corners on Ag3PO4 CRD was carved out a parallelepiped, then every Ag3PO4 CRD crystal is more lighter than Ag3PO4 RD. So the numbers of Ag3PO4 CRD crystals are more than that of Ag3PO4 RD in the same unit mass and the surface area of Ag3PO4 RD was somewhat smaller than that of Ag3PO4 CRD, which also demonstrated that the improved catalytic activity of Ag3PO4 RD was not caused by the specific surface area to a certain extent.
On the basis of previous reports, the separation efficiency of electrons and holes played an important role and the photoelectrochemical parameters could be used to investigate the excitation and transfer of photo-generated charge carriers in the photocatalytic process.22,23 To better confirm the reason why the same face with different morphology and photocatalytic properties of Ag3PO4 CRD and Ag3PO4 RD, the photocurrent responses in an electrolyte under visible light and EIS (Nyquist plot) using a three-electrode system in 0.5 M Na2SO4 solution were performed, which may indirectly correlate with the generation and transfer to the photoinduced charge carriers in the photocatalytic process of Ag3PO4 CRD and Ag3PO4 RD. The current abruptly increased and decreased when the light source was switched on and off. Remarkably, the Ag3PO4 RD exhibited an obviously enhanced photocurrent response compared with Ag3PO4 CRD as shown in Fig. 5a, which implied that less efficient separation of the photogenerated electron–hole pairs and slow transfer of photoinduced charge carriers occurred in the Ag3PO4 CRD. Moreover, the EIS measurements were used to study the charge transfer and recombination proces.24,25 As shown in Fig. 5b, the diameter of the arc radius on the EIS Nyquist plot of the Ag3PO4 CRD was much larger than Ag3PO4 RD, which indicated that the more effective separation of photo-generated electron–hole pairs and fast interface charge transfer occurred in the Ag3PO4 RD.26
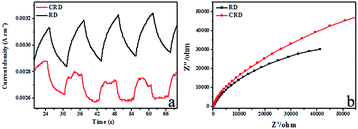 | ||
| Fig. 5 Transient photocurrent of Ag3PO4 CRD and Ag3PO4 RD under visible light irradiation (a) and electrochemical impedance spectra (Nyquist plot) of Ag3PO4 CRD and Ag3PO4 RD (b). | ||
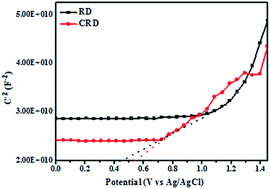
The Mott–Schottky plots under dark conditions were also investigated to compare the charge carrier density of Ag3PO4 CRD and Ag3PO4 RD, which were scanned using the voltage in the range of 0–1.5 eV and the frequency of 1000 Hz. The platinum wire and Ag/AgCl electrode were served as the counter electrode and reference electrode, respectively. The positive slop of the C−2–E as shown in Fig. 6 indicated that the Ag3PO4 RD and Ag3PO4 CRD belonged to n-type semiconductors.27 By plotting 1/C2 versus E, the flat-band potential of the n-type semiconductor could be quantified from the slope, which was found to shift from 0.54 eV for the Ag3PO4 CRD to 0.46 eV for the Ag3PO4 RD, and the more negative potential shift demonstrated that the separation efficiency of charge carrier was improved in the Ag3PO4 RD.28
Over all, easier generation, more efficient separation and enhanced transfer efficiency of photogenerated electron–hole pairs could be achieved in Ag3PO4 RD than Ag3PO4 CRD for degradation of RhB under visible light irradiation.
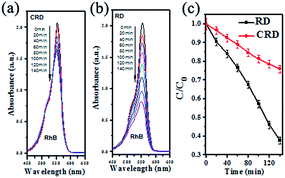
Fig. 7 shows the photocatalytic activity of the Ag3PO4 CRD and Ag3PO4 RD for RhB degradation under visible light irradiation. It can be seen that about 63% of RhB solution was degraded in 140 min for Ag3PO4 RD. However, only 24% of RhB was degraded for Ag3PO4 CRD with the same visible light irradiation. The dramatically decrease of photocatalytic activity of Ag3PO4 CRD is mainly attributed to the morphology change. With the concave geometrical shapes, the recombination of photogenerated holes and electrons become easier and the reaction with organic molecules could be harder due to the space limitation and shadow effect.
In this work, holes (h+), superoxide radicals (˙O2−) or hydroxyl radicals (˙OH) might be active species in photocatalytic process. To further explore the major specie, radical-trapping experiments with different scavengers were performed as shown in Fig. 8. In this way, 1 mM ethylenediamine tetraacetic acid disodium (EDTA-2Na, a scavenger of h+), 1 mM benzoquinone (BQ, a scavenger of ˙O2−), and 1 mM tertiary butanol (t-BuOH, a scavenger of ˙OH) were adopted in the degradation of RhB (3.5 ppm, 80 mL), which indicated that ˙OH radicals were not the dominant active species compared to no scavenger. The degradation efficiency was almost decreased completely after addition of EDTA-2Na and was decreased remarkably when the BQ added, which suggest that the h+ was the main active specie and ˙O2− also played a certain role in the photocatalytic degradation of RhB solution.
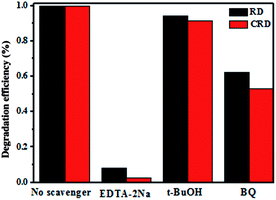 | ||
| Fig. 8 Trapping experiment of active species during the photocatalytic degradation of RhB over Ag3PO4 CRD and Ag3PO4 RD under visible light irradiation. | ||
Conclusion
In this work, a novel and interesting Ag3PO4 CRD was synthesized via a facile wet chemical method. Both enclosed by {110}, the Ag3PO4 CRD showed different geometrical shape, lower intensity ratios of XRD peaks (110)/(222), blue shift of absorption edge, less efficient separation and enhanced transfer efficiency of photogenerated electron–hole pairs compared with the Ag3PO4 RD. It was found that the Ag3PO4 CRD was formed through carving out four parallelepiped at special junctions. Furthermore, the photocatalytic activity of Ag3PO4 CRD dramatically decreased compared with Ag3PO4 RD, which was attributed to the concave nature-induced easier recombination of photogenerated holes and electrons and harder reaction with organic molecules with the space limitation and shadow effect. Apart from other reported vital factors, this work indicated that the geometrical distribution of crystal facets also plays an important role in photocatalytic property during the multielement determination.Acknowledgements
The research leading to these results has received funding from the National Natural Science Foundation of China (Projects 21471071, 21431002).Notes and references
- M. Yang, N. Zhang, M. Pagliaro and Y. Xu, Chem. Soc. Rev., 2014, 43, 8240 RSC.
- S. J. Moniz, S. A. Shevlin, D. J. Martin, Z. X. Guo and J. Tang, Energy Environ. Sci., 2015, 8, 731 CAS.
- M. Yang and Y. Xu, Nanoscale Horiz., 2016, 1, 185 RSC.
- H. Li, Y. Zhou, W. Tu, J. Ye and Z. Zou, Adv. Funct. Mater., 2015, 25, 998 CrossRef CAS.
- L. Zeng, X. Guo, C. He and C. Duan, ACS Catal., 2016, 6, 7935 CrossRef CAS.
- J. Hu, A. Liu, H. Jin, D. Ma, D. Yin, P. Ling, S. Wang, Z. Lin and J. Wang, J. Am. Chem. Soc., 2015, 137, 11004 CrossRef CAS PubMed.
- S. Bao, Z. Wang, X. Gong, C. Zeng, Q. Wu, B. Tian and J. Zhang, J. Mater. Chem. A, 2016, 4, 18570 CAS.
- H. Chen, G. Yu, G. D. Li, T. Xie, Y. Sun, J. Liu, H. Li, X. Huang, D. Wang, T. Asefa, W. Chen and X. Zou, Angew. Chem., Int. Ed., 2016, 55, 11442 CrossRef CAS PubMed.
- J. Ding, L. Zhang, Q. Liu, W. L. Dai and G. Guan, Appl. Catal., B, 2017, 203, 335 CrossRef CAS.
- Y. Bi, S. Ouyang, N. Umezawa, J. Cao and J. Ye, J. Am. Chem. Soc., 2011, 133, 6490 CrossRef CAS PubMed.
- B. Zheng, X. Wang, C. Liu, K. Tan, Z. Xie and L. Zheng, J. Mater. Chem. A, 2013, 1, 12635 CAS.
- P. Dong, Y. Wang, H. Li, H. Li, X. Ma and L. Han, J. Mater. Chem. A, 2013, 1, 4651 CAS.
- J. Wang, S. Lou, P. Sun, L. Wang, Y. Teng, M. Chen and F. Teng, ChemCatChem, 2014, 6, 2021 CrossRef CAS.
- G. Botelho, J. Andres, L. Gracia, L. S. Matos and E. Longo, ChemPlusChem, 2016, 81, 202 CrossRef CAS.
- M. Li, M. Chen, J. Wang and F. Teng, CrystEngComm, 2014, 16, 1237 RSC.
- L. Wang, N. Li, Q. Zhang, S. Lou, Y. Zhao, M. Chen and F. Teng, CrystEngComm, 2014, 16, 9326 RSC.
- B. Wang, L. Wang, Z. Hao and Y. Luo, Catal. Commun., 2015, 58, 117 CrossRef CAS.
- Z. Jiao, Y. Zhang, H. Yu, G. Lu, J. Ye and Y. Bi, Chem. Commun., 2013, 49, 636 RSC.
- U. Sulaeman, F. Febiyanto, S. Yin and T. Sato, Catal. Commun., 2016, 85, 22 CrossRef CAS.
- J. Wang, F. Teng, M. Chen, J. Xu, Y. Song and X. Zhou, CrystEngComm, 2013, 15, 39 RSC.
- H. Wang, L. He, L. Wang, P. Hu, L. Guo, X. Han and J. Li, CrystEngComm, 2012, 14, 8342 RSC.
- J. Yu, G. Dai and B. Cheng, J. Phys. Chem. C, 2010, 114, 19378 CAS.
- N. Zhang, S. Liu, X. Fu and Y. Xu, J. Mater. Chem., 2012, 22, 5042 RSC.
- S. Yang, Y. Gong, J. Zhang, L. Zhan, L. Ma, Z. Fang, R. Vajtai, X. Wang and P. Ajayan, Adv. Mater., 2013, 25, 2452 CrossRef CAS PubMed.
- L. Huang, H. Xu, Y. Li, H. Li, X. Cheng, J. Xia, Y. Xu and G. Cai, Dalton Trans., 2013, 42, 8606 RSC.
- L. Liu, Y. Qi, J. Lu, S. Lin, W. An, Y. Liang and W. Cui, Appl. Catal., B, 2016, 183, 133 CrossRef CAS.
- P. Debabrara, K. Susanta, T. Simon, M. Mano and T. Kam, Mater. Express, 2011, 1, 59 CrossRef.
- I. M. P. Silva, G. Byzynski, C. Ribeiro and E. Longo, J. Mol. Catal. A: Chem., 2016, 417, 89 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |