Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A hybrid macrocyclic anion receptor exploiting the pyrrole-2,5-diacetamide unit†
Janusz
Jurczak
 *,
Pawel
Dydio‡
,
Pawel
Stepniak
*,
Pawel
Dydio‡
,
Pawel
Stepniak
 and
Tomasz
Zielinski§
and
Tomasz
Zielinski§
Institute of Organic Chemistry, Polish Academy of Sciences, Warsaw, Poland. E-mail: jurczak_group@icho.edu.pl
First published on 20th April 2016
Abstract
We report the synthesis of both macrocyclic and linear derivatives of pyrrole-2,5-diacetic bisamides, and the studies of their anion binding properties, which are further compared with the properties of similar derivatives bearing dipicolinic or isophthalic moieties. The new macrocyclic compound built on pyrrole-2,5-diacetic bisamide was found to be an efficient receptor for H2PO4− and PhCOO−, even in DMSO-d6 + 0.5% H2O (v/v), with changed relative selectivity toward a series of anions with respect to its parental analogues. The anion binding properties of the linear derivatives of pyrrole-2,5-diacetic bisamide, in turn, were found to strongly depend on the pKa of the amide NH protons, which can be modulated through the introduction of various substituents.
Introduction
Anion recognition is a robustly developing branch of supramolecular chemistry,1 owing to potential applications in the construction of molecular devices, such as sensors, responsive materials, extraction systems, simple and transmembrane transporters, transition metal catalysts and organocatalysts.2 Various strategies exploiting weak interactions have been evaluated for achieving selective and efficient anion recognition.3 Among the interactions harvested for the receptor–anion binding, the hydrogen bond is arguably the most attractive.4 Such interactions are of relatively high energy and they can be readily multiplied using several H-bond donating groups. Finally, they can be easily introduced and manipulated by tailoring the structure of the putative receptor. Importantly, hydrogen bonding is spatially sensitive, as it is formed only if the H-bond donor is positioned accurately in respect to the H-bond acceptor. This feature allows for the design of receptors characterized by high selectivity for anions that match the size and the shape of the binding pocket. Consequently, hydrogen bonding has been broadly explored in studies of anion recognition with synthetic anion receptors.5The hydrogen bond donating groups for this purpose frequently used include amide, sulfonamide, urea, hydrazide, hydrazine, and pyrrole moieties.1 Unlike the other groups, the pyrrole unit can act only as a donor, diminishing the risk of formation of intramolecular hydrogen bonding, unfavorable for anion binding.6 Consequently, pyrrole derivatives have attracted particular attention, with various linear and cyclic structures being explored as potential anion receptors.7 Alternatively, the pyrrole moiety has been used as a scaffold bearing additional hydrogen bond donors.8 This strategy has led to the formation of efficient and selective linear anion receptors, such as pyrrole-2,5-dicarboxyamides9 and pyrrole-2,5-diacetamides.10
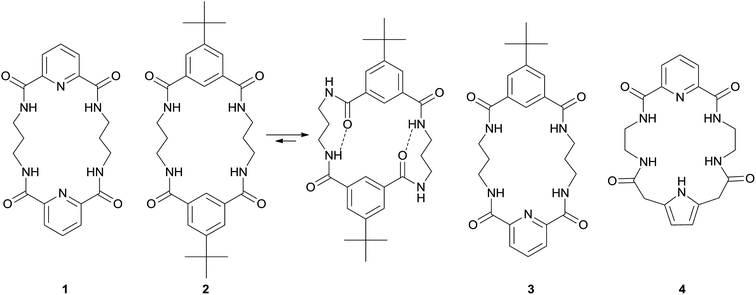
The macrocyclic topology of putative receptors allows many H-bonding sites to be gathered in close proximity and arranged in a convergent manner, as required for effective host–guest interactions. Previously, we have shown that macrocyclic tetraamides, such as 1 and 2 (Fig. 1), are superior anion receptors over their linear analogues.11 We also found that the dipicolinic amide moiety, while itself interacting rather weakly with anionic guests, serves as a powerful unit in preorganizing hydrogen bonding sites.12 For instance, hybrid macrocycle 3, containing dipicolinic and isophthalic subunits, is a better anion receptor than both its parental congeners 1 and 2 (Fig. 1). We envisioned that introducing additional hydrogen bonding groups to such receptors may have the result of both strengthening the host–guest interaction and modulating the relative selectivity toward different anionic guest species. Here, we report on the synthesis and anion binding properties of hybrid receptor 4 which combines the potency of both dipicolinic amide and pyrrole-2,5-diacetamide building blocks, having a macrocyclic cavity of the same size as receptors 1–3 (20-membered ring).¶
Results and discussion
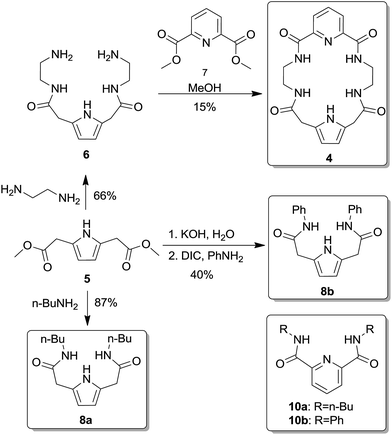
As shown in Scheme 1, the desired macrocyclic compound 4 was readily synthesized from known pyrrole-2,5-diacetic dimethyl ester (5).13 Diester 5 was reacted with ethylenediamine to form diamide 6, which was then subjected to cyclization with dipicolinic methyl diester (7). The reaction proceeded very slowly in methanol, but when 1,8-diazabicycloundec-7-ene (DBU) was added, the desired tetraamide 4 was formed with 15% yield within 2 weeks.In addition, we prepared acyclic derivative 8a by simple amidation of diester 5 with n-butylamine, and also its 8b analogue by a two-step procedure (hydrolysis to diacid 9, followed by amidation with aniline and DIC) (Scheme 1).
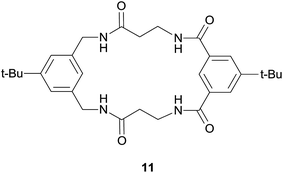
Linear receptors 8a and 8b as well as dipicolinic acid bisamides (10a–b) (Scheme 1) were evaluated in terms of the relative contribution of the two aromatic moieties in receptor 4 (the dipicolinic amide and pyrrole-2,5-diacetamide building blocks) to its binding properties. Furthermore, this allowed the influence of the macrocyclic topology of 4 on its anion binding properties to be estimated. Finally, we compared binding properties of receptors 4 and 11 (Fig. 2) which allowed us to abstract contribution of topological differences of hydrogen bond donors position and assess role of the pyrrole moiety.
The anion binding measurements of the putative receptors were carried out in DMSO-d6 + 0.5% H2O (v/v), using the 1H NMR titration technique, with appropriate TBA salts as sources of anions. The association constants (Ka) measured for 8a and 8b were substantially higher than those of dipicolinic bisamide 10, showing that the pyrrolediacetamide is a superior building block over the dipicolinic moiety for the construction of linear receptors (Table 1). Receptor 8b, bearing phenyl substituents instead of the n-butyl groups of 8a, binds anions with three-fold higher Ka.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes of 1–4, 8a–b, 10a–b and 11 with various anionsa
1 complexes of 1–4, 8a–b, 10a–b and 11 with various anionsa
| 1 b , c | 2 d | 3 e | 4 | 8a | 8b | 10a | 10b | 11 d | |
|---|---|---|---|---|---|---|---|---|---|
a Measured in DMSO-d6 + 0.5% H2O. TBA salts were used as anion sources, uncertainties <10%.
b Measured in DMSO-d6.
c
Ref. 11a.
d
Ref. 11c.
e
Ref. 12.
f Data did not fit 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 model.
g Not determined. 1 model.
g Not determined.
|
|||||||||
| PhCOO− | 2283 | 601 | 3612 | 260 | 31 | 87 | 6.3 | 4.2 | ndg |
| H2PO4− | 7410 | —f | >8000 | 617 | 108 | 577 | 12 | 12 | 327 |
| Cl− | 1930 | 378 | 2148 | 204 | 3 | 8 | <5 | <5 | 29 |
| Br− | 150 | <5 | 175 | 8 | <5 | <5 | ndg | <5 | ndg |
The macrocyclic topology of 4, by contrast, imposes a conformation suitable for the anion binding process, with all amide and pyrrole protons convergent. This structure led to a 70-fold higher Ka for chlorides in comparison to Ka measured for 8a (cf.Table 1). Binding to both benzoate and dihydrogen phosphate follows a similar tendency, with about one order of magnitude higher Ka's for macrocyclic 4 as compared with linear 8a. Interestingly, bromides are weakly bound by receptor 4, indicating that such macrocycles are not planar, but rather form bent conformations which allow interaction with larger guests. This is supported by crystal structure analysis carried out for complexes of analogous molecules, were diaromatic macrocycles often form bent π-stacked structures.11b
Comparison of the binding properties of macrocycle 4 with those of previously evaluated receptors 1–3 shows that the presence of the pyrrole unit modifies the relative selectivity for binding of different anions (Table 1). Interestingly, the incorporation of the additional binding site – the pyrrole NH proton – does not result in increased association constants of receptor 4. On the contrary, receptor 4 binds anions with about one order of magnitude lower Ka's than receptors 1–3. Hybrid dipicolinic–isophtalic derivative 3 remains the most efficient receptor of this type. However, comparison of 4 with more structurally related macrocycle 1111c shows that the additional hydrogen bonding NH group not only modifies the relative selectivity but also leads to a substantial increase in the binding of the chloride anion, with a 10-fold higher Ka.
Finally, in order to compare the binding properties of linear receptors 8 with the analogues obtained previously by Gale and coworkers,10 we performed additional titrations in MeCN-d3 (Fig. 3 and Table 2). As could be expected,14 binding constants measured in acetonitrile-d3 are higher than those measured in DMSO-d6. Compound 8b binds chlorides with the highest Ka in this series of receptors. We found that the trend of increasing strength of the host–guest interaction follows the increasing acidity of the NH amide proton (defined by the chemical shifts found in 1H NMR spectra) in the order: alkyl, benzyl, and phenyl amide derivatives. Similar trends were observed in benzoate anion binding, which was bound the weakest by aliphatic derivative 8a, and the strongest by phenyl derivative 8b and 2-pyridylmethylene derivative 12c, with 4150 M−1 and 5270 M−1 association constants, respectively.
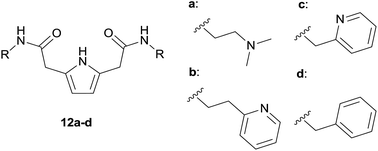 | ||
| Fig. 3 Receptors used investigated by Gale and coworkers.10 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes of 8a–b, and 12a–d with various anionsa
1 complexes of 8a–b, and 12a–d with various anionsa
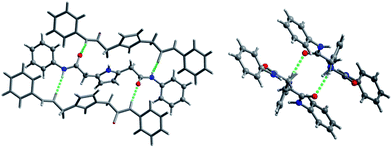
In order to confirm our rationalization of the linear binding system, we performed crystal structure analysis of receptor 8b. This showed that amide groups of the linear receptors are not coplanar with the pyrrole ring, but adopt the anti–anti conformation (Fig. 4). In consequence, contrary to its dipicolinic analogue (10), receptor 8b is not pre-organized for anion binding, hence the binding process requires a conformational change to adopt a pseudo syn–syn arrangement.
Summary
In conclusion, we have synthesized and studied the anion binding properties of both linear and macrocyclic receptors that incorporate the pyrrole-2,5-diacetamide building block. The linear receptors revealed relatively strong affinity for anions even in demanding solvents, e.g. DMSO + 0.5% H2O. The anion affinity of the linear pyrroleamide receptors strongly depends on the acidity of the amide NH protons, which can be controlled by different substitution of the side amide groups. The hybrid macrocyclic receptor constructed from both dipicolinic and pyrrole-2,5-diacetamidic building blocks showed superior affinity for anions as compared to the simple linear receptors, but inferior affinity as compared to the other hybrid receptors of this class. Overall, therefore, pyrrole-2,5-diacetamide further expands the repertoire of building blocks for the construction of linear and macrocyclic anion binding receptors.References
- (a) M. E. Moragues, R. Martínez-Máñez and F. Sancenón, Chem. Soc. Rev., 2011, 40(5), 2593–2643 RSC; (b) P. A. Gale and T. Gunnlaugsson, Chem. Soc. Rev., 2010, 39(10), 3595 RSC; (c) B. P. Hay, Chem. Soc. Rev., 2010, 39(10), 3700–3708 RSC; (d) P. A. Gale, Chem. Soc. Rev., 2010, 39(10), 3746 RSC.
- (a) N. Busschaert, C. Caltagirone, W. Van Rossom and P. A. Gale, Chem. Rev., 2015, 115(15), 8038–8155 CrossRef CAS PubMed; (b) P. Dydio, R. J. Detz, B. de Bruin and J. N. H. Reek, J. Am. Chem. Soc., 2014, 136, 8418–8429 CrossRef CAS PubMed; (c) P. Dydio, M. Ploeger and J. N. H. Reek, ACS Catal., 2013, 3, 2939–2942 CrossRef CAS; (d) P. Dydio, R. J. Detz and J. N. H. Reek, J. Am. Chem. Soc., 2013, 135, 10817–10828 CrossRef CAS PubMed; (e) P. Dydio and J. N. H. Reek, Angew. Chem., Int. Ed., 2013, 52, 3878–3882 CrossRef CAS PubMed; (f) P. Dydio, C. Rubay, T. Gadzikwa, M. Lutz and J. N. H. Reek, J. Am. Chem. Soc., 2011, 133, 17176–17179 CrossRef CAS PubMed; (g) P. Dydio, W. I. Dzik, M. Lutz, B. de Bruin and J. N. H. Reek, Angew. Chem., Int. Ed., 2011, 50, 396–400 CrossRef CAS PubMed.
- (a) F. Zapata, L. Gonzalez, A. Caballero, I. Alkorta, J. Elguero and P. Molina, Chem.–Eur. J., 2015, 21, 9797–9808 CrossRef CAS PubMed; (b) I. Saha, J. T. Lee and C.-H. Lee, Eur. J. Org. Chem., 2015, 2015, 3859–3885 CrossRef CAS; (c) S. J. Edwards, H. Valkenier, N. Busschaert, P. A. Gale and A. P. Davis, Angew. Chem., Int. Ed., 2015, 54, 4592–4596 CrossRef CAS PubMed; (d) G. Alibrandi, V. Amendola, G. Bergamaschi, L. Fabbrizzi and M. Licchelli, Org. Biomol. Chem., 2015, 13, 3510–3524 RSC; (e) L. Fabbrizzi and A. Poggi, Chem. Soc. Rev., 2013, 42, 1681–1699 RSC; (f) J. Cai and J. L. Sessler, Chem. Soc. Rev., 2014, 43, 6198 RSC; (g) S. K. Kim, V. M. Lynch and J. L. Sessler, Org. Lett., 2014, 16, 6128–6131 CrossRef CAS PubMed; (h) J. Y. C. Lim and P. D. Beer, Chem. Commun., 2015, 51, 3686–3688 RSC.
- (a) S. W. Robinson, C. L. Mustoe, N. G. White, A. Brown, A. L. Thompson, P. Kennepohl and P. D. Beer, J. Am. Chem. Soc., 2015, 137(1), 499–507 CrossRef CAS PubMed; (b) A. E. Hargrove, S. Nieto, T. Zhang, J. L. Sessler and E. V. Anslyn, Chem. Rev., 2011, 111(11), 6603–6782 CrossRef CAS PubMed; (c) P. A. Gale, Acc. Chem. Res., 2011, 44(3), 216–226 CrossRef CAS PubMed; (d) J. Cai and J. L. Sessler, Chem. Soc. Rev., 2014, 43, 6198–6213 RSC.
- V. Amendola, L. Fabbrizzi and L. Mosca, Chem. Soc. Rev., 2010, 39, 3889 RSC.
- T. Zieliński, P. Dydio and J. Jurczak, Tetrahedron, 2008, 64, 568–574 CrossRef.
- D. S. Kim and J. L. Sessler, Chem. Soc. Rev., 2015, 44(2), 532–546 RSC.
- (a) V. Amendola, L. Fabbrizzi and L. Mosca, Chem. Soc. Rev., 2010, 39(10), 3889–3915 RSC; (b) J. W. Steed, Chem. Soc. Rev., 2010, 39(10), 3686 RSC; (c) V. Blažek Bregović, N. Basarić and K. Mlinarić-Majerski, Coord. Chem. Rev., 2015, 295, 80–124 CrossRef.
- (a) T. Zieliński, M. Kedziorek and J. Jurczak, Chem.–Eur. J., 2007, 14, 838–846 Search PubMed; (b) L. S. Evans, P. A. Gale, M. E. Light and R. Quesada, New J. Chem., 2006, 30, 1019 RSC.
- R. Li, L. S. Evans, D. S. Larsen, P. a. Gale and S. Brooker, New J. Chem., 2004, 28, 1340 RSC.
- (a) M. J. Chmielewski and J. Jurczak, Tetrahedron Lett., 2004, 45(31), 6007–6010 CrossRef CAS; (b) M. J. Chmielewski and J. Jurczak, Chem.–Eur. J., 2005, 11, 6080–6094 CrossRef CAS PubMed; (c) M. J. Chmielewski and J. Jurczak, Chem.–Eur. J., 2006, 12, 7652–7667 CrossRef CAS PubMed.
- M. J. Chmielewski and J. Jurczak, Tetrahedron Lett., 2005, 46, 3085–3088 CrossRef CAS.
- (a) W. Flitsch and F.-J. Lüttig, Liebigs Ann. Chem., 1987, 893 CrossRef CAS; (b) F. Johnson, K. G. Paul, D. Favara, R. Ciabatti and U. Guzzi, J. Am. Chem. Soc., 1982, 104, 2190 CrossRef CAS; (c) R. Li, D. S. Larsen and S. Brooker, New J. Chem., 2003, 27, 1353 RSC.
- P. Dydio, D. Lichosyt and J. Jurczak, Chem. Soc. Rev., 2011, 40, 2971 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1457776. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra05804c |
| ‡ Present address: Department of Chemistry, University of California, Berkeley, California 94720, USA. |
| § Present address: SynthSys and School of Biological Sciences, University of Edinburgh, C. H. Waddington Building, Edinburgh EH9 3JD, UK. |
| ¶ Synthetic attempts to obtain the analogues of 4 that contain the pyrrole-2,5-dicarboxyamide unit in place of the pyrrole-2,5-diacetamide moiety were previously unsuccessful, hindering the evaluation of such putative anion receptors; for details, see: T. Zielinski, PhD Thesis, Institute of Organic Chemistry, Polish Academy of Sciences, Warsaw, 2006. |
| This journal is © The Royal Society of Chemistry 2016 |