Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Physicochemical properties of [Cnmim][TFA] (n = 2, 3, 4, 5, 6) ionic liquids†
Da-Wei Fang ,
Fang Zhang,
Rui Jia,
Wei-jun Shan,
Li-xin Xia* and
Jia-zhen Yang
,
Fang Zhang,
Rui Jia,
Wei-jun Shan,
Li-xin Xia* and
Jia-zhen Yang
Institute of Rare and Scattered Elements, College of Chemistry, Liaoning University, Shenyang, 110036, P. R. China
First published on 15th February 2017
Abstract
A series of ionic liquids based on trifluoroacetic acid, namely, [Cnmim][TFA] (n = 2, 3, 4, 5, 6) (1-alkyl-3-methylimidazolium trifluoroacetate), were designed and synthesized. The density, surface tension and refractive index were measured in the temperature range of 293.15 to 343.15 ± 0.05 K, and some physicochemical properties of the ILs were calculated. Using the concept of molar surface Gibbs free energy, the traditional Eötvös equation was improved into a modified Eötvös equation, in which the intercept and the slope represented the molar surface enthalpy and the molar surface entropy, respectively, for [Cnmim][TFA] (n = 2, 3, 4, 5, 6). The thermal expansion coefficient (α) of [Cnmim][TFA] was calculated according to the interstitial model, and the order of magnitude of the calculated values was in good agreement with the corresponding experimental values. A new hypothesis was proposed, stating that the interstitial molar surface Gibbs free energy (gs) is not determined by the type of IL. From the refractive index and the molar surface Gibbs free energy, an equation to predict the surface tension of ILs was derived and the predicted values were highly correlated with the corresponding experimental values. Finally, a new polarity scale for ILs was developed, and the polarity order of the [Cnmim][TFA] (n = 2, 3, 4, 5, 6) ILs was estimated.
1. Introduction
Ionic liquids (ILs) are room-temperature molten organic salts that have attracted considerable attention from the industrial and academic community because of their special physicochemical properties, such as low melting temperature, negligible vapor pressure, high electrical conductivity, large liquid range, selective solubility and electrochemical stability. As green environmentally friendly solvents, ILs are considered to be the best alternative to traditional solvents.1–5 The predicted results for the physicochemical properties of ILs were not fully consistent with the literature values, but the level of agreement is sufficient as reference data to select the ionic liquids for industry applications.6–9As a continuation of our previous study,10–13 and to expand our knowledge on IL chemistry, we synthesized a new series of ILs, namely, [Cnmim][TFA] (n = 2, 3, 4, 5, 6) (1-alkyl-3-methylimidazolium trifluoroacetate), and then measured their density, surface tension and refractive index in the temperature range of 293.15 to 343.15 K, at intervals of 5 K. Several important physical parameters, discussed in this article, were also estimated by semi-empirical methods.14–16
2. Experimental
2.1 Chemicals
Ethyl acetate, acetone and acetonitrile (all from Shanghai Reagent Co. Ltd.) were distilled and then stored over molecular sieves in tightly-sealed glass bottles. The precursor 1-methylimidazole, of AR grade reagent, was obtained from ACROS and vacuum distilled prior to use. Moreover, 1-bromoethane, 1-bromopropane, 1-bromobutane, 1-bromopentane, and 1-bromohexane (99.8%), all from Shanghai Reagent Co. Ltd., were refined by redistillation before use. Trifluoroacetate (99.5%) was from Alading Reagent Co. Ltd.2.2 Preparation of [Cnmim][TFA] (n = 2, 3, 4, 5, 6) ILs
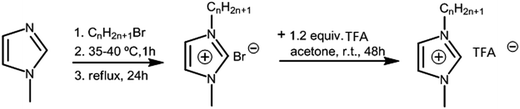
The intermediates 1-alkyl-3-methylimidazolium bromide ([Cnmim]Br, n = 2, 3, 4, 5, 6) were synthesized according to the procedure reported in ref. 17. The yields were approximately 80%.In our laboratories, a series of imidazolium trifluoroacetate, namely [Cnmim][TFA] (n = 2, 3, 4, 5, 6), have been synthesized (Fig. 1). A typical preparation of ILs is as follows: 1-alkyl-3-methylimidazolium bromide ([CnH2n+1mim]Br) and 1.2 equiv. of TFA are mixed in acetone under argon and stirred at room temperature for 48 h. The slurry is then filtrated with a Gooch funnel to remove the precipitates. Subsequently, acetone is removed, and the obtained target products are washed 3 times with n-hexane and dried under vacuum.
The obtained [Cnmim][TFA] (n = 2, 3, 4, 5, 6) ILs were characterized by 1H NMR and 9F NMR spectroscopy. The water content was determined with a Karl Fischer moisture titrator (ZSD-2 type), and all ILs showed a water content of less than 500 ppm. The Br− content was determined by dripping a silver nitrate solution, i.e., dissolving 0.5 mL of the product in water and then dripping aqueous silver nitrate; no yellow precipitates were observed. The structure of the synthesized ILs was confirmed by NMR, and this data can be found in the ESI.†
2.3 Density, surface tension and refractive index measurements
The density of degassed water was measured with a Westphal balance at 293.15 ± 0.05 K and was in good agreement with the value reported in ref. 18, within an experimental error of ±0.0003 g cm−3. Then, the densities of the samples were measured in the temperature range from 293.15 to 343.15 K. A sample was placed in a jacketed cell and then thermostated at each temperature with an accuracy of ±0.05 K.The surface tension of degassed water was measured in the temperature range from 293.15 to 343.15 K by the forced bubble method with a tensiometer (DPAW type produced by Sang Li Electronic Co.), and the results were in good agreement with the value reported in ref. 18, within an experimental error of ±0.1 mJ m−2. Then, the surface tension of the samples was measured by the same method in the same temperature range from 293.15 to 343.15 K.
The refractive index of the ILs was measured with an Abbe refractometer. The refractive index of degassed water, as measured by the Abbe refractometer, is 1.3329 ± 0.0001, which is consistent with the value of 1.33299 reported in ref. 18. The refractive indices of a series of samples were measured in the temperature range from 293.15 to 343.15 K at intervals of 5 K.
3. Results and discussion
The density, surface tension and refractive index values for the [Cnmim][TFA] (n = 2, 3, 4, 5, 6) samples are listed in Table 1, and each value is the average of triplicate measurements.| T/K | [C2mim][TFA] | [C3mim][TFA] | [C4mim][TFA] | [C5mim][TFA] | [C6mim][TFA] |
|---|---|---|---|---|---|
| Density ρ (g cm−3) of the [Cnmim][TFA] (n = 2, 3, 4, 5, 6) ILs | |||||
| 293.15 | 1.2772 | 1.2503 | 1.2242 | 1.1980 | 1.1705 |
| 298.15 | 1.2733 | 1.2462 | 1.2201 | 1.1939 | 1.1661 |
| 303.15 | 1.2705 | 1.2435 | 1.2159 | 1.1883 | 1.1622 |
| 308.15 | 1.2672 | 1.2405 | 1.2119 | 1.1831 | 1.1583 |
| 313.15 | 1.2632 | 1.2364 | 1.2078 | 1.1792 | 1.1542 |
| 318.15 | 1.2601 | 1.2328 | 1.2042 | 1.1758 | 1.1502 |
| 323.15 | 1.2562 | 1.2293 | 1.2003 | 1.1711 | 1.1467 |
| 328.15 | 1.2524 | 1.2259 | 1.1960 | 1.1661 | 1.1431 |
| 333.15 | 1.2489 | 1.2225 | 1.1921 | 1.1617 | 1.1394 |
| 338.15 | 1.2453 | 1.2190 | 1.1880 | 1.1572 | 1.1354 |
| 343.15 | 1.2420 | 1.2159 | 1.1841 | 1.1522 | 1.1320 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Surface tension γ (mJ m−2) of the [Cnmim][TFA] (n = 2, 3, 4, 5, 6) ILs | |||||
| 293.15 | 49.3 | 46.4 | 44.1 | 41.3 | 40.0 |
| 298.15 | 49.0 | 46.1 | 43.8 | 41.0 | 39.7 |
| 303.15 | 48.6 | 45.8 | 43.5 | 40.8 | 39.5 |
| 308.15 | 48.3 | 45.4 | 43.2 | 40.6 | 39.2 |
| 313.15 | 48.0 | 45.1 | 42.9 | 40.3 | 39.0 |
| 318.15 | 47.8 | 44.8 | 42.6 | 40.1 | 38.8 |
| 323.15 | 47.5 | 44.6 | 42.2 | 39.9 | 38.6 |
| 328.15 | 47.2 | 44.2 | 41.9 | 39.6 | 38.3 |
| 333.15 | 46.9 | 43.9 | 41.6 | 39.4 | 38.1 |
| 338.15 | 46.5 | 43.7 | 41.2 | 39.2 | 37.9 |
| 343.15 | 46.2 | 43.5 | 41.0 | 39.1 | 37.8 |
3.1 Estimation of the volumetric properties of the [Cnmim][TFA] (n = 2, 3, 4, 5, 6) ILs
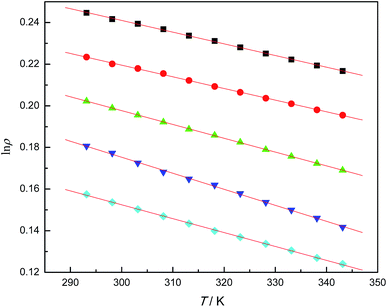
When plotting the ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ρ values against T, a straight line was obtained (see Fig. 2) for each IL, and the empirical linear equation is as follows:
ρ values against T, a straight line was obtained (see Fig. 2) for each IL, and the empirical linear equation is as follows:
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ρ = b − αT ρ = b − αT
| (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ρ/∂T)p, is the thermal expansion coefficient of the ILs. The αexp values are listed in Table 7. The correlation coefficient of all ln
ρ/∂T)p, is the thermal expansion coefficient of the ILs. The αexp values are listed in Table 7. The correlation coefficient of all ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ρ vs. T linear fittings was larger than 0.99 and standard deviations were within the experimental error.
ρ vs. T linear fittings was larger than 0.99 and standard deviations were within the experimental error.
The molecular volume (Vm) of ILs is the sum of the cation and anion volumes. The Vm value for the [Cnmim][TFA] ILs was calculated using the following equation:
| Vm = M/(Nρ) | (2) |
| IL | Vm (nm3) | S0 (J K−1 mol−1) | 103Sa (mJ K−1 m−2) | Ea (mJ m−2) | UPOT (kJ mol−1) |
|---|---|---|---|---|---|
| [C2mim][TFA] | 0.2923 | 393.9 | 60.5 | 67.1 | 457 |
| [C3mim][TFA] | 0.3174 | 425.1 | 59.5 | 63.8 | 448 |
| [C4mim][TFA] | 0.3432 | 457.3 | 63.5 | 62.7 | 439 |
| [C5mim][TFA] | 0.3703 | 491.0 | 45.1 | 54.4 | 431 |
| [C6mim][TFA] | 0.3991 | 526.9 | 44.7 | 53.0 | 422 |
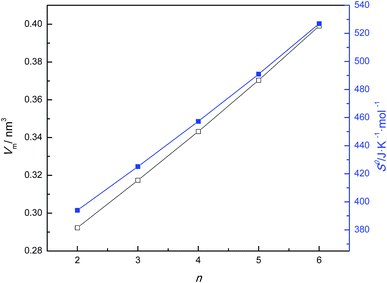
According to the Glasser's theory,14 the standard entropy values, S0 (298), expressed in J K−1 mol−1, for the [Cnmim][TFA] ILs, can be estimated using eqn (3), and the results are listed in Table 2. As calculated by the least-squares method, the linear regression slope of S0 (298) vs. the number of carbons (n) is 33.2 J K−1 mol−1 (see Fig. 3), which represents the contribution of each methylene group to the standard entropy of the ILs. This value is in agreement with the value of 33.9 J K−1 mol−1 for [Cnmim][BF4].14
| S0 (298) (J K−1 mol−1) = 1246.5 Vm (nm3) + 29.5 | (3) |
The crystal energy (UPOT) of the ILs can be estimated by the Glasser's empirical equation:14
| UPOT (kJ mol−1) = 1981.2(ρ/M)1/3 + 103.8 | (4) |
The UPOT values were calculated and listed in Table 2. From Table 2, it can be seen that the crystal energies of [Cnmim][TFA] are much lower than those of inorganic fused salts, for example, the UPOT of fused CsI18 is 613 kJ mol−1, which is the lowest crystal energy among alkali halides. Their low crystal energy is the underlying reason why ILs can be formed at room temperature.
3.2 Estimation of surface properties for the [Cnmim][TFA] (n = 2, 3, 4, 5, 6) ILs
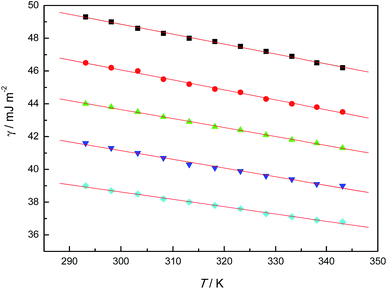
The γ experimental values for each [Cnmim][TFA] were fitted against T by the least-squares method to a linear equation, and several good straight lines were obtained. All correlation coefficients of the fittings were larger than 0.99, and the standard deviations were within the experimental error (see Fig. 4).From the fitting line slopes, the surface entropy (Sa) values were obtained and are listed in Table 2. In addition, the surface energy (Ea) values at 298.15 K may be obtained from the surface tension using the following equation: Ea = γ − T(∂γ/∂T)p and are also listed in Table 2. In comparison with fused salts (for example, Ea is 146 mJ m−2 for fused NaNO3), Ea values for [Cnmim][TFA] ILs are much lower and are closer to those of organic liquids (for example, Ea for benzene is 67 mJ m−2, and for n-octane is 51.1 mJ m−2).18 The surface excess energy depends on the interaction energy between ions, and hence these results show that the interaction energy between ions in the [Cnmim][TFA] ILs is much lower than in inorganic fused salts, which in turn suggests that the crystal energy of the [Cnmim][TFA] ILs is much lower than that of inorganic fused salts.
3.3 Eötvös equation and molar surface Gibbs free energy of the [Cnmim][TFA] (n = 2, 3, 4, 5, 6) ILs
In general, the surface tension (γ) of several liquids decreases almost linearly as the temperature increases and the relationship is expressed by the Eötvös equation:19| γV2/3 = k(Tc − T) | (5) |
The molar enthalpy of vaporization, ΔglH0m (298 K), of ILs can be estimated according to the Kabo's empirical equation:15
| ΔglH0m (298 K) = 0.01121(γV2/3N1/3) + 2.4 kJ mol−1 | (6) |
Rebelo23 proposed another equation to estimate the molar enthalpy of vaporization of ILs from the product of the hypothetical normal boiling point (Tb) and the Trouton constant (≈90 J mol−1 K−1):
| ΔglH0m (Tb) = 90Tb | (7) |
The empirical relationship between the hypothetical boiling point (Tb) and the critical temperature (Tc) is as follows:
| Tb = 0.6Tc | (8) |
The vaporization enthalpies of the ILs were calculated by the above two methods, and the results are listed in Table 3. The difference between the ΔglH0m (Tb) as estimated by the Rebelo's equation and the ΔglH0m (298 K) as estimated by the Kabo's equation is due to the heat capacity difference between the liquid and gas phases at different temperatures.
| IL | Tc (K) | Tb (K) | ΔglH0m (Tb) (kJ mol−1) | ΔglH0m (298 K) (kJ mol−1) |
|---|---|---|---|---|
| [C2mim][TFA] | 1437 | 862 | 77.6 | 148 |
| [C3mim][TFA] | 1369 | 821 | 73.9 | 147 |
| [C4mim][TFA] | 1271 | 763 | 68.6 | 147 |
| [C5mim][TFA] | 1956 | 1173 | 105.6 | 145 |
| [C6mim][TFA] | 1731 | 1039 | 93.5 | 148 |
As regards the classical Eötvös equation, the relationship between surface tension and temperature is well reflected and the critical temperature can be calculated from it; however a drawback is that the units of J mol−2/3 for γV2/3 make it complex and the significance of the slope is not clear. After adjustments, a modified Eötvös equation16 was derived and the concept of molar surface Gibbs free energy was proposed as follows:
| g = γV2/3N1/3 = a0 − a1T | (9) |
| T/K | [C2mim][TFA] | [C3mim][TFA] | [C4mim][TFA] | [C5mim][TFA] | [C6mim][TFA] | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 105γV2/3 (J mol−2/3) | g (kJ mol−1) | 105γV2/3 (J mol−2/3) | g (kJ mol−1) | 105γV2/3 (J mol−2/3) | g (kJ mol−1) | 105γV2/3 (J mol−2/3) | g (kJ mol−1) | 105γV2/3 (J mol−2/3) | g (kJ mol−1) | |
| 293.15 | 15.46 | 13.05 | 15.36 | 12.97 | 15.38 | 12.99 | 15.15 | 12.80 | 15.42 | 13.03 |
| 298.15 | 15.39 | 13.00 | 15.30 | 12.92 | 15.31 | 12.93 | 15.08 | 12.73 | 15.35 | 12.96 |
| 303.15 | 15.29 | 12.91 | 15.22 | 12.85 | 15.24 | 12.87 | 15.05 | 12.71 | 15.30 | 12.92 |
| 308.15 | 15.22 | 12.85 | 15.11 | 12.76 | 15.17 | 12.81 | 15.02 | 12.69 | 15.22 | 12.86 |
| 313.15 | 15.16 | 12.80 | 15.04 | 12.71 | 15.10 | 12.75 | 14.94 | 12.62 | 15.18 | 12.82 |
| 318.15 | 15.12 | 12.77 | 14.97 | 12.65 | 15.02 | 12.69 | 14.90 | 12.58 | 15.14 | 12.78 |
| 323.15 | 15.06 | 12.72 | 14.93 | 12.61 | 14.92 | 12.60 | 14.86 | 12.55 | 15.09 | 12.74 |
| 328.15 | 14.99 | 12.66 | 14.83 | 12.52 | 14.85 | 12.54 | 14.79 | 12.49 | 15.00 | 12.67 |
| 333.15 | 14.92 | 12.60 | 14.75 | 12.46 | 14.77 | 12.47 | 14.76 | 12.46 | 14.96 | 12.63 |
| 338.15 | 14.83 | 12.52 | 14.72 | 12.43 | 14.66 | 12.38 | 14.72 | 12.43 | 14.91 | 12.60 |
| 343.15 | 14.76 | 12.46 | 14.67 | 12.39 | 14.62 | 12.35 | 14.72 | 12.43 | 14.90 | 12.59 |
| 107k/J mol−2/3 K−1 | a0/kJ mol−1 | 107k/J mol−2/3 K−1 | a0/kJ mol−1 | 107k/J mol−2/3 K−1 | a0/kJ mol−1 | 107k/J mol−2/3 K−1 | a0/kJ mol−1 | 107k/J mol−2/3 K−1 | a0/kJ mol−1 | |
| 1.347 | 16.38 | 1.427 | 16.48 | 1.569 | 16.89 | 0.909 | 15.06 | 1.075 | 15.66 | |
| Tc/K | a1/kJ mol−1 K−1 | Tc/K | a1/kJ mol−1 K−1 | Tc/K | a1/kJ mol−1 K−1 | Tc/K | a1/kJ mol−1 K−1 | Tc/K | a1/kJ mol−1 K−1 | |
| 1437 | 0.0114 | 1369 | 0.0120 | 1271 | 0.0133 | 1956 | 0.0078 | 1731 | 0.0090 | |
| r | 0.994 | 0.992 | 0.990 | 0.991 | 0.997 | 0.996 | 0.983 | 0.980 | 0.989 | 0.987 |
| 105s/J mol−2/3 | s/kJ mol−1 | 105s/J mol−2/3 | s/kJ mol−1 | 105s/J mol−2/3 | s/kJ mol−1 | 105s/J mol−2/3 | s/kJ mol−1 | 105s/J mol−2/3 | s/kJ mol−1 | |
| 0.0174 | 0.0167 | 0.0238 | 0.0183 | 0.0149 | 0.0132 | 0.0197 | 0.0184 | 0.0186 | 0.0170 | |
In order to further confirm the reliability of the modified Eötvös equation, we conducted an extensive literature review,20,24–28 and the molar surface Gibbs free energy (g/kJ mol−1) values of other ILs are shown in Table 5. The k and Tc values were calculated according to the Eötvös equation and the parameters a0 and a1 were determined according to the modified Eötvös equation, and results are included in Table 6. The empirical parameters a0 and a1 of each IL can be easily compared in Table 6.
| IL | T (K) | ||||
|---|---|---|---|---|---|
| 298.15 | 308.15 | 318.15 | 328.15 | 338.15 | |
| [C2mim][OAc] | 9.04 | 8.92 | 8.74 | 8.60 | 8.46 |
| [C3mim][OAc] | 9.34 | 9.11 | 8.97 | 8.82 | 8.67 |
| [C4mim][OAc] | 9.50 | 9.37 | 9.21 | 8.94 | 8.81 |
| [C5mim][OAc] | 9.75 | 9.58 | 9.38 | 9.15 | 8.98 |
| [C6mim][OAc] | 9.95 | 9.77 | 9.53 | 9.32 | 9.10 |
| [C2mim][Ala] | 11.96 | 11.84 | 11.72 | 11.55 | 11.45 |
| [C3mim][Ala] | 12.07 | 11.94 | 11.81 | 11.63 | 11.52 |
| [C4mim][Ala] | 12.14 | 11.99 | 11.86 | 11.67 | 11.55 |
| [C5mim][Ala] | 12.30 | 12.16 | 12.01 | 11.81 | 11.69 |
| [C3mim][Gly] | 12.12 | 12.01 | 11.85 | 11.73 | |
| [C4mim][Gly] | 12.23 | 12.10 | 11.98 | 11.85 | |
| [C5mim][Gly] | 12.29 | 12.15 | 12.02 | 11.82 | 11.71 |
| [C6mim][Gly] | 12.75 | 12.58 | 12.43 | 12.24 | 12.11 |
| [C2mim][Lact] | 11.35 | 11.23 | 11.07 | 10.95 | 10.81 |
| [C4mim][Lact] | 12.81 | 12.67 | 12.48 | 12.35 | 12.18 |
| [C5mim][Lact] | 13.01 | 12.79 | 12.62 | 12.49 | 12.29 |
| [C2mim][Thr] | 15.59 | 15.48 | 15.31 | 15.17 | |
| [C4mim][Thr] | 16.18 | 16.09 | 15.89 | 15.73 | |
| [C2mim][Pro] | 9.62 | 9.50 | 9.38 | 9.21 | 9.09 |
| [C3mim][Pro] | 10.09 | 9.99 | 9.87 | 9.74 | 9.62 |
| [C4mim][Pro] | 10.29 | 10.16 | 10.02 | 9.86 | 9.72 |
| [C5mim][Pro] | 10.48 | 10.37 | 10.26 | 10.08 | 9.91 |
| [C6mim][Pro] | 10.77 | 10.61 | 10.49 | 10.31 | 10.16 |
| IL | a0 (kJ mol−1) | a1 (kJ mol−1 K−1) | R | s (kJ mol−1) | 107k (J mol−2/3 K−1) | Tc (K) |
|---|---|---|---|---|---|---|
| [C2mim][OAc] | 13.50 | 0.0149 | 0.998 | 0.0129 | 1.7660 | 905 |
| [C3mim][OAc] | 14.19 | 0.0164 | 0.996 | 0.0217 | 1.9410 | 865 |
| [C4mim][OAc] | 14.95 | 0.0182 | 0.994 | 0.0288 | 2.1522 | 823 |
| [C5mim][OAc] | 15.79 | 0.0202 | 0.998 | 0.0208 | 2.3892 | 783 |
| [C6mim][OAc] | 16.49 | 0.0218 | 0.999 | 0.0139 | 2.5857 | 755 |
| [C2mim][Ala] | 15.74 | 0.0127 | 0.997 | 0.0179 | 1.5019 | 1241 |
| [C3mim][Ala] | 16.12 | 0.0136 | 0.997 | 0.0194 | 1.6099 | 1186 |
| [C4mim][Ala] | 16.47 | 0.0145 | 0.997 | 0.0202 | 1.7208 | 1133 |
| [C5mim][Ala] | 16.87 | 0.0154 | 0.997 | 0.0213 | 1.8204 | 1098 |
| [C3mim][Gly] | 15.98 | 0.0129 | 0.998 | 0.0098 | 1.5331 | 1234 |
| [C4mim][Gly] | 15.95 | 0.0125 | 0.999 | 0.0124 | 1.4793 | 1277 |
| [C5mim][Gly] | 16.60 | 0.0145 | 0.997 | 0.0211 | 1.7131 | 1148 |
| [C6mim][Gly] | 17.63 | 0.0164 | 0.999 | 0.0145 | 1.9390 | 1077 |
| [C2mim][Lact] | 16.75 | 0.0140 | 0.999 | 0.0112 | 1.6541 | 1199 |
| [C4mim][Lact] | 17.61 | 0.0161 | 0.999 | 0.0133 | 1.9033 | 1095 |
| [C5mim][Lact] | 18.91 | 0.0174 | 0.998 | 0.0188 | 2.0662 | 1042 |
| [C2mim][Thr] | 19.80 | 0.0141 | 0.996 | 0.0130 | 1.6750 | 1401 |
| [C4mim][Thr] | 20.69 | 0.0151 | 0.990 | 0.0230 | 1.7890 | 1371 |
| [C2mim][Pro] | 13.57 | 0.0132 | 0.999 | 0.0119 | 1.5658 | 1026 |
| [C3mim][Pro] | 13.72 | 0.0122 | 0.999 | 0.0082 | 1.4374 | 1131 |
| [C4mim][Pro] | 14.47 | 0.0140 | 0.999 | 0.0132 | 1.6612 | 1031 |
| [C5mim][Pro] | 14.76 | 0.0143 | 0.995 | 0.0262 | 1.6875 | 1035 |
| [C6mim][Pro] | 15.24 | 0.0150 | 0.999 | 0.0140 | 1.7776 | 1015 |
3.4 The interstitial model for ILs
For pure ILs, a new theoretical model,29 obtained by classical statistical mechanics, provides an expression for the calculation of the interstitial volume (ν):| ν = 0.6791(kbT/γ)3/2 | (10) |
There are some papers about studies on voids in ILs,30,31 in which the voids are defined by the volume occupied by an ion pair, i.e., the sum V+ + V− of the cationic and anionic volumes (known from the crystal structure). The void can also be defined by the mean hole volume and the hole free volume. However, these are different from our interstitial model. For pure ionic liquids, our model is proposed on the basis of the following assumptions: (1) due to their large size and asymmetric shape, the ions may not be closely packed, generating lots of interstices between ions; (2) in order to calculate the volume easily, the interstice is regarded as a bubble; (3) there are 2N interstices for 1 mol of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ionic liquid, where N is the Avogadro's constant; (4) the interstice in ILs can move around in the same way as an ion or another particle, and in the movement the interstice does not vanish and instead it can be compressed and expanded, which is an additional feature of interstitial motion called the breathing motion. According to the hole model of molten salts, the interstitial volume (ν) can be calculated using eqn (10).
1 ionic liquid, where N is the Avogadro's constant; (4) the interstice in ILs can move around in the same way as an ion or another particle, and in the movement the interstice does not vanish and instead it can be compressed and expanded, which is an additional feature of interstitial motion called the breathing motion. According to the hole model of molten salts, the interstitial volume (ν) can be calculated using eqn (10).
The average interstitial volume values for the [Cnmim][TFA] ILs at different temperatures were determined using eqn (10) and are listed in Table 7.
| Ionic liquid | 10−24ν (cm3) | ∑ν (cm3) | V (cm3 mol−1) | 102∑ν/V | 104αest (K−1) | 104αexp (K−1) |
|---|---|---|---|---|---|---|
| [C2mim][TFA] | 16.53 | 19.91 | 176.1 | 11.3 | 5.69 | 5.62 |
| [C3mim][TFA] | 18.12 | 21.82 | 191.1 | 11.4 | 5.74 | 5.60 |
| [C4mim][TFA] | 19.56 | 23.56 | 206.7 | 11.4 | 5.74 | 6.63 |
| [C5mim][TFA] | 21.60 | 26.01 | 223.0 | 11.7 | 5.87 | 7.70 |
| [C6mim][TFA] | 22.67 | 27.30 | 240.4 | 11.4 | 5.72 | 6.66 |
The molar volume of the interstice (∑ν = 2Nν) and the volume fraction of the interstice (∑ν/V) range from 11.3% to 11.7%. These results are in good agreement with the values for the majority of materials that exhibit a 10–15% volume expansion during the solid to liquid state transition32 and suggest that the interstitial model is reasonable.
The IL volume (V) consists of the inherent volume (Vi) and the total volume of all interstices:
| V = Vi + 2Nν | (11) |
If the increase of the IL volume only results from the expansion of the interstices when temperature increases, then the expression for the calculation of the thermal expansion coefficient (α) can be derived from the interstitial model:
| α = (1/V)(∂V/∂T)p = 3Nν/VT | (12) |
The αest values, calculated using eqn (12), and the corresponding experimental values (αexp) for the [Cnmim][TFA] ILs at 298.15 K are listed in Table 7. From Table 7, it can be observed that the order of magnitude of the αest values is in good agreement with the αexp values, indicating that the interstitial model is reasonable.
We assumed that the interstices in the ILs are particles with zero static mass, and therefore the molar interstitial volume can be expressed as the product of the interstitial volume (ν) and the Avogadro's constant (N). The definition of the interstitial molar surface Gibbs free energy of the ILs can be expressed as follows:
| gs = N1/3γ(Nν)2/3 | (13) |
According to the interstitial model of ILs, the following formula can be introduced:
| γ(Nν)2/3 = (0.6791N)2/3kbT | (14) |
Combining eqn (13) and (14), the interstitial molar surface Gibbs free energy of the [Cnmim][TFA] ILs is ultimately expressed as follows:
| gs = 0.67912/3RT | (15) |
The interstitial molar surface entropy of the ILs is calculated as ss = −0.67912/3R = −6.42 J K−1 mol−1, which is a constant. Thus, the interstitial molar surface enthalpy can be obtained according to the following equation:
| hs = gs + Tss | (16) |
It is clear that the interstitial molar surface enthalpy values of the ILs are approximately equal to zero. Thus, it can be concluded that the interstitial molar surface Gibbs free energy (gs), the interstitial molar surface entropy (ss) and the interstitial molar surface enthalpy (hs) are independent of the type of the ILs formed in the interstice.
From the IL interstitial model, we can conclude that the nature of the interstice is only related to the volume and the shape of the interstice, but not to the type of IL. Thus, we hypothesized that the Schottky defects in crystals, holes in molten salts and interstices in thin films have similar properties.32
3.5 Molar refraction and improved Lorentz–Lorenz equation
The refractive index values for the [Cnmim][TFA] (n = 2, 3, 4, 5, 6) ILs are listed in Table 8. Each value in the table is the average of triplicate measurements.| T/K | [C2mim][TFA] | [C3mim][TFA] | [C4mim][TFA] | [C5mim][TFA] | [C6mim][TFA] |
|---|---|---|---|---|---|
| 293.15 | 1.5669 | 1.5309 | 1.4978 | 1.4677 | 1.4428 |
| 298.15 | 1.5651 | 1.5298 | 1.4962 | 1.4656 | 1.4409 |
| 303.15 | 1.5632 | 1.5282 | 1.4943 | 1.4640 | 1.4398 |
| 308.15 | 1.5611 | 1.5262 | 1.4915 | 1.4614 | 1.4388 |
| 313.15 | 1.5588 | 1.5244 | 1.4896 | 1.4595 | 1.4363 |
| 318.15 | 1.5566 | 1.5222 | 1.4879 | 1.4574 | 1.4352 |
| 323.15 | 1.5554 | 1.5203 | 1.4858 | 1.4555 | 1.4341 |
| 328.15 | 1.5530 | 1.5183 | 1.4836 | 1.4539 | 1.4328 |
| 333.15 | 1.5517 | 1.5163 | 1.4815 | 1.4514 | 1.4308 |
| 338.15 | 1.5501 | 1.5144 | 1.4792 | 1.4498 | 1.4295 |
| 343.15 | 1.5484 | 1.5126 | 1.4779 | 1.4493 | 1.4281 |
The Lorentz–Lorenz relationship28 between the refractive index and the mean molecular polarisability (αp) leads to the definition of the molar refraction (Rm):
| Rm = [(nD2 − 1)/(nD2 + 2)](M/ρ) = (4πN/3)αp | (17) |
The Rm and αp values were calculated according to eqn (17), from the nD values of [Cnmim][TFA] (see Table 9).
| IL | T (K) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 293.15 | 298.15 | 303.15 | 308.15 | 313.15 | 318.15 | 323.15 | 328.15 | 333.15 | 338.15 | 343.15 | ||
| [C2mim][TFA] | Rm | 57.33 | 57.36 | 57.32 | 57.30 | 57.28 | 57.24 | 57.31 | 57.28 | 57.33 | 57.36 | 57.36 |
| 1024αp | 22.72 | 22.73 | 22.72 | 22.71 | 22.70 | 22.69 | 22.72 | 22.70 | 22.72 | 22.73 | 22.74 | |
| γest | 49.2 | 49.0 | 48.7 | 48.3 | 48.0 | 47.7 | 47.5 | 47.1 | 46.9 | 46.6 | 46.4 | |
| [C3mim][TFA] | Rm | 58.94 | 59.03 | 59.00 | 58.96 | 58.99 | 58.95 | 58.94 | 58.91 | 58.88 | 58.87 | 58.84 |
| 1024αp | 23.36 | 23.40 | 23.39 | 23.37 | 23.38 | 23.37 | 23.36 | 23.35 | 23.34 | 23.33 | 23.32 | |
| γest | 46.4 | 46.2 | 45.9 | 45.6 | 45.4 | 45.0 | 44.7 | 44.4 | 44.1 | 43.8 | 43.5 | |
| [C4mim][TFA] | Rm | 60.37 | 60.41 | 60.42 | 60.34 | 60.33 | 60.34 | 60.32 | 60.29 | 60.27 | 60.22 | 60.29 |
| 1024αp | 23.93 | 23.95 | 23.95 | 23.92 | 23.91 | 23.92 | 23.91 | 23.90 | 23.89 | 23.87 | 23.90 | |
| γest | 44.1 | 43.8 | 43.5 | 43.1 | 42.8 | 42.5 | 42.2 | 41.8 | 41.5 | 41.2 | 40.9 | |
| [C5mim][TFA] | Rm | 61.75 | 61.73 | 61.83 | 61.80 | 61.78 | 61.72 | 61.75 | 61.82 | 61.76 | 61.81 | 62.01 |
| 1024αp | 24.48 | 24.47 | 24.51 | 24.50 | 24.49 | 24.46 | 24.48 | 24.50 | 24.48 | 24.50 | 24.58 | |
| γest | 41.3 | 41.0 | 40.8 | 40.5 | 40.2 | 40.0 | 39.7 | 39.5 | 39.2 | 39.0 | 38.9 | |
| [C6mim][TFA] | Rm | 63.46 | 63.46 | 63.54 | 63.62 | 63.53 | 63.61 | 63.67 | 63.70 | 63.65 | 63.71 | 63.71 |
| 1024αp | 25.15 | 25.15 | 25.18 | 25.22 | 25.18 | 25.21 | 25.24 | 25.25 | 25.23 | 25.25 | 25.25 | |
| γest | 39.8 | 39.6 | 39.4 | 39.2 | 38.9 | 38.8 | 38.6 | 38.4 | 38.1 | 37.9 | 37.7 | |
A short calculation revealed that the contribution value of each methylene (–CH2–) group to the molar refraction is almost equal to that in the [Cnmim][TFA] (n = 2, 3, 4, 5, 6) homologue, which also suggests that all methylene (–CH2–) groups in the alkyl chains of the imidazolium-based ILs have very similar chemical environments.
The new concept of molar surface Gibbs free energy (g/kJ mol−1) was applied to improve the Lorentz–Lorenz equation:33
| γ3/2 = [g3/2/(N1/3Rm)][(nD2 − 1)/(nD2 + 2)] | (18) |
The surface tension (γest) of the [Cnmim][TFA] ILs was predicted based on eqn (18), and results are listed in Table 9.
As can be seen in Table 9, Rm and αP are temperature-independent physical properties. When plotting the estimated γest values of the [Cnmim][TFA] (n = 2, 3, 4, 5, 6) ILs against the corresponding experimental γexp values, a good straight line was obtained (see Fig. 5). The γest and γexp values are correlated, with a correlation coefficient of 0.999, and the standard deviation is within the experimental range (Fig. 6).
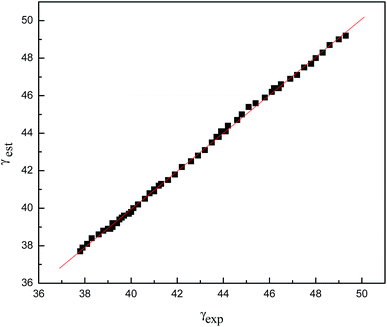 | ||
| Fig. 5 Plot of γest vs. γexp for the [Cnmim][TFA] ILs. γest = 1.01439γexp − 0.6287; r = 0.999, s = 0.1059. | ||
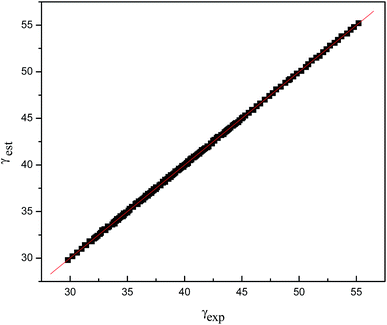 | ||
| Fig. 6 Plot of γest vs. γexp for other ILs. γest = 0.99957γexp − 2.02 × 10−2; r = 0.99, s = 5.82 × 10−2. | ||
In order to further explore the applicability of eqn (18), we estimated the surface tension of other ILs20,24–28 using the improved Lorentz–Lorenz equation, fitting γest and the corresponding experimental results γexp as obtained from the literature, which shows that the values are in good agreement, with a correlation coefficient of up to 0.99 (Table 10).
| IL | T/K | |||||||
|---|---|---|---|---|---|---|---|---|
| 298.15 | 303.15 | 308.15 | 313.15 | 318.15 | 323.15 | 328.15 | 333.15 | |
| [C2mim][OAc] | 38.2 | 37.8 | 37.4 | 37.0 | 36.7 | 36.3 | 35.9 | 35.5 |
| [C3mim][OAc] | 36.7 | 36.3 | 35.9 | 35.5 | 35.1 | 34.8 | 34.4 | 34.0 |
| [C4mim][OAc] | 35.3 | 34.9 | 34.5 | 34.1 | 33.7 | 33.4 | 33.0 | 32.6 |
| [C5mim][OAc] | 34.2 | 33.8 | 33.4 | 33.0 | 32.6 | 32.2 | 31.8 | 31.4 |
| [C6mim][OAc] | 33.1 | 32.7 | 32.3 | 31.8 | 31.4 | 31.0 | 30.6 | 30.2 |
| [C2mim][Ala] | 44.8 | 44.5 | 44.2 | 43.9 | 43.6 | 43.2 | 42.9 | 42.6 |
| [C3mim][Ala] | 42.6 | 42.3 | 42.0 | 41.7 | 41.4 | 41.0 | 40.7 | 40.4 |
| [C4mim][Ala] | 40.6 | 40.3 | 40.0 | 39.7 | 39.4 | 39.0 | 38.7 | 38.4 |
| [C5mim][Ala] | 39.1 | 38.8 | 38.5 | 38.2 | 37.9 | 37.5 | 37.2 | 36.9 |
| [C3mim][Gly] | 45.6 | 45.3 | 45.0 | 44.6 | 44.3 | 44.0 | 43.7 | 43.4 |
| [C4mim][Gly] | 43.5 | 43.2 | 42.9 | 42.6 | 42.3 | 42.0 | 41.7 | 41.4 |
| [C5mim][Gly] | 41.5 | 41.2 | 40.9 | 40.6 | 40.2 | 39.9 | 39.6 | 39.3 |
| [C6mim][Gly] | 40.9 | 40.6 | 40.2 | 39.9 | 39.6 | 39.2 | 38.9 | 38.6 |
| [C2mim][Lact] | 48.8 | 48.4 | 48.1 | 47.7 | 47.4 | 47.0 | 46.6 | 46.3 |
| [C4mim][Lact] | 44.0 | 43.7 | 43.3 | 43.0 | 42.6 | 42.3 | 41.9 | 41.6 |
| [C5mim][Lact] | 42.3 | 41.9 | 41.6 | 41.2 | 40.9 | 40.5 | 40.1 | 39.8 |
| [C2mim][Thr] | 54.8 | 54.5 | 54.1 | 53.8 | 53.4 | 53.1 | 52.8 | 52.4 |
| [C4mim][Thr] | 51.2 | 50.8 | 50.5 | 50.1 | 49.8 | 49.5 | 49.1 | 48.8 |
| [C2mim][Pro] | 39.5 | 39.2 | 38.9 | 38.5 | 38.2 | 37.9 | 37.6 | 37.2 |
| [C3mim][Pro] | 38.7 | 38.5 | 38.2 | 37.9 | 37.6 | 37.3 | 37.0 | 36.7 |
| [C4mim][Pro] | 37.1 | 36.8 | 36.5 | 36.2 | 35.8 | 35.5 | 35.2 | 34.9 |
| [C5mim][Pro] | 35.8 | 35.5 | 35.2 | 34.9 | 34.6 | 34.3 | 34.0 | 33.7 |
| [C6mim][Pro] | 34.8 | 34.5 | 34.2 | 33.9 | 33.6 | 33.3 | 33.0 | 32.7 |
3.6 Polarity of the [Cnmim][TFA] (n = 2, 3, 4, 5, 6) ILs
There is no widely accepted standard for evaluating the polarity of liquids, and it is common to determine whether a substance has polarity or not according to its dielectric constant, but as indicated in the related literature, this approach is not feasible for ILs.16 For example, the dielectric constant of [C4mim][NTf2],31 as measured by Daguenet et al. is 11.7. Wakai et al. measured the dielectric constants of a different IL, [C4mim][BF4],34 and yet obtained the same result. However, there is a great difference between the polarity of these two ILs. [C4mim][NTf2] is a hydrophobic IL, while [C4mim][BF4] is hydrophilic. Based on the Hildebrand's theory,35 we proposed a new standard, δμ, to describe the polarity of an IL:36| δμ2 = ΔHvμ/V − (1 − x)RT/V | (19) |
| ΔHvμ = ΔglH0m (298) − ΔHvn | (20) |
The ΔglH0m values have been calculated according to the Kabo's empirical eqn (6)15,37 and listed in Table 3.
The ΔHvn value is obtained according to the Lawson–Ingham equation:38
| ΔHvn = C[(nD2 − 1)/(nD2 + 2)]V | (21) |
| IL | ΔHvn, kJ mol−1 | ΔHvμ, kJ mol−1 | δμ, J1/2 cm−3/2 |
|---|---|---|---|
| [C2mim][TFA] | 74.39 | 64.45 | 19.13 |
| [C3mim][TFA] | 76.56 | 61.44 | 17.93 |
| [C4mim][TFA] | 78.36 | 69.02 | 18.27 |
| [C5mim][TFA] | 80.06 | 56.00 | 15.85 |
| [C6mim][TFA] | 82.31 | 56.14 | 15.28 |
From Table 11, it can be observed that the polarity (δμ) of the [Cnmim][TFA] (n = 2, 3, 4, 5, 6) ILs decreases with the increase of the number of methylene (–CH2–) groups in the alkyl chains of the ILs and this behavior is consistent with the literature.28
According to Seddon39 et al., the δμ value for [C4mim][BF4] was calculated to be 20.42 J1/2 cm−3/2, which is greater than the polarity of [C4mim][NTf2] (δμ = 10.23 J1/2 cm−3/2) and this result is consistent with our experience, i.e., [C4mim][BF4] is hydrophilic and [C4mim][NTf2] is hydrophobic. These observations prove that the new polarity scale for ILs has certain reliability. In our experiment, the polarity value obtained for [C4mim][TFA] was between that of [C4mim][BF4] and [C4mim][NTf2], which is reasonable.
4. Conclusion
In summary, a series of 1-alkyl-3-methylimidazolium trifluoroacetate ionic liquids were synthesized and characterized. Their density, surface tension and refractive index were measured in the temperature range from 293.15 to 343.15 ± 0.05 K. The standard molar entropy and lattice energy of the ILs were calculated according to the Glasser's theory. The molar enthalpy of vaporization (ΔglH0m) of the ILs at 298 K was estimated by the Kabo's method. The Eötvös equation was modified by introducing the molar surface Gibbs free energy. In the modified Eötvös equation, the slope represents the molar surface entropy and the intercept represents the molar surface enthalpy. The interstitial model was applied to calculate the thermal expansion coefficient (α) of the [Cnmim][TFA] (n = 2, 3, 4, 5, 6) ILs, and the order of magnitude for the calculated and experimental coefficients was the same. According to the interstitial model, a new concept was proposed, that is the interstitial molar surface Gibbs free energy (gs), which is independent of the type of IL. Similarly, the molar surface Gibbs free energy was applied to improve the Lorentz–Lorenz equation, which can predict the surface tension, and the predicted values for [Cnmim][TFA] ILs and other ILs were in good agreement with the corresponding experimental values. The polarity of the [Cnmim][TFA] ILs was estimated by the new polarity scale for ILs, and the results obtained show the reliability of this scale.Acknowledgements
This project was supported by the NSFC (21373005, 21471073, 21671089), the Education Bureau of the Liaoning Province (LZ2014001), and the Liaoning BAIQIANWAN Talent research funding 2014.References
- J. S. Wilkes, J. A. Levisky, R. A. Wilson and C. L. Hussey, Inorg. Chem., 1982, 21, 1263–1264 CrossRef CAS.
- J. Robinson and R. A. Osteryoung, J. Am. Chem. Soc., 1979, 101, 323–327 CrossRef CAS.
- J. Robinson, R. C. Bugle, H. L. Chum and D. Koran, J. Am. Chem. Soc., 1979, 101, 3776–3779 CrossRef CAS.
- N. W. N. Muhammad, Z. Omar, M. A. Man, S. Bustam, Y. Rafiq and Y. Uemura, Ind. Eng. Chem. Res., 2012, 51, 2280–2289 CrossRef CAS.
- F. Endres, ChemPhysChem, 2002, 3, 144–154 CrossRef CAS.
- J. S. Wilkes and M. J. J. Zaworotko, J. Chem. Soc., Chem. Commun., 1992, 965 RSC.
- J. Dupout, R. F. de Souza and P. A. Z. Suarez, Chem. Rev., 2002, 102, 3667–3692 CrossRef.
- M. D. Zhou, J. Zhao, S. L. Zang and K. E. Fritz, Chem.–Eur. J., 2007, 13, 158–166 CrossRef CAS PubMed.
- M. D. Zhou, Y. Yu, S. L. Zang and K. E. Fritz, Chem.–Asian J., 2009, 4, 411–418 CrossRef CAS PubMed.
- J. Z. Yang, Y. Jin, W. G. Xu, Q. G. Zhang and S. L. Zang, Fluid Phase Equilib., 2005, 227, 41–46 CrossRef CAS.
- D. W. Fang, J. Tong, W. Guan, H. Wang and J. Z. Yang, J. Phys. Chem. B, 2010, 114, 13808–13814 CrossRef CAS PubMed.
- J. Tong, Q. S. Liu, W. G. Xu, D. W. Fang and J. Z. Yang, J. Phys. Chem. B, 2008, 112, 4381–4386 CrossRef CAS PubMed.
- D. W. Fang, X. J. Gu, Y. Xiong, S. Yue, J. Li and S. L. Zang, J. Chem. Eng. Data, 2010, 55, 4440–4443 CrossRef CAS.
- L. Glasser, Thermochim. Acta, 2004, 421, 87–93 CrossRef CAS.
- D. H. Zaitsau, G. J. Kabo, A. A. Strechan, Y. U. Paulechka, A. Tschersich, S. P. Verevkin and A. Heintz, J. Phys. Chem. A, 2006, 110, 7303–7306 CrossRef CAS PubMed.
- J. Tong, H.-X. Yang, R.-J. Liu, C. Li, L. X. Xia and J.-Z. Yang, J. Phys. Chem. B, 2014, 118, 12972–12978 CrossRef CAS PubMed.
- J. G. Huddleston, A. E. Visser, W. M. Reichert and H. D. Willauer, Green Chem., 2001, 3, 156–164 RSC.
- D. R. Lide, Handbook of Chemistry and Physics, CRC Press, Boca Raton, 90th edn, 2010, pp. 1528–1529 Search PubMed.
- Z. F. Zhang, J. B. Li, J. Tong and J. Z. Yang, Chin. J. Appl. Chem., 2009, 26, 426–430 CAS.
- D. W. Fang, W. Guan, J. Tong, Z. W. Wang and J. Z. Yang, J. Phys. Chem. B, 2008, 112, 7499–7505 CrossRef CAS PubMed.
- J. M. Slattery, C. Daguenet, J. Paul, D. Thomas, J. S. Schubert and I. Krossing, Angew. Chem., Int. Ed., 2007, 46, 5384–5388 CrossRef CAS PubMed.
- J. Z. Yang, J. Tong, J. B. Li, J. G. Li and J. Tong, J. Colloid Interface Sci., 2007, 313, 374–377 CrossRef CAS PubMed.
- L. P. N. Rebelo, J. N. Canongia, J. M. S. S. Esperança and E. Filipe, J. Phys. Chem. B, 2005, 109, 6040–6043 CrossRef CAS PubMed.
- K. Fukumoto, M. Yoshizawa and H. Ohno, J. Am. Chem. Soc., 2005, 127, 2398–2399 CrossRef CAS PubMed.
- H. Ohno and K. Fukumoto, Acc. Chem. Res., 2007, 40, 1122–1129 CrossRef CAS PubMed.
- X.-X. Ma, J. Wei, Q.-B. Zhang, F. Tian, Y.-Y. Feng and W. Guan, Ind. Eng. Chem. Res., 2013, 52, 9490–9496 CrossRef CAS.
- M. Hong, S. Ao, L. Chun, W. Guan, J. Tong and J. Z. Yang, Ind. Eng. Chem. Res., 2013, 52, 15679–15683 CrossRef CAS.
- J. Tong, L. F. Wang and D. L. Liu, J. Chem. Thermodyn., 2016, 97, 221–227 CrossRef CAS.
- J. Z. Yang, X. M. Lu and J. S. Gui, Green Chem., 2004, 6, 541–543 RSC.
- C. Larriba, Y. Yoshida and J. F. Mora, J. Phys. Chem. B, 2008, 112, 12401–12407 CrossRef CAS PubMed.
- W. Beichel, Y. Yu, G. Dlubek, R. K. Rehberg, J. Pionteck, D. P. S. Bulut, D. Bejan, C. Friedrich and I. Krossing, Phys. Chem. Chem. Phys., 2013, 15, 8821–8830 RSC.
- R. J. Gale and R. A. Osteryoung, Inorg. Chem., 1980, 19, 2240–2242 CrossRef CAS.
- C. Daguenet, P. J. Dyson, I. Krossing, A. Oleinikova, J. Slattery, C. Wakai and H. Weingärtner, J. Phys. Chem. B, 2006, 110, 12682–12688 CrossRef CAS PubMed.
- C. Wakai, A. Oleinikova, M. Ott and H. Weingärtner, J. Phys. Chem. B, 2005, 109, 17028–17030 CrossRef CAS PubMed.
- J. H. Hildebrand and R. L. Scott, The Solubility of Nonelectrolytes, Reinhold, New York, 3rd edn, 1950 Search PubMed.
- T. I. Morrow and E. J. Maginn, J. Phys. Chem. B, 2002, 106, 12807–12813 CrossRef CAS.
- A. A. Strechan, G. J. Kabo and Y. U. Paulechka, Fluid Phase Equilib., 2006, 250, 125–130 CrossRef CAS.
- D. D. Lawson, Nature, 1969, 223, 614–615 CrossRef CAS.
- M. Deetlefs, K. R. Seddon and M. Shara, Phys. Chem. Chem. Phys., 2006, 8, 642–649 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra00197e |
| This journal is © The Royal Society of Chemistry 2017 |