Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enantioselective Barbier-type allylation of ketones using allyl halide and indium in water†
Shuichi Nakamura *ab,
Yoshichika Haraa,
Takashi Furukawaa and
Tsunehisa Hirashitaa
*ab,
Yoshichika Haraa,
Takashi Furukawaa and
Tsunehisa Hirashitaa
aDepartment of Life Science and Applied Chemistry, Graduate School of Engineering, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555, Japan. E-mail: snakamur@nitech.ac.jp
bFrontier Research Institute for Material Science, Nagoya Institute of Technology Gokiso, Showa-ku, Nagoya 466-8555, Japan
First published on 9th March 2017
Abstract
We disclose herein an efficient enantioselective Barbier-type allylation of ketones using allyl halide and indium metal in water. The reaction was catalysed by chiral bis(imidazoline) to afford homoallylic alcohols having quaternary stereocenters in good yield with moderate to good enantioselectivity. Based on experimental investigation, a possible transition state has been proposed to explain the origin of the asymmetric induction.
Optically active tertiary homoallylic alcohols are an important class of synthetic intermediates because they often act as useful chiral building blocks for the synthesis of biologically active compounds. One of the most efficient methods for the synthesis of optically active tertiary homoallylic alcohols would be the catalytic enantioselective allylation of ketones. Although there are many papers on the catalytic enantioselective allylation of aldehydes, catalytic enantioselective allylation of ketones have been far less explored probably due to their low reactivity and the difficulty in enantiofacial discrimination of ketones.1 Recently, catalytic enantioselective allylations of ketones using stannanes,2 silanes,3 boron reagents,4 allylalcohols,5 and manganese compounds6 using various chiral catalysts have been reported. However, these reactions rely on strictly anhydrous conditions or on the use of corrosive or toxic reagents. On the other hand, Barbier-type allylation using allyl halide and indium metal has shown to be an effective method for the synthesis of homoallylic alcohols, because organoindium compounds have low toxicity, and they have the ability to tolerate the reaction in water.7 Therefore, there are several papers on the enantioselective Barbier-type allylation of ketones with allyl halides using a stoichiometric amount of chiral additives and indium metal in an organic solvent.8 However, there is no report on the catalytic enantioselective Barbier-type allylation of ketones in water using indium metal and allyl halides.9 Recently, Kobayashi and co-workers first reported the catalytic enantioselective allylation of ketones in water using allylboronate and a catalytic amount of indium(0) and bis(oxazoline) catalyst to give a product with 52% ee.10 Despite the pioneering progress achieved in enantioselective reaction of allylation with ketones in water, the development of novel catalyst systems with acceptable catalytic activity and stereoselectivity still remains a major challenge. On the other hand, we recently reported the enantioselective three-component synthesis of optically active propargylamines in water11 and the enantioselective allylation of ketimines using chiral bis(imidazoline) catalysts.12 Therefore, our research interest was expanded to the catalytic enantioselective Barbier-type allylation using allyl halide and indium metal using chiral bis(imidazoline) catalysts in water.
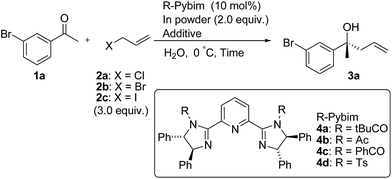
First, we examined the enantioselective Barbier-type allylation of 3-bromoacetophenone with various allyl halides (3.0 equiv.) and indium powder (2.0 equiv.) using 10 mol% of chiral bis(imidazoline) ligand in water. The results are shown in Table 1.
| Run | X | 4 | Additive (equiv.) | Time (h) | Yield (%) | ee (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.10 mmol), allyl halide (3.0 equiv.), In (2.0 equiv.), and 4 (10 mol%) in water (0.10 M) were used.b In THF.c Sodium dodecyl sulfate. | ||||||
| 1 | Cl | 4a | — | 18 | 0 | — |
| 2 | Br | 4a | — | 18 | 10 | 54 |
| 3 | I | 4a | — | 18 | 80 | 76 |
| 4 | I | 4b | — | 18 | 5 | 16 |
| 5 | I | 4c | — | 18 | 80 | 60 |
| 6 | I | 4d | — | 18 | 85 | 27 |
| 7b | I | 4a | — | 24 | 75 | 16 |
| 8 | I | 4a | SDSc (0.2) | 18 | 99 | 30 |
| 9 | Br | 4a | NaI (3.9) | 18 | 99 | 86 |
To our delight, the reaction using allyl iodide as allyl halide afforded product 3a in high yield with moderate enantioselectivity, although the reaction using allyl chloride or -bromides gave product 3a in low yield (Table 1, entries 1–3). Although we investigated the effect of the substituent on imidazoline catalysts, changing the substituent on nitrogen in imidazoline catalysts from a tert-butylcarbonyl group to an acetyl, benzoyl or tosyl group could not improve the enantioselectivity of product 3a (Table 1, entries 4–6). When the reaction was carried out in THF instead of water, the enantioselectivity was significantly reduced (Table 1, entry 7). In order to improve yield and enantioselectivity, we added some additives. Sodium dodecyl sulfate (SDS) was added to the reaction mixture as a surfactant, but stereoselectivity could not be improved (Table 1, entry 8).13 On the other hand, the addition of 3.9 equiv. of NaI to the reaction of allyl bromide 2b and 1a improved the yield and enantioselectivity of 3a (Table 1, entry 9 vs. 2).
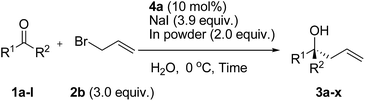
Having established the reaction conditions, Barbier-type allylation of various ketones with allyl bromide, indium powder and NaI using 10 mol% of chiral bis(imidazoline) ligand 4a in water was examined (Table 2). The reaction of acetophenone 1b afforded product 3b in good yield with moderate enantioselectivity (Table 2, entry 2). The reaction of electron-deficient ketone 1c–g having fluoro, chloro, or bromo groups in the para or meta position were tolerated in this reaction condition and gave products 3c–g with good stereoselectivity (59–89% ee, Table 2, entries 3–7), although the reaction of ortho-substituted ketone 1f gave product 3f in low yield and enantioselectivity (Table 2, entry 6). Ketones 1h, i bearing an electron-donating methyl and methoxy group gave corresponding products 3h, i in high yield with good enantioselectivity (Table 2, entries 8 and 9). Ketones 1j, k having a naphthyl or heteroaryl group such as the thienyl group also afforded products 3j, k in moderate yield with good enantioselectivity (Table 2, entries 10 and 11). These reaction conditions were also applicable to the reaction of trifluoromethyl ketones 1l (Table 2, entry 12). The absolute configurations of products 3a–d, f–l were determined in comparison with the value of the specific rotation reported in the literature (see ESI†). To our knowledge, these results are the first examples for the indium-mediated catalytic enantioselective Barbier-type allylation of ketones in water.
| Entry | 1 | R1 | R2 | Time (h) | Yield (%) | ee (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 1 (0.10 mmol), 2b (3.0 equiv.), NaI (3.9 equiv.), In (2.0 equiv.), and 4a (10 mol%) in water (0.10 M) were used. | ||||||
| 1 | 1a | 3-BrC6H4 | CH3 | 18 | 99 | 86 |
| 2 | 1b | Ph | CH3 | 18 | 86 | 65 |
| 3 | 1c | 3-FC6H4 | CH3 | 18 | 89 | 80 |
| 4 | 1d | 3-ClC6H4 | CH3 | 48 | 76 | 76 |
| 5 | 1e | 3-IC6H4 | CH3 | 18 | 99 | 89 |
| 6 | 1f | 2-BrC6H4 | CH3 | 48 | 40 | 59 |
| 7 | 1g | 4-BrC6H4 | CH3 | 48 | 78 | 65 |
| 8 | 1h | 3-MeOC6H4 | CH3 | 48 | 92 | 74 |
| 9 | 1i | 3-MeC6H4 | CH3 | 18 | 80 | 84 |
| 10 | 1j | 3-Thienyl | CH3 | 24 | 90 | 55 |
| 11 | 1k | 2-Naphthyl | CH3 | 24 | 94 | 71 |
| 12 | 1l | Ph | CF3 | 18 | 77 | 86 |
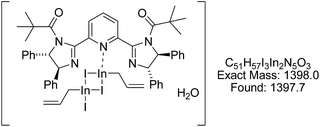
In order to clarify the reaction mechanism, we conducted spectroscopic analysis. The 1H NMR spectrum for the mixture of 2b, 4a, NaI, and indium powder showed a new methylene signal at 2.85 ppm (see ESI†). Chan and co-workers reported that 1H NMR peaks for allylindium(III) and allylindium(I) in water were observed at 2.8 ppm and 1.7 ppm, respectively, and that allylindium(III) make a allylindium sesquihalide species.14 Furthermore, the ESI-mass spectroscopic analysis for the reaction mixture of 2b, 4a, NaI, and indium powder showed complex A (Fig. 1: cation mode, calcd for C51H57I3InN5O3 as complex A: 1398.0 found: 1397.7). This signal implied a complex between allylindium(III) sesquihalide and 2b.15
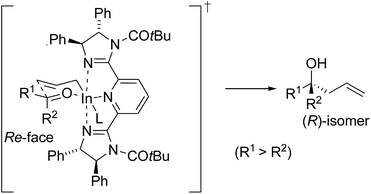
From the above consideration and absolute stereochemistry of the products, the assumed transition state for the enantioselective Barbier-type allylation of ketones using 4a in water is shown in Fig. 2. The allylation of ketones would proceed through a six-membered transition state including In(III) species.16 Allylindium sesquihalide dissociates to monomeric allylindium species by coordination to bis(imidazoline), then indium(III) cation coordinates to ketones. In this transition state, indium(III) makes an octahedral structure,17 and the allyl group approaches the Re-face of ketones avoiding steric repulsion between the phenyl group in 4a and substituent for ketones to give (R)-homoallylic alcohols. Further studies are required to fully elucidate the mechanistic detail of the Barbier-type allylation reaction of ketones with 4a.
In conclusion, we developed an enantioselective allylation of ketones using chiral bis(imidazoline) catalysts. To our knowledge, this is the first example of the highly enantioselective allylation of ketones using Barbier-type allylation of allyl halide and indium in water. Further experiments are in progress to study the scope of the asymmetric synthesis in water using bis(imidazoline) catalyst to other reactions.
Acknowledgements
This work was partly supported by partially supported by a Grant-in-Aid for Scientific Research from the MEXT (Japan) and Tokuyama Science Foundation.Notes and references
- For asymmetric allylation of ketones, see reviews: (a) M. Yus, J. C. González-Gómez and F. Foubelo, Chem. Rev., 2011, 111, 7774 CrossRef CAS PubMed; (b) M. Hatano and K. Ishihara, Synthesis, 2008, 1647 CAS; (c) L. F. Tietze, T. Kinzel and C. C. Brazel, Acc. Chem. Res., 2009, 42, 367 CrossRef CAS PubMed.
- (a) S. Casolari, D. D. Addario and E. Tagliavini, Org. Lett., 1999, 1, 1061 CrossRef CAS; (b) K. M. Waltz, J. Gavenonis and P. J. Walsh, Angew. Chem., Int. Ed., 2002, 41, 3697 CrossRef CAS; (c) H. Hanawa, S. Kii and K. Maruoka, Adv. Synth. Catal., 2001, 343, 57 CrossRef CAS; (d) A. Cunningham and S. Woodward, Synlett, 2002, 43 CrossRef CAS; (e) S. Kii and K. Maruoka, Chirality, 2003, 15, 68 CrossRef CAS PubMed; (f) J. G. Kim, K. M. Waltz, I. F. Garcia, D. Kwiatkowski and P. J. Walsh, J. Am. Chem. Soc., 2004, 126, 12580 CrossRef CAS PubMed; (g) A. Cunningham, V. Mokal-Parekh, C. Wilson and S. Woodward, Org. Biomol. Chem., 2004, 2, 741 RSC; (h) J. Lu, S.-J. Ji, Y.-C. Teo and T.-P. Loh, Tetrahedron Lett., 2005, 46, 7435 CrossRef CAS; (i) J. Lu, M.-L. Hong, S.-J. Ji, Y.-C. Teo and T.-P. Loh, Chem. Commun., 2005, 4217 RSC; (j) Y.-C. Teo, J.-D. Goh and T.-P. Loh, Org. Lett., 2005, 7, 2743 CrossRef CAS PubMed; (k) O. Prieto and S. Woodward, J. Organomet. Chem., 2006, 691, 1515 CrossRef CAS; (l) J. G. Kim, E. H. Camp and P. J. Walsh, Org. Lett., 2006, 8, 4413 CrossRef CAS PubMed; (m) A. J. Wooten, J. G. Kim and P. J. Walsh, Org. Lett., 2007, 9, 381 CrossRef CAS PubMed; (n) X. Zhang, D. Chen, X. Liu and X. Feng, J. Org. Chem., 2007, 72, 5227 CrossRef CAS PubMed; (o) J. Yadav, G. R. Stanton, X. Fan, J. R. Robinson, E. J. Schelter, P. J. Walsh and M. A. Pericas, Chem.–Eur. J., 2014, 20, 7122 CrossRef CAS PubMed; (p) J. Lu and S.-J. Ji, Chin. J. Chem., 2006, 24, 1439 CrossRef CAS.
- (a) S. Yamasaki, K. Fujii, R. Wada, M. Kanai and M. Shibasaki, J. Am. Chem. Soc., 2002, 124, 6536 CrossRef CAS PubMed; (b) M. Wadamoto and H. Yamamoto, J. Am. Chem. Soc., 2005, 127, 14556 CrossRef CAS PubMed; (c) N. V. Hanhan, Y. C. Tang, N. T. Tran and A. K. Franz, Org. Lett., 2012, 14, 2218 CrossRef CAS PubMed; (d) Z.-Y. Cao, J.-S. Jiang and J. Zhou, Org. Biomol. Chem., 2016, 14, 5500 RSC.
- (a) R. Wada, K. Oisaki, M. Kanai and M. Shibasaki, J. Am. Chem. Soc., 2004, 126, 8910 CrossRef CAS PubMed; (b) S. Lou, P. N. Moquist and S. E. Schaus, J. Am. Chem. Soc., 2006, 128, 12660 CrossRef CAS PubMed; (c) D. S. Barnett, P. N. Moquist and S. E. Schaus, Angew. Chem., Int. Ed., 2009, 48, 8679 CrossRef CAS PubMed; (d) S.-L. Shi, L.-W. Xu, K. Oisaki, M. Kanai and M. Shibasaki, J. Am. Chem. Soc., 2010, 132, 6638 CrossRef CAS PubMed; (e) Y. Zhang, N. Li, B. Qu, S. Ma, H. Lee, N. C. Gonnella, J. Gao, W. Li, Z. Tan, J. T. Reeves, J. Wang, J. C. Lorenz, G. Li, D. C. Reeves, A. Premasiri, N. Grinberg, N. Haddad, B. Z. Lu, J. J. Song and C. H. Senanayake, Org. Lett., 2013, 15, 1710 CrossRef CAS PubMed; (f) R. Alam, T. Vollgraff, L. Eriksson and K. J. Szabó, J. Am. Chem. Soc., 2015, 137, 11262 CrossRef CAS PubMed.
- X.-C. Qiao, S.-F. Zhu and Q.-L. Zhou, Tetrahedron: Asymmetry, 2009, 20, 1254 CrossRef CAS.
- (a) J. J. Miller and M. S. Sigman, J. Am. Chem. Soc., 2007, 129, 2752 CrossRef CAS PubMed; (b) J. J. Miller and M. S. Sigman, Angew. Chem., Int. Ed., 2008, 47, 771 CrossRef CAS PubMed; (c) X.-R. Huang, C. Chen, G.-H. Lee and S.-M. Peng, Adv. Synth. Catal., 2009, 351, 3089 CrossRef CAS; (d) R.-Y. Chen, A. P. Dhondge, G.-H. Lee and C. Chen, Adv. Synth. Catal., 2015, 357, 961 CrossRef CAS.
- For first indium-mediated allylation of carbonyl compounds, see: (a) S. Araki, H. Ito and Y. Butsugan, J. Org. Chem., 1988, 53, 1831 CrossRef CAS. Reviews for indium-mediated allylation of carbonyl compounds, see; (b) U. K. Roy and S. Roy, Chem. Rev., 2010, 110, 2472 CrossRef CAS PubMed; (c) Z. L. Shen, S. Y. Wang, Y. K. Chok, Y. H. Xu and T.-P. Loh, Chem. Rev., 2013, 113, 271 CrossRef CAS PubMed.
- (a) T.-P. Loh, J.-R. Zhou and X.-R. Li, Tetrahedron Lett., 1999, 40, 9333 CrossRef CAS; (b) V. Thornqvist, S. Manner and T. Frejd, Tetrahedron: Asymmetry, 2006, 17, 410 CrossRef CAS; (c) T. D. Haddad, L. C. Hirayama, P. Taynton and B. Singaram, Tetrahedron Lett., 2008, 49, 508 CrossRef CAS; (d) T. D. Haddad, L. C. Hirayama and B. Singaram, J. Org. Chem., 2010, 75, 642 CrossRef CAS PubMed. For enantioselective Barbier-type allylation of aldehydes using chiral catalysts in an organic solvent, see: (e) R. P. A. Melo, J. A. Vale, G. Zeni and P. H. Menezes, Tetrahedron Lett., 2006, 47, 1829 CrossRef CAS. For enantioselective Barbier-type allylation of aldehydes using stoichiometric amount of chiral additives, see: (f) T.-P. Loh, J.-R. Zhou and Z. Yin, Org. Lett., 1999, 1, 1855 CrossRef CAS; (g) T.-P. Loh and J. R. Zhou, Tetrahedron Lett., 1999, 40, 9115 CrossRef CAS; (h) L. C. Hirayama, S. Gamsey, D. Knueppel, D. Steiner, K. D. Torre and B. Singaram, Tetrahedron Lett., 2005, 46, 2315 CrossRef CAS.
- For Barbier-type allylation of ketones in water, see: (a) L. A. Paquette, G. D. Bennett, M. B. Isaac and A. Chhatriwalla, J. Org. Chem., 1998, 63, 1836 CrossRef CAS; (b) L. A. Paquette and P. C. Lobben, J. Org. Chem., 1998, 63, 5604 CrossRef CAS; (c) C. J. Li and T. H. Chan, Tetrahedron Lett., 1991, 32, 7017 CrossRef CAS; (d) T. H. Chan and Y. Yang, J. Am. Chem. Soc., 1999, 121, 3228 CrossRef CAS. For Barbier-type allylation of imines in water, see: (e) W. Lu and T. H. Chan, J. Org. Chem., 2000, 65, 8589 CrossRef CAS PubMed; (f) X.-W. Sun, M. Liu, M.-H. Xu and G.-Q. Li, Org. Lett., 2008, 10, 1259 CrossRef CAS PubMed. For catalytic enantioselective allylation of aldehyde in water, see: (g) M. Ueno, A. Tanoue and S. Kobayashi, Chem. Lett., 2014, 43, 1867 CrossRef. For catalytic enantioselective Barbier-type allylation of hydrazones, see: (h) G. R. Cook, R. Kargbo and B. Maity, Org. Lett., 2005, 7, 2767 CrossRef CAS PubMed; (i) R. Kargbo, Y. Takahashi, S. Bhor, G. R. Cook, G. C. Lloyd-Jones and I. R. Shepperson, J. Am. Chem. Soc., 2007, 129, 3846 CrossRef CAS PubMed; (j) K. L. Tan and E. N. Jacobsen, Angew. Chem., Int. Ed., 2007, 46, 1315 CrossRef CAS PubMed.
- (a) U. Schneider, M. Ueno and S. Kobayashi, J. Am. Chem. Soc., 2008, 130, 13824 CrossRef CAS PubMed. See also: (b) U. Schneider and S. Kobayashi, Angew. Chem., Int. Ed., 2007, 46, 5909 CrossRef CAS PubMed; (c) U. Schneider and S. Kobayashi, Acc. Chem. Res., 2012, 45, 1331 CrossRef CAS PubMed; (d) U. Schneider, I.-H. Chen and S. Kobayashi, Org. Lett., 2008, 10, 737 CrossRef CAS PubMed; (e) S. Kobayashi, H. Konishi and U. Schneider, Chem. Commun., 2008, 2313 RSC.
- M. Ohara, Y. Hara, T. Ohnuki and S. Nakamura, Chem.–Eur. J., 2014, 20, 8848 CAS.
- (a) S. Nakamura, K. Hyodo, M. Nakamura, D. Nakane and H. Masuda, Chem.–Eur. J., 2013, 19, 7304 CrossRef CAS PubMed. For related recent studies for imidazoline catalysts from our group, see: (b) S. Nakamura, K. Hyodo, Y. Nakamura, N. Shibata and T. Toru, Adv. Synth. Catal., 2008, 350, 1443 CrossRef CAS; (c) S. Nakamura, M. Ohara, Y. Nakamura, N. Shibata and T. Toru, Chem.–Eur. J., 2010, 16, 2360 CrossRef CAS PubMed; (d) M. Ohara, S. Nakamura and N. Shibata, Adv. Synth. Catal., 2011, 353, 3285 CrossRef CAS; (e) K. Hyodo, S. Nakamura, K. Tsuji, T. Ogawa, Y. Funahashi and N. Shibata, Adv. Synth. Catal., 2011, 353, 3385 CrossRef CAS; (f) K. Hyodo, S. Nakamura and N. Shibata, Angew. Chem., Int. Ed., 2012, 51, 10337 CrossRef CAS PubMed; (g) K. Hyodo, M. Kondo, Y. Funahashi and S. Nakamura, Chem.–Eur. J., 2013, 19, 4128 CrossRef CAS PubMed; (h) S. Nakamura, K. Hyodo, M. Nakamura, D. Nakane and H. Masuda, Chem.–Eur. J., 2013, 19, 7304 CrossRef CAS PubMed; (i) S. Nakamura, M. Ohara, M. Koyari, M. Hayashi, K. Hyodo, N. R. Nabisaheb and Y. Funahashi, Org. Lett., 2014, 16, 4452 CrossRef CAS PubMed; (j) M. Kondo, N. Kobayashi, T. Hatanaka, Y. Funahashi and S. Nakamura, Chem.–Eur. J., 2015, 21, 9066 CrossRef CAS PubMed; (k) M. Kondo, T. Nishi, T. Hatanaka, Y. Funahashi and S. Nakamura, Angew. Chem., Int. Ed., 2015, 54, 8198 CrossRef CAS PubMed; (l) S. Nakamura, J. Synth. Org. Chem., Jpn., 2015, 73, 1062 CrossRef CAS; (m) S. Nakamura, N. Matsuda and M. Ohara, Chem.–Eur. J., 2016, 22, 9478 CrossRef CAS PubMed.
- We also examined reactions with various additives, such as ammonium bromide, cetyltrimethylammonium bromide and 18-crown-6, however the yield and enantioselectivity of the product was low (see ESI†).
- T. H. Chan and Y. Yang, J. Am. Chem. Soc., 1999, 121, 3228 CrossRef CAS.
- We also examined the enantioselective allylation reaction using various ratios of allyl bromide and indium. The best result was obtained in the reaction using allyl bromide and indium in a 3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio (see ESI†). This result supports the formation of indium(III) sesquihalide species.
2 ratio (see ESI†). This result supports the formation of indium(III) sesquihalide species. - (a) J. H. Dam, P. Fristrup and R. Madsen, J. Org. Chem., 2008, 73, 3228 CrossRef CAS PubMed; (b) I. A. Olson, A. M. Sessler, J. L. Connell, E. Giordano, Y. Y. Baez Sosa, S. W. Zavaleta and W. J. Bowyer, J. Phys. Chem. A, 2009, 113, 2801 CrossRef CAS PubMed; (c) K. Koszinowski, J. Am. Chem. Soc., 2010, 132, 6032 CrossRef CAS PubMed . See also ref. 7b and c.
- (a) M. Yasuda, M. Haga and A. Baba, Organometallics, 2009, 28, 1998 CrossRef CAS; (b) M. Yasuda, K. Kiyokawa, K. Osaki and A. Baba, Organometallics, 2009, 28, 132 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra01038a |
| This journal is © The Royal Society of Chemistry 2017 |