Selective hydrogenation of paracetamol to acetamidocyclohexanone with silylated SiO2 supported Pd-based catalysts
Abstract
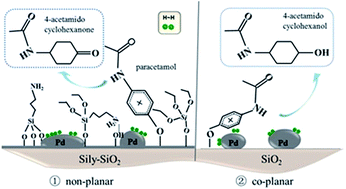
A series of catalysts comprising well-distributed Pd nanoparticles incorporated on silylated SiO2 were fabricated by the wet impregnation method and investigated in the selective hydrogenation of paracetamol to 4-acetamidocyclohexanone. The catalysts calcined at different temperatures were characterized by TG, FT-IR, N2 physisorption, TPR and XPS. The results showed that organic modification led to a catalyst surface composed of stable Si–(CH3)3 species even after calcination at 300 °C. Also, changes occurred in the size and electronic properties of the Pd particles through the different amounts of grafted groups on the SiO2 support. The mode of adsorption of the paracetamol molecule was influenced by the quite bulky organic groups on the support, resulting in a significant improvement in selectivity towards 4-acetamidocyclohexanone and preventing full hydrogenation to some extent. The best result was obtained on the silylated Pd catalyst calcined at 500 °C, with 64.9% selectivity to keto at the paracetamol conversion of 60.5%, while the non-silylated SiO2 supported Pd catalyst gave 4-acetamidocyclohexanone selectivity of 29.1% at 53.8% conversion.

 Please wait while we load your content...
Please wait while we load your content...