Open Access Article
Open Access ArticleDirect reuse of two deep-dewatered sludge cakes without a solidifying agent as landfill cover: geotechnical properties and heavy metal leaching characteristics†
Jiakuan Yang*ab,
Shinan Zhanga,
Yafei Shi*ac,
Chao Lia,
Wenbo Yua,
Ruonan Guana,
Jun Xiaoa,
Sha Lianga,
Jingping Hua,
Huijie Houa and
Jiukun Hud
aSchool of Environmental Science and Engineering, Huazhong University of Science and Technology, Wuhan, Hubei 430074, P. R. China. E-mail: jkyang@hust.edu.cn; yfshi@hust.edu.cn; Fax: +86 27 87792101; Tel: +86 27 87792102
bState Key Laboratory of Coal Combustion, Huazhong University of Science and Technology, Wuhan, Hubei 430074, P. R. China
cSchool of Civil Engineering, Architecture and Environment, Hubei University of Technology, Wuhan 430068, Hubei, P. R. China
dDongjiang Environmental Co., Ltd., Shenzhen, Guangdong 518057, P. R. China
First published on 13th January 2017
Abstract
Two types of deep-dewatered sewage sludge cakes were produced from pilot-scale experiments by using two composite conditioners: FeCl3 + quick lime (Fe–Lime) and Fenton's reagent + red mud (Fenton–RM). The feasibility of direct reuse without any solidifying agents of these two deep-dewatered cakes, with water content of about 60 wt%, as landfill cover materials was investigated. Geotechnical properties of these two sludge cakes were found appropriate for reuse as landfill covers. Their plasticity index values increased significantly from 11.6 (raw sludge) to 23.8 (Fe–Lime) and 35.4 (Fenton–RM). The unconfined compressive strength and direct shear strength of the two deep-dewatered sludge cakes could meet or exceed the requirement of landfill cover materials after a certain curing time. Microstructural analyses of scanning electron microscopy (SEM) and X-ray diffraction (XRD) showed that their microstructures were more porous than that of raw sludge since the skeleton builders played a role in building the rigorous framework. There was negligible leaching of Cu, Zn, Pb, Cd and Cr from the deep-dewatered sludge cake from the toxicity characteristic leaching procedure and column leaching test. Both deep-dewatered sludge cakes could be reused as effective landfill cover materials with a suitable curing time.
1. Introduction
A tremendous amount of sewage sludge is being produced worldwide from wastewater treatment plants (WWTPs). In China alone, the annual sludge production has reached up to 24 million tons of dry sludge.1 Its treatment and disposal is costly and difficult. At present, sanitary landfilling is still the major disposal method for sewage sludge in China; while incineration and land application have a few applications.2Increasing sludge production creates great needs for cost-effective alternatives, in which the most favorable one is beneficial reuse. Reuse of sewage sludge as daily or final cover materials for landfill has attracted substantial attention, and it has been recommended by U.S. EPA.3,4 Commonly, the water content of mechanically dewatered sewage sludge is typically about 75–85 wt% with chemical conditioning with polymers (e.g., polyacrylamide (PAM)). To reduce the high water content of dewatered sludge so that it can be beneficially reused as a landfill cover material, three approaches are often taken. The first approach is sludge drying,5 which is extremely expensive and energy-consuming with a risk of emissions of unpleasant odors. The second approach is blending the dewatered sludge cake with soil. However, the proper type of soil is often not readily available. The third approach is to add solidifying agents into the dewatered sludge cake. With addition of solidifying agents such as Portland cement,6 fluidized bed combustion ash,7 converter slag,8 fly ash and lime,9 calcined aluminum salts,1 or magnesium oxychloride cement,10 the solidified sewage sludge specimens were found effective as landfill cover materials. Unfortunately, the mass ratio of the soil or solidifying agents to the dry solid of the dewatered sludge often needs to be 100–200%. Consequently, the increased volume would take away lots of valuable landfill capacity. Direct reuse of dewatered sewage sludge as landfill cover materials without any soil or solidifying agents is seldom reported in literature.11
For landfill disposal, water content of sludge should be low; and the maximum water content limit is normally 60 wt% in China.12 Many attempts have been made to improve sludge dewatering by employing pretreatment. The pretreatment methods include physical and thermal processes,13,14 chemical processes,15 and biological processes.16 Deep dewatering of sewage sludge using new types of chemical conditioners instead of the traditional polymer conditioners, have been extensively investigated and considered as a cost-effective alternative. In deep dewatering, water content of the dewatered sludge cake could be less than 60 wt%, which makes it more suitable for subsequent reuses or disposal. FeCl3 combined with quick lime (referred as Fe–Lime) is a typical conditioner recommended by U.S. EPA in sewage sludge dewatering.17 In a Fe–Lime system, Fe3+ acts as a coagulant to provide polyvalent cations, and lime plays as a skeleton builder.18–20 Recently, an innovative composite conditioner of combined Fenton's reagent and red mud (referred as Fenton–RM) has been studied by this research group.21 In Fenton–RM system, Fenton reagent acts as an oxidant which destroys the extracellular polymeric substances structure and improves the release of the bond water in sewage sludge; while red mud (RM) plays as a skeleton builder to promote a rigid and permeable structure of dewatered sludge that improves sludge compressibility. In these two conditioning systems, skeleton builders (quick lime and RM) were added during dewatering so that they could be homogeneously dispersed into the dewatered sludge. The skeleton builders also play an important role in the subsequent solidification/stabilization stage for the dewatered sewage sludge. By using either of these two systems, the water content of the treated sludge can be reduced to less than 60 wt%.
In the previous paper,22 pilot-scale sewage sludge dewatering experiments were conducted using both composite conditioners: Fe–Lime and Fenton–RM. Consequently, two types of deep-dewatered sewage sludge cakes were obtained with water content of 52.8 and 47.7 wt%, respectively. It was found that most of the heavy metals in sewage sludge were retained in the dewatered sludge cake.22 However, feasibility of direct reuse of those two deep-dewatered sewage sludge cakes as landfill cover materials has not been fully investigated.
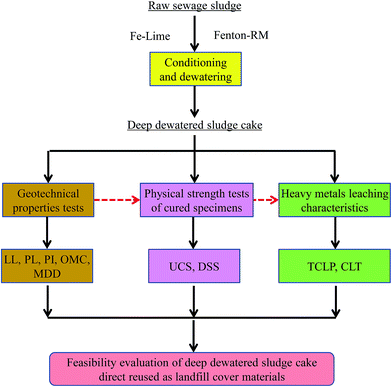
The objective of this study was to evaluate geotechnical properties and heavy metal leaching characteristics of these two deep-dewatered sludge cakes. Two issues would be further investigated in this study: (1) how the skeleton builders would behave to improve the geotechnical properties of sludge cakes; (2) what are the leaching behaviors of heavy metals retained in the sludge cakes. Finally, feasibility of direct reuse of them as landfill cover materials would also be evaluated. The schematic of this study is depicted in Fig. 1.
2. Materials and methods
2.1 Materials
The raw sludge (RS) used in this study was a mixture of primary and secondary sludge after a gravity-thickening process from Tangxunhu WWTP in Wuhan, China. Table 1 summarizes some main characteristics of the RS sample. Two batches of RS were investigated in this study. RS-I was sampled in September of 2013; RS-II was sampled in April of 2014.| Batch | pH | Water content (wt%) | VSS/TSS (%) | Cu (mg kg−1)* | Zn (mg kg−1)* | Pb (mg kg−1)* | Cd (mg kg−1)* | Cr (mg kg−1)* |
|---|---|---|---|---|---|---|---|---|
| a On the dry solid basis. * Milligrams per kilogram of dry solid (mg kg−1). | ||||||||
| RS-I | 7.2 | 96.0 ± 0.1 | 41.3 ± 0.3 | 132.1 ± 2.9 | 279.6 ± 12.9 | 39.8 ± 0.5 | 4.5 ± 0.2 | 73.3 ± 0.9 |
| RS-II | 6.9 | 96.9 ± 0.1 | 59.6 ± 0.2 | 192.0 ± 2.0 | 1139.3 ± 10.3 | 23.3 ± 0.3 | 2.9 ± 0.1 | 66.7 ± 0.6 |
As presented in Table 1, the volatile suspended solid/total suspended solid (VSS/TSS) of RS-II is higher than that of RS-I since different influent load of sewage mixing with rainwater in various seasons. In the following experiments, RS-I was used in geotechnical tests, and both RS-I and RS-II were used in the leaching properties tests. Both RS samples had a pH close to neutral, and the concentrations of heavy metals (Cu, Zn, Pb, Cd and Cr) are in the order of Zn > Cu > Cr > Pb > Cd.
FeCl3, FeSO4·7H2O and H2O2 (27.5% v/v) of industrial grade were obtained from a commercial company in China. H2SO4 was used to adjust the initial pH of the RS samples to the optimal value of 5.0 before the addition of Fenton's reagent.20 All the acids (including H2SO4, HNO3, HF and HClO4) used for sludge sample digestion were of analytical grade.
Quick lime and RM, which were used as skeleton builders, were dried, milled and sieved to less than 1 mm in particle size before use. The quick lime was obtained from a local factory. The RM was supplied by an alumina plant employing the Bayer process in Zhengzhou, China. Their chemical compositions are presented in Table S1 as ESI.†
2.2 Dewatering process and dewatered sludge cake
Two composite conditioning systems (Fe–Lime and Fenton–RM) were used in this study. The sludge conditioning and dewatering process is presented in Fig. S1,† studied in our previous study.22 The scheme of sludge conditioning has been optimized in our previous work and is presented in Table S2.†21,23 After conditioning, the sludge was dewatered by a pilot-scale diaphragm filter press in the Tangxunhu WWTP (Fig. 2). For each batch, 500–800 kg of RS was pumped into a conditioning tank. A more detailed description of the dewatering process was depicted elsewhere.22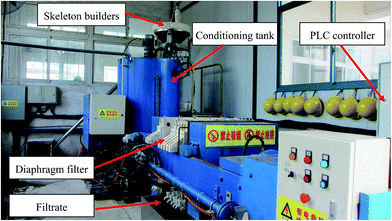 | ||
| Fig. 2 The photo of the pilot-scale facility for sewage sludge conditioning and deep dewatering in the Tangxunhu WWTP. | ||
Main characteristics of two types of deep-dewatered sludge cakes are presented in Table 2. After the deep-dewatering process, the water contents of the dewatered sludge cakes from both systems were reduced to less than 60 wt%, which is favorable for subsequent disposal, especially for landfilling. VSS/TSS ratios also decreased due to the addition of inorganic skeleton builders. As reported earlier, most of the heavy metals remained in the sludge cake during the dewatering process.22
| Conditioning system | Water content (wt%) | VSS/TSS (wt%) | Cu (mg kg−1)* | Zn (mg kg−1)* | Pb (mg kg−1)* | Cd (mg kg−1)* | Cr (mg kg−1)* |
|---|---|---|---|---|---|---|---|
| a On the dry solid basis. * Milligrams per kilogram of dry solid (mg kg−1). | |||||||
| Fe–Lime-I | 52.8 | 35.2 | 91.7 ± 0.9 | 251.3 ± 7.0 | 30.3 ± 1.3 | 2.4 ± 0.4 | 59.9 ± 3.4 |
| Fenton–RM-I | 47.7 | 37.8 | 101.6 ± 1.6 | 253.6 ± 5.6 | 36.0 ± 0.0 | 4.8 ± 0.1 | 117.4 ± 0.9 |
| Fenton–RM-II | 53.7 | 45.7 | 93.7 ± 0.5 | 797.2 ± 4.3 | 30.5 ± 0.5 | 3.0 ± 0.1 | 77.7 ± 0.3 |
The traditional dewatered sludge after conditioned with PAM (referred to RS-PAM) was comparatively investigated in the geotechnical tests. RS-PAM was produced from a centrifugal dewatering process after conditioning with PAM in the Tangxunhu WWTP. The water content of RS-PAM is 81.7 wt%, and its VSS/TSS is about 41 wt%, which is almost the same as that of the RS-I sample.
2.3 Geotechnical tests
The dewatered sludge cakes were divided into two parts; one was tested for the geotechnical properties, and the other was used for mechanical strength tests. Geotechnical properties of the dewatered cakes from two composite conditioning systems (Fe–Lime and Fenton–RM) were compared to RS-PAM and standard clay specimen which was provided by Hubei Province Geological Experiment Institute, China.The geotechnical tests for the samples were conducted in accordance with the Chinese standards for soil laboratory test methods (GB/T 50123-1999) for water content, liquid limit (LL), plastic limit (PL) and plasticity index (PI).24 The classification of the solidified sludge was determined in accordance with the Unified Soil Classification System.25 The dewatered sludge specimens were molded in a self-made compactor device to determine the optimum moisture content (OMC) and the maximum dry density (MDD). A heavy compaction procedure (5 layers, 27 times compaction for each layer), as specified in Chinese test methods of soils for highway engineering, was used to estimate the characteristics of compaction.26 The permeability of the dewatered sludge specimens under optimum compacted conditions was also measured by a variable-head permeability test following the Chinese standard.24
Mechanical strength properties, i.e., unconfined compressive strength (UCS) and direct shear strength (DSS), were also determined according to the standard.24 The cylinder specimens for the UCS test were 3.91 cm in diameter and 8.0 cm in length, and the ones for the DSS test were 6.18 cm in diameter and 2.0 cm in length with a relative compaction of 90%. The specimens were cured in a standard curing chamber at 20 ± 2 °C with humidity >90 wt%. Triplicate samples were immersed in water for 24 h, and used to evaluate the mechanical strengths of these specimens cured for 0, 3, 7, 14, 21, 28, 60, 90 days.
2.4 Leaching tests of heavy metals
The leaching tests for heavy metals were performed according to two specifications: a standard toxicity characteristic leaching procedure (TCLP) as a short-term leaching test and a column leaching test (CLT) for the long-term leaching characteristics. In the Fe–Lime system, the dewatered sludge cake of Fe–Lime-I was investigated on leaching tests for heavy metals, and RS-I was studied as a control. In Fenton–RM system, the dewatered sludge cake of Fenton–RM-II was investigated on leaching tests for heavy metals, and RS-II was studied as a control.According to TCLP,27 50 g air-dried sludge specimens (Fe–Lime-I and RS-I) was grinded and sieved to achieve particle sizes smaller than 9.5 mm and then placed in a polyethylene bottle, followed by an addition of one liter acetic acid solution (5.7 mL acetic acid in 1 L of distilled water, pH = 2.88). The bottle was placed in a rotary extractor and rotated for 18 h at 30 ± 2 rpm. After rotation, the sample was filtered through a 0.45 μm membrane filter, and then the filtrate was collected and analyzed for metal concentrations by using an atomic absorption spectrometer (Analytik Jena AG NovAA 400, Germany).
The schematic of the CLT is presented in Fig. S2.† As a dynamic test, CLT reportedly has a better leaching effect.28 Eighty grams of air-dried sludge specimens (Fenton–RM-II and RS-II) were grinded and sieved to achieve particle sizes smaller than 4 mm, and placed in a glass column (5 cm inside diameter and 35 cm height) and a perforated plate on both ends. The dewatered sludge was leached from the bottom up by a continuous flow of the acetic acid solution (pH = 2.88) using a peristaltic pump. A flowing rate of 5 mL h−1, commonly used in literature, was adopted here; and a rate of 10 mL h−1 was also applied for a comparison.
The leachate was sampled at different liquid-to-solid ratios (L/S, mL g−1). L represents the volume of the acetic acid solution passed through the column, in mL; and S represents the mass of the sludge sample in the column, in g. Heavy metals in the leachate were analyzed. The extraction percentage of each heavy metal is the mass ratio of heavy metal leached out to the total mass of heavy metal in the original sludge sample.
2.5 Microstructural analysis
Microstructural characteristics of the dewatered sludge samples were investigated by X-ray diffraction (XRD) and scanning electron microscopy (SEM). The specimens were immersed in ethanol to end the hydration reaction and then dried in an oven at 40 °C for 24 h. XRD analyses were carried out using Cu Kα radiation, operated at 40 kV, with a scan speed of 0.2785° s−1 and in the 2θ range from 5° to 75°. Morphology studies were carried out using a Sirion 200 scanning microscope after the samples were coated with gold.3. Results and discussion
3.1 Geotechnical properties of solidified sludge
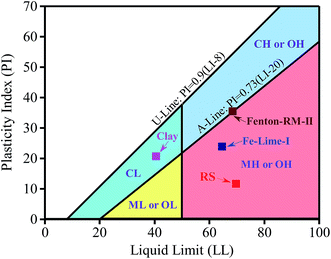
The geotechnical properties of the clay, RS-I and the dewatered sludge cakes are presented in Table 3. As shown, when compared to RS-I, the PL of the Fe–Lime-I decreased significantly from 58.1 wt% to 40.9 wt%, while the PI increased from 11.6 wt% to 23.8 wt%. As for the Fenton–RM-I, its PL and PI were 33.1 wt% and 35.4 wt%, respectively. Moreover, the PL and PI of RS-PAM were close to those of RS. The changes can be seen more readily in Fig. 3. The LL values of the dewatered sludge cakes were nearly the same as that of the RS, but PL values of the dewatered sludge cakes were significantly less than that of the RS. Thus the PI values increased obviously from RS to the sludge cakes since PI equals to LL minus PL, as presented in Table 3. PI value was in the order of RS < Fe–Lime < Fenton–RM. According to the classification of ASTM, the RS belongs to high-plasticity silty while the sludge cake of Fenton–RM is on the boundary of silty and clay. When compared to deep-dewatered sludge cakes (Fe–Lime and Fenton–RM), the standard clay has a smaller plasticity index values.| Specimens | LL (wt%) | PL (wt%) | PI (wt%) | OMC (wt%) | MDD (g cm−3) | Coefficient of permeability (cm s−1) |
|---|---|---|---|---|---|---|
| Clay | 40.6 | 20.0 | 20.6 | 12.4 | 1.890 | 3.31 × 10−8 |
| RS-I | 69.7 | 58.1 | 11.6 | 37.5 | 1.581 | 6.45 × 10−8 |
| RS-PAM | 69.3 | 59.8 | 9.5 | 36.9 | 1.561 | 6.71 × 10−8 |
| Fe–Lime-I | 64.7 | 40.9 | 23.8 | 25.5 | 1.421 | 5.90 × 10−7 |
| Fenton–RM-I | 68.5 | 33.1 | 35.4 | 23.0 | 1.435 | 7.20 × 10−6 |
In addition, RS-I showed a high OMC of 37.5 wt% and an MDD of 1.581 g cm−3. While for both deep-dewatered sludge cakes, the values of OMC and MDD decreased considerably. It implied that deep-dewatered sludge cakes have lower water-absorption capacity, when compared with RS and RS-PAM.
It can also be seen from Table 3 that the deep-dewatered sludge cakes had higher permeability coefficient values (>10−7 cm s−1) than that of RS (6.45 × 10−8 cm s−1). These results validate the roles of skeleton builders in the conditioning stage that increased the porosity of dewatered sludge. No existing Chinese standards define acceptable parameters with regards to the permeability coefficient of landfill cover. In USA, the permeability coefficients of lime-stabilized sludge as landfill cover range from 1 × 10−3 to 10−6 cm s−1.29 In general, the permeability coefficient values of the deep-dewatered sludge and the RS are less than the reported permeability coefficient values of lime-stabilized sludge. Both deep-dewatered sludge cakes had a permeability coefficient value (between 10−6 and 10−8 cm s−1), which is smaller to the reported permeability coefficient of the lime-stabilized sludge.
3.2 Mechanical strength properties
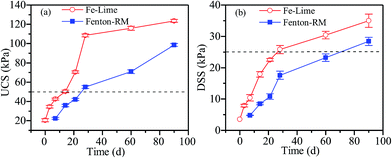
The relationship between the UCS and the curing age is shown in Fig. 4(a). As shown, the UCS values of the dewatered sludge cakes increase with the curing age. In general, a minimum UCS of 50 kPa is the permissible limit in Chinese standard for an effective landfill cover material.12 It took 14 days and 28 days for the dewatered sludge of Fe–Lime and the Fenton–RM systems to go above this minimum limit, respectively. As shown in Fig. 4(b), the DSS values of the dewatered sludge cakes increase with the curing age, which is consistent with the case for the UCS. In general, the required minimum limit of DSS for an effective landfill cover material is 25 kPa. It took 28 and 90 days for the dewatered sludge of Fe–Lime and Fenton–RM systems to go above this minimum limit, respectively. The results imply that lime could enhance the mechanical strengths of the dewatered sludge in Fe–Lime system more effectively than red mud (a skeleton builder in Fenton–RM system), and it took a shorter curing time for Fe–Lime system to attain the required mechanical strength.3.3 Microstructure characteristics
Fig. 5 shows the SEM images and XRD patterns of the RS-I and the dewatered sludge cakes (Fe–Lime-I, and Fenton–RM-I). As shown in Fig. 5(a), the morphological structure of the RS is flake-like, dense and compact, since more organic matters in the RS filled most of voids in the dried RS specimen. The XRD pattern indicates that the RS consists mainly of quartz and mica. Microstructure characteristics of the RS are consistent with those found in the previous studies.20,21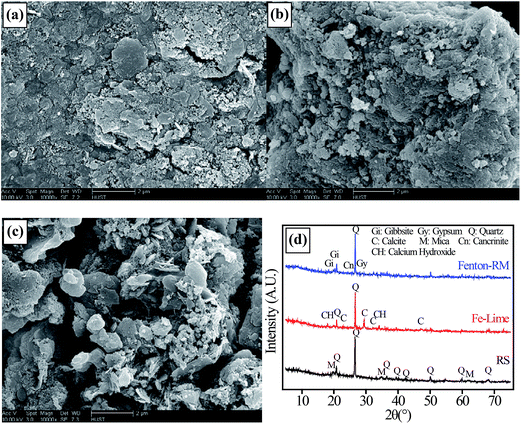 | ||
| Fig. 5 SEM images of (a) RS-I, and the (b) dewatered sludge cakes of Fe–Lime-I, (c) Fenton–RM-I; and (d) the XRD patterns of these samples. | ||
The dewatered sludge cake of Fe–Lime-I consists of smaller particulates and it is more porous with an irregular shape (Fig. 5(b)). It indicates that the raw sludge with a high organic content and poor dewaterability can be improved with skeleton builders to generate a porous, but relatively incompressible structure. The XRD pattern indicates the Fe–Lime-I consists mainly of quartz, calcium hydroxide and calcite.
Some irregularly shaped crystals embedded among the sludge particles were found in Fenton–RM-I (Fig. 5(c)). This dewatered sludge cake appears to be more porous. The XRD patterns indicate that it consists mainly of quartz, mica, gypsum, cancrinite, and gibbsite.
3.4 Leaching tests for heavy metals
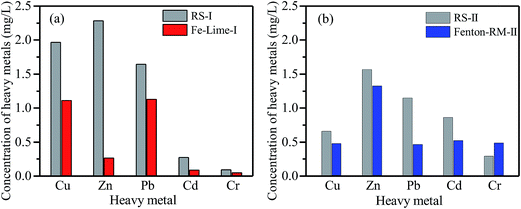 | ||
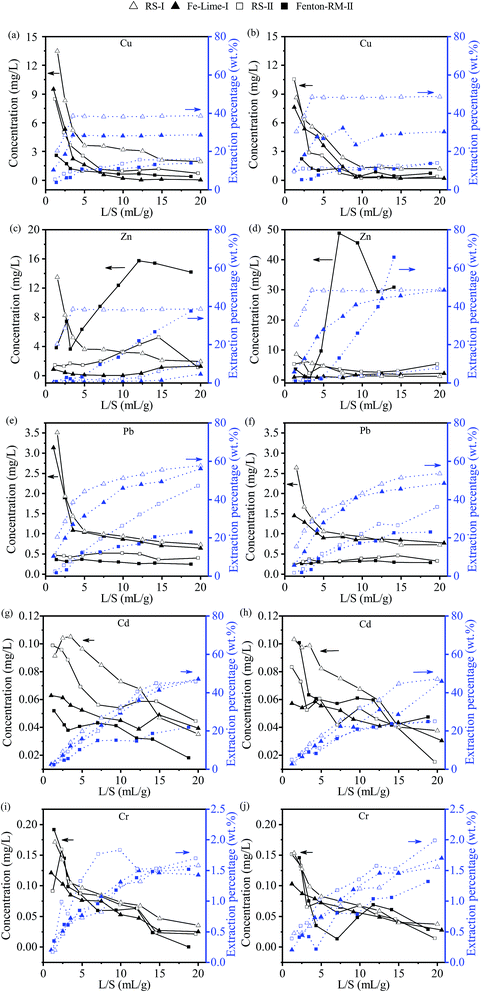
| Fig. 6 Concentrations of heavy metal in the leachates from the TCLP tests: (a) RS-I, and Fe–Lime-I; (b) RS-II, and Fenton–RM-II. | ||
As shown in Fig. 6(a) and (b), leached concentrations of Cu, Zn and Pb from the two dewatered sludge cakes of Fe–Lime-I are significantly lower than those of the RS-I; while Cr concentration in the dewatered sludge of Fenton–RM-II are the highest among all of RS and dewatered sludge specimens (Fig. 6(b)). The higher concentrations of Cr might come from the red mud which was added as the conditioner. In general, the sludge cake from the Fe–Lime system has a good retention for heavy metals.
Typical acceptable TCLP concentrations of heavy metals effluent discharge standards of municipal solid waste landfills are 40, 100, 0.25, 0.15 and 4.5 mg L−1 of Cu, Zn, Pb, Cd, and Cr (GB16889-2008), respectively.30 The results show that Pb in Fe–Lime-I exceeded the acceptable limit, while the retention capability of Fenton–RM-II on Pb and Cd needs to be improved. In order to facilitate technology adaptability, two improvements could be used to promote the solidification effects of the dewatered sludge cakes. Firstly, during the conditioning and dewatering process, a little more dosage of conditioner (lime or cement) could be added to further improve the dewatering effects and the heavy metal solidification behavior. Besides, to avoid the risk of heavy metal release in the landfill process, small amount of cement could be mixed with the dewatered sludge cakes to strengthen the heavy metal solidification effects, given the good cooperation of alkali cement and red mud.
The results of this CLT study indicate that the deep-dewatered sludge cakes conditioned with skeleton builders are relative stable for a long period of time, with regards to heavy metal leaching, when they are used as daily or final landfill cover.
4. Conclusion
In this study, feasibility of reusing deep-dewatered sludge cakes without addition of solidifying agents as landfill cover materials was evaluated. Results of geotechnical tests indicate that deep-dewatered sludge cakes, conditioned with skeleton builders, improved the geotechnical properties of the dewatered sludge, when compared with RS and RS-PAM. The deep-dewatered sludge cakes can attain the required USC and DSS needed for landfill covers within a short curing time. The XRD patterns and SEM images validated the framework effect of the skeleton builders and showed that these cakes are less compressible. Results of leaching tests using both TCLP and CLT revealed that both deep-dewatered sludge cakes could retain heavy metals for a long period of time.In summary, the skeleton builders introduced in the conditioning stage during deep-dewatering process improve the geotechnical properties and heavy metal leaching characteristics of the dewatered sludge cakes which make them appropriate for re-use as landfill cover materials.
Abbreviation
| CLT | Column leaching test |
| DS | Dry solid |
| DSS | Direct shear strength |
| LL | Liquid limit |
| L/S | Liquid-to-solid ratio |
| MDD | Maximum dry density |
| OMC | Optimum moisture content |
| PAM | Polyacrylamide |
| PI | Plasticity index |
| PL | Plastic limit |
| RM | Red mud |
| RS | Raw sludge |
| SEM | Scanning electron microscopy |
| TCLP | Toxicity characteristic leaching procedure |
| TSS | Total suspended solids |
| UCS | Unconfined compressive strength |
| VSS | Volatile suspended solids |
| WWTP | Wastewater treatment plant |
| XRD | X-ray diffraction |
Acknowledgements
The research was supported by the National Natural Science Foundation of China (51508214), the Research Project of Chinese Ministry of Education (113046A), Project of Innovative and Interdisciplinary Team of Huazhong University of Science and Technology (2015ZDTD027) and the Foundation of State Key Laboratory of Coal Combustion (FSKLCCA1604). The authors would like to thank Analytical and Testing Center of Huazhong University of Science and Technology for providing detecting instruments.References
- G. Zhen, X. Yan, H. Zhou, H. Chen, T. Zhao and Y. Zhao, J. Environ. Sci., 2011, 23, 1225–1232 CrossRef CAS.
- L. Jin, G. Zhang and H. Tian, Water Res., 2014, 66, 85–98 CrossRef CAS PubMed.
- U. S. EPA, 40 CFR Part 257, 1993.
- E. H. Kim, J. K. Cho and S. Yim, Chemosphere, 2005, 59, 387–395 CrossRef CAS PubMed.
- H. Liu, P. Liu, H. Hu, Q. Zhang, Z. Wu, J. Yang and H. Yao, Chemosphere, 2014, 117, 559–566 CrossRef CAS PubMed.
- S. Valls and E. Vàzquez, Cem. Concr. Res., 2000, 30, 1671–1678 CrossRef CAS.
- V. Bednarik, M. Vondruska, M. Sild and K. Marek, J. Environ. Eng., 2004, 130, 1527–1533 CrossRef CAS.
- E.-H. Kim, J.-K. Cho and S. Yim, Chemosphere, 2005, 59, 387–395 CrossRef CAS PubMed.
- P. Samaras, C. A. Papadimitriou, I. Haritou and A. I. Zouboulis, J. Hazard. Mater., 2008, 154, 1052–1059 CrossRef CAS PubMed.
- M. Jianli, Z. Youcai, W. Jinmei and W. Li, Constr. Build. Mater., 2010, 24, 79–83 CrossRef.
- Y. L. Li, J. W. Liu, J. Y. Chen, Y. F. Shi, W. Mao, H. Liu, Y. Li, S. He and J. K. Yang, Int. J. Environ. Sci. Technol., 2014, 11, 233–240 CrossRef CAS.
- Standardization Administration of the People's Republic of China, GB/T 23485-2009, 2009.
- G. Zhang, J. He, P. Zhang and J. Zhang, J. Hazard. Mater., 2009, 164, 1105–1109 CrossRef CAS PubMed.
- K. Hu, J. Q. Jiang, Q. L. Zhao, D. J. Lee, K. Wang and W. Qiu, Water Res., 2011, 45, 5969–5976 CrossRef CAS PubMed.
- K. Song, X. Zhou, Y. Liu, G.-J. Xie, D. Wang, T. Zhang, C. Liu, P. Liu, B. Zhou and Q. Wang, Chem. Eng. J., 2016, 295, 436–442 CrossRef CAS.
- H. Liu, S. Yang, J. Shi, X. Xu, H. Liu and B. Fu, Sep. Purif. Technol., 2016, 165, 53–59 CrossRef CAS.
- U. S. EPA, EPA-625/1-82/014, 1987.
- S. Deneux-Mustin, B. S. Lartiges, G. Villemin, F. Thomas, J. Yvon, J. L. Bersillon and D. Snidaro, Water Res., 2001, 35, 3018–3024 CrossRef CAS PubMed.
- Y. Qi, K. B. Thapa and A. F. Hoadley, Chem. Eng. J., 2011, 171, 373–384 CrossRef CAS.
- H. Liu, J. Yang, N. Zhu, H. Zhang, Y. Li, S. He, C. Yang and H. Yao, J. Hazard. Mater., 2013, 258, 144–150 CrossRef PubMed.
- H. Zhang, J. Yang, W. Yu, S. Luo, L. Peng, X. Shen, Y. Shi, S. Zhang, J. Song, N. Ye, Y. Li, C. Yang and S. Liang, Water Res., 2014, 59, 239–247 CrossRef CAS PubMed.
- C. Li, S. Zhang, J. Yang, Y. Shi, W. Yu, S. Liang, J. Song, Q. Xu, Y. Chen, J. Hu, Y. Li and C. Yang, RSC Adv., 2015, 5, 102332–102339 RSC.
- H. Liu, Y. Li, Y. Shi, Y. Li, S. He and J. Yang, Environ. Chem., 2011, 30, 1877–1882 CAS.
- Ministry of Construction of the People's Republic of China, GB/T 50123-1999, 1999.
- ASTM, D2487-93, 1997.
- Ministry of Transport of the People's Republic of China, JTG E40-2007, 2007.
- U. S. EPA, Test method 1311, 1996.
- NEN 7343, Netherlands Standardisation Institute, 1995.
- A. Jacobs and M. Silver, Water Sci. Technol., 1990, 22, 93–106 CAS.
- Ministry of Environmental Protection of the People's Republic of China, GB 16889-2008, 2008.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra26480h |
| This journal is © The Royal Society of Chemistry 2017 |