Crystalline structures in tetracosane–asphaltene films
Abstract
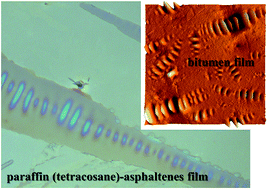
Waxes and asphaltenes are well known compounds in crude oil and related materials such as bituminous materials. In this study, the solid-like structures formed in mixtures of tetracosane, C24H50 paraffin, and asphaltenes were studied. The undulated structures that formed in films casted from dispersions of tetracosane and asphaltenes in toluene are identical to the undulated structures found on the surface of annealed bitumen films. Additionally, the growth of other crystalline forms was observed and the resulting structures also showed similarities with crystalline structures found in bitumen upon heating and cooling or increase in tetracosane content. The appearance of the undulated structures both after annealing of bitumen films and from solvent casting indicates that the undulated pattern is a result of phase behaviour and not of other physical phenomena such as buckling or wrinkling. To confirm this, in this work it is shown that tetracosane and asphaltenes segregate or demix, and mainly asphaltenes form the hills in the undulated structures. The results from this work will contribute to the understanding of the mechanism of the formation of wax–asphaltene structures which would be greatly appreciated by different communities working with petroleum-related materials.

 Please wait while we load your content...
Please wait while we load your content...