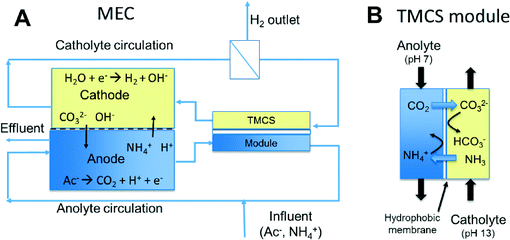
Gas-permeable hydrophobic membranes enable transport of CO2 and NH3 to improve performance of bioelectrochemical systems
Tom H. J. A.
Sleutels
*a,
Biense J.
Hoogland
a,
Philipp
Kuntke
a,
Annemiek
ter Heijne
b,
Cees J. N.
Buisman
ab and
Hubertus V. M.
Hamelers
a
aWetsus, European Centre of Excellence for Sustainable Water Technology, Oostergoweg 9, 8911 MA Leeuwarden, The Netherlands. E-mail: tom.sleutels@wetsus.nl
bSub-Department of Environmental Technology, Wageningen University, Bornse Weilanden 9, P.O. Box 17, 6700 AA Wageningen, The Netherlands
First published on 10th June 2016
Abstract
Application of bioelectrochemical systems (BESs), for example for the production of hydrogen from organic waste material, is limited by a high internal resistance, especially when ion exchange membranes are used. This leads to a limited current density and thus to large footprint and capital costs. Ion transport between anode and cathode compartment is one of the factors determining the internal resistance. The aim of this study was to reduce the resistance for ion transport in a microbial electrolysis cell (MEC) through the ion exchange membrane by shuttling of CO2 and NH3 between anode and cathode. The transport of these chemical species was enabled through the use of a hydrophobic TransMembraneChemiSorption module (TMCS) that was placed between anolyte and catholyte circulation outside the cell. The driving force for transport was the pH difference between both solutions. The transport of CO2 and NH3 resulted in an increase in current density from 2.1 to 4.1 A m−2 for a cation exchange membrane (CEM) and from 2.5 to 13.0 A m−2 for an anion exchange membrane (AEM) at 1 V applied voltage. The increase in current density was the result of a lower ion transport resistance through the membrane; this resistance was 60% lower for the CEM, as a result of NH3 recycling from cathode to anode, and 82% for the AEM, as a result of CO2 recycling from anode to cathode with TMCS, compared to experiments without TMCS.
Water impactA high internal resistance in bioelectrochemical systems (BESs) leads to energetic losses and are a major challenge for the widespread application of BESs. These energetic losses result in a lower energy production of microbial fuel cells and a higher energy demand of microbial electrolysis cells. The main factor influencing the internal resistance is the transport of ions through ion exchange membranes (IEMs). IEMs are employed in BESs to separate anode and cathode reaction and are essential to obtain high efficiencies. The application of gas permeable hydrophobic membranes for shuttling of reactive gaseous species (CO2 and NH3) between anode and cathode compartments can significantly lower this internal resistance of BESs. |
1. Introduction
Bioelectrochemical systems (BESs) are a promising emerging technology for the recovery of chemical energy present in, for example wastewaters,1,2 to produce useful products like electricity and hydrogen gas.3,4 In recent years, the range of products being produced from the energy from waste has extended to methane,5 hydrogen peroxide,6 caustics7 and metals.8,9 More recently these systems serve as a platform technology for electrosynthesis to produce a wide range of chemicals.10,11One of the main challenges for BESs being applied in practice is the high internal resistance of the system. For microbial fuel cells (MFCs) this internal resistance leads to limited energy recovery, while for microbial electrolysis cells (MECs) the production rate is too low leading to a high footprint and consequently to high investment costs.12
Previously it has been shown that addition of CO2 to the catholyte significantly improved performance of these systems.13–15 The added CO2 dissolves to form carbonic acid that is in equilibrium with carbonate or bicarbonate (depending on pH) and releases protons into solution. It was suggested that this release of protons reduces the pH gradient over the membrane and therefore the internal resistance was reduced.13,16 It has been suggested by Torres et al.13 that the CO2 produced at the anode could be shuttled to the cathode. So far, however, this concept has not been demonstrated.
Here, we present the use of a hydrophobic membrane module (TransMembraneChemiSorption or TMCS) to transport the CO2 and NH3 between anolyte and catholyte (Fig. 1B). This type of commercially available module is commonly used for the transfer of gas (e.g. CO2,17 NH3 (ref. 18) and H2S (ref. 17)) into or from water. The anolyte and catholyte are circulated though the two compartments of such a module separated by a hydrophobic layer. The driving force for the exchange of NH3 and CO2 between anolyte and catholyte in this process is the pH and concentration difference between the two solutions. Another advantage of using the TMCS module is that exchange of species is possible without direct contact between anolyte and catholyte and this way substrate/product crossover can be prevented.
We used the TMCS module in an MEC equipped with an anion exchange membrane (AEM) and a cation exchange membrane (CEM). We studied the transport of ions through the ion exchange membranes and the exchange of uncharged species through this TMCS module. The impact of the transport of ions and other species on the performance of the system was studied through an analysis of the partial internal resistances. All these analyses were done under steady state conditions.
2. Experimental section
2.1 Electrochemical cells
Two identical MECs were used, consisting of an anode and a cathode compartment separated by a CEM or an AEM (Ralex® CMH-PES and AMH-PES, MEGA a.s., Stráž pod Ralskem, Czech Republic). Each MEC had a membrane surface area of 100 cm2, same as the projected surface area for the electrodes. The anode consisted of a 4 mm thick and 95% porous carbon cloth (FMI Composites Ltd., Galashiels, Scotland) connected to the external circuit through a platinum/iridium wire (Pt80/Ir20). The anolyte flow was directed through this electrode by using a 1.75 mm thick spacer.19 The cathode consisted of a platinum coated titanium electrode with a mesh structure (Magneto Special Anodes, Schiedam, The Netherlands).The anodes were inoculated with the effluent from an MEC running on acetate. The influent fed to the anodes contained: 1.36 g L−1 NaCH3COO·3H2O, 0.74 g L−1 KCL, 0.58 g L−1 NaCl, 0.28 g L−1 NH4Cl, 0.1 g L−1 CaCl2 2H2O, 0.01 g L−1 MgSO4·7H2O, 0.87 g L−1 KH2PO4, 0.68 g L−1 K2HPO4 and 0.1 ml L−1 trace element mixture20 at a flow rate of 3 ml min−1. The total anolyte and catholyte volume was 700 ml, and both were recirculated at a rate of 100 ml min−1 (in case the TMCS was used also over the module). The initial catholyte contained 10 mM NaCl (pH 7). Data of pH, cell voltage, electrode potentials, and current were recorded continuously All potentials are measured and reported vs. Ag/AgCl (+205 mV vs. NHE), inserted in both electrolytes. The temperature of the cell was controlled at 303 K.
2.2 Experimental procedures and steady state conditions
With each reactor (one with AEM, one with CEM), two runs were performed. The first run was without, the second run with TMCS module. The TMCS module (TMCS MicroModule® 0.5 × 1 membrane contactor (Membrana, Wuppertal, Germany), 200 ml min−1 capacity) was connected outside the reactor, and anolyte and catholyte were recirculated on each side of the module to allow gas exchange between both liquids (Fig. 1).During start-up the bioanode was allowed to develop and reach stable anode potential and current production. After start-up, each experiment was started by refreshing the catholyte with a fresh 10 mM NaCl solution and applying −1 V between anode and cathode using a power source (Delta Elektronika ES 030-5). The experiment was finished when steady state conditions were reached; these steady state data are used in this manuscript. We defined steady state as the situation in which current density, pH and all other ion concentrations in anode and cathode compartment were constant, as previously extensively described in Sleutels et al. (2013).21
2.3 Analytical methods
During the experiment, ion composition of both anolyte and catholyte were analysed daily. Inorganic carbon (IC) was analysed using a TOC analyzer (Shimadzu TOC-VCPH) and acetate was analysed using an ion chromatograph (Metrohm Compact IC 761). Anolyte and catholyte were analysed for anion and cation content using ion chromatography (Metrohm Compact IC Flex 930). Total ammonium content was measured using Hach Lange (LCK303).2.4 Internal resistance calculations
Internal resistances were calculated to determine energetic losses in MECs as previously described by Sleutels et al. (2009).22 The reactions at the electrodes in an MEC are driven by applying a voltage to the system (Vapp; V) which results in a produced current density (I; A m−2). This applied voltage is partly reversible (Veq; V), i.e. harvested in the form of hydrogen, and partly irreversible, i.e. to drive the reactions at the electrodes (electrode overpotentials) and to transport ions through the solution and membrane (ohmic and transport losses). These irreversible losses can be considered as partial internal resistances of the system according to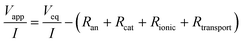 | (1) |
The reversible part of the voltage is harvested in the form of produced hydrogen gas and is determined by the thermodynamic potential difference between the oxidation (Ean; V) and reduction reaction (Ecat; V) calculated at pH 7 at the respective electrodes
| Eeq = Ecat − Ean | (2) |
The anode and cathode potential can be calculated using the Nernst equation as thoroughly explained by Logan et al. 2008.4 Here we used the actual concentrations of reactants and products, except for the pH which was set to 7.
These anode and cathode potentials can also be used to calculate the resistance of these reactions compared to the measured values of the electrodes (Ean, measured and Ecat, measured) using
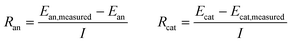 | (3) |
The ionic resistance is calculated as
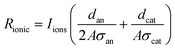 | (4) |
Finally, energy is lost to transport ions through the membrane (Rtransport); this resistance is calculated from all the other calculated and measured values. All these partial internal resistances together determine the total internal resistance of an MEC and will give a response in current density when a voltage is applied to the system.
3. Results and discussion
3.1 Recycling of CO2 and NH3 with the TMCS enhances current production
Fig. 2 shows the current density at −1 V applied voltage with and without TMCS, for both membranes. For the MEC with CEM, the TMCS module led to an increase in current density from 2.1 to 4.1 A m−2. For the MEC with AEM, the TMCS module led to an increase in current density from 2.5 to 13.0 A m−2. The increased current density for the MEC with AEM is in line with previous work13,15 where CO2 was added to the cathode. With the TMCS however, we show an effective means of transporting the CO2 produced at the anode to the cathode.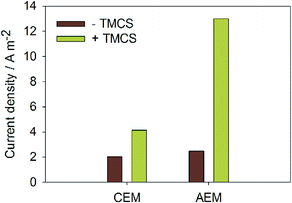 | ||
| Fig. 2 Produced current densities (A m−2) for both AEM and CEM, with and without TMCS module. Transport of CO2 and NH3via the TMCS module results in a higher current at the same applied voltage. | ||
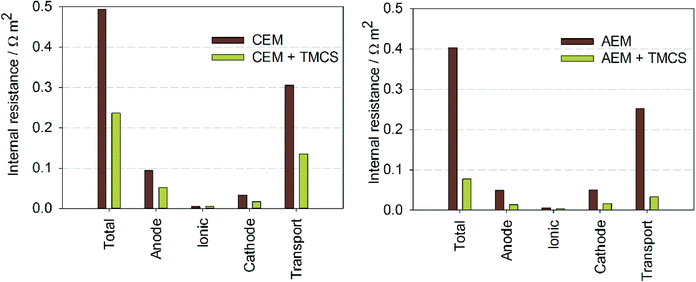
Internal resistances were calculated for all experiments (Fig. 3) to study which resistance was mostly affected by the TMCS. The total internal resistance was reduced from 0.49 to 0.34 Ω m2 by the TMCS for the CEM, and from 0.40 to 0.077 Ω m2 for the AEM. Fig. 3 also shows the partial internal resistances of the system for all experiments. Both for the CEM and the AEM, the change in internal resistance with TMCS is mainly caused by the decrease in the ion transport resistance, although also a slight decrease in anode and cathode resistance was observed. Although it has been shown before that MECs with an AEM have a lower internal resistance compared to systems with a CEM,22,23 the use of a CEM still finds a promising application in, for example, the recovery of ammonia from urine.24,25
3.2 Transport of uncharged species that can carry protons reduces the ion transport resistance
In steady state conditions protons are transported through the CEM from anode to cathode and hydroxyl is transported through the AEM from cathode to anode.22 By connecting the TMCS module between anolyte and catholyte, uncharged chemical species, like CO2 and NH3, can be transported without contributing to the charge transport. This transport is thus not driven by the electric field, but by concentration gradient, which is for both species directly related to pH. For the CEM, we found that the transport through the TMCS was mostly in the form of NH3, while for the AEM it was mostly in the form of CO2, although for both membranes a combination of NH3 and CO2 was transported.Fig. 4A shows the mechanisms of NH3 transport. During MEC operation with a CEM, NH4+ is transported from anode to cathode, driven by the electric current. Because pH in the cathode was around 13 at steady state, NH4+ dissociates into NH3 and H+. NH3, in gaseous form, is then transported back to the anode through the TMCS module. The driving force for NH3 transport is the lower pH 7 at the anode, resulting in low NH3 concentration in the anolyte compared to the catholyte. In the anolyte, NH3 forms NH4+ and becomes available for ion transport through the CEM again. Table 1 shows an overview of the steady state concentrations (in mM) of inorganic carbon (HCO3−/CO32−), NH4+/NH3, and pH in anode and cathode. Without TMCS module, NH4+/NH3 accumulates in the cathode and reaches a concentration of 49 mM. With the TMCS module, the final concentration of NH4+/NH3 is only 0.6 mM. Partly, the NH3 is transported back to the anolyte, although also part of this lower concentration can be explained by the equilibrium between gaseous and dissolved NH3. With the TMCS module, not only NH4+/NH3 decreases, but also the HCO3−/CO32− concentration increases in the catholyte (1.6 mM without TMCS and 95 mM with TMCS). The higher concentration in the catholyte results in a reduced cathodic resistance, likely as a result of higher buffer capacity for the cathodic reduction reaction26 (Fig. 3).
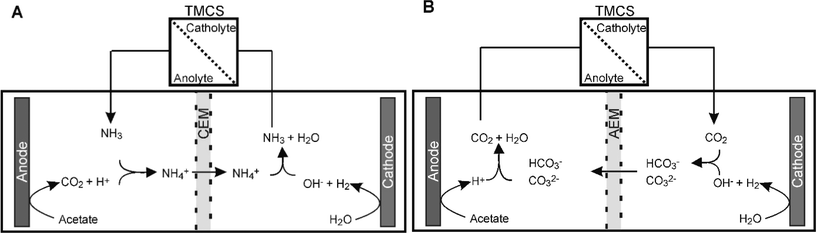 | ||
| Fig. 4 NH3 transport in an MEC with a CEM (A) and CO2 transport in an MEC with an AEM (B). These ions are exchanged between anolyte and catholyte in a hydrophobic membrane module. | ||
| CEM | CEM + TMCS | AEM | AEM + TMCS | |||||
|---|---|---|---|---|---|---|---|---|
| A | C | A | C | A | C | A | C | |
| A: anode compartment. C: cathode compartment. | ||||||||
| HCO3−/CO32− | 3.1 | 1.6 | 6.2 | 95.0 | 7.1 | 0.9 | 10.3 | 18.9 |
| NH4+ | 3.4 | 49.1 | 3.3 | 0.6 | 4.3 | 39.8 | 4.0 | 10.3 |
| pH | 6.38 | 12.6 | 7.03 | 13.4 | 6.05 | 12.7 | 6.66 | 12.91 |
The ammonium transport was further investigated by introducing the TMCS module after the steady state period of the control experiment without TMCS. In this control experiment, the NH4+/NH3 concentration in the catholyte was 49 mM without the TMCS module and after one day with the TMCS module, the NH4+/NH3 concentration decreased to 3.4 mM (not shown in table). This shows that NH4+/NH3 was indeed removed from the cathode and transported through the TMCS back to the anode compartment.
In case the TMCS module was used, hardly any crossover of hydrogen was detected between anode and cathode because of the low solubility of hydrogen in water (saturated concentration of 0.7 mM at 303 K) and because the module was placed after the recycling vessel where hydrogen was released from the system (Fig. 1). Even if hydrogen would pass through the TMCS module, this could not explain the increase in current densities in these experiments.
For the AEM, a similar effect was observed. The NH4+ concentration in the cathode was higher without (39.8 mM) than with (10.3 mM) TMCS module, indicating that ammonia is transported from catholyte to anolyte via the TMCS. The CO2 concentration in the cathode was higher with (18.9 mM) than without (0.9 mM) TMCS module, indicating that CO2 was transported from anode to cathode. For the AEM however, higher concentrations of CO32−, a bivalent negatively charged ion, in the cathode, results in lower ion transport resistance (Fig. 3), because CO32− is easily transported through an AEM. Also, because this bivalent ion is transported back directly from cathode to anode, the overall concentration in the cathode is relatively low compared to the concentration of ammonia.
To further prove that CO32− was transported from the cathode to the anode through the AEM, the TMCS was removed after the steady state period. In a 2 day period after the TMCS was removed, the CO32− concentration in the catholyte decreased from 18.9 mM to 11.5 mM (not shown in table). This decrease in CO32− concentration corresponded to 12% of the total ion transport through the AEM compared to the produced current density, showing that indeed, CO32− was one of the main species transported through the AEM in addition to hydroxyl.
One could expect that the transport of NH3 and CO2 between anode and cathode could decrease the pH gradient over the membrane. In all experiments presented here however, the cell voltage was controlled at −1 V. In case the TMCS module was used, and the internal resistance decreased due to the enhanced ion transport, an increase in current production was observed. As a result of this higher current, the pH gradient over the membrane remains comparable to the pH gradient when the module was not used.
4. Conclusions
We show that the transport of CO2 from anode to cathode via a TMCS module decreases the internal resistance of MECs. The mechanism is that chemical species are exchanged between anolyte and catholyte, thereby introducing extra ionic species as charge carrier in the electrolyte. To further optimize and understand the effect of TMCS on ion transport, future work will combine experimental data with the recently developed theoretical framework27 that describes ion transport as function of pH and different membranes.Here, the TMCS module improved MEC performance but application of the module could easily be extended to the MFC since the ion transport through the system relies on the same principles; also for MFCs, the transport of CO2 and NH3 may enhance energy efficiency. Using the TMCS to enhance ion transport in BESs shows great promise for practical application.
Acknowledgements
This work was performed in the cooperation framework of Wetsus, European Centre of Excellence for Sustainable Water Technology (http://www.wetsus.eu). Wetsus is co-funded by the Dutch Ministry of Economic Affairs and Ministry of Infrastructure and Environment, the Province of Fryslân, and the Northern Netherlands Provinces. The authors like to thank the participants of the Resource Recovery research theme for the fruitful discussions and their financial support.References
- K. Rabaey and W. Verstraete, Microbial fuel cells: novel biotechnology for energy generation, Trends Biotechnol., 2005, 23(6), 291–298 CrossRef CAS PubMed.
- K. Rabaey, L. Angenent, U. Schröder and J. Keller, Bioelectrochemical systems: from extracellular electron transfer to biotechnological application, Integr. Environ. Technol. Ser. 2010, No. July 2015, p. 488 Search PubMed.
- B. E. Logan, B. Hamelers, R. Rozendal, U. Schröder, J. Keller, S. Freguia, P. Aelterman, W. Verstraete and K. Rabaey, Microbial fuel cells: Methodology and technology, Environ. Sci. Technol., 2006, 40(17), 5181–5192 CrossRef CAS PubMed.
- B. E. Logan, D. Call, S. Cheng, H. V. M. Hamelers, T. H. J. A. Sleutels, A. W. Jeremiasse and R. A. Rozendal, Microbial Electrolysis Cells for High Yield Hydrogen Gas Production from Organic Matter, Environ. Sci. Technol., 2008, 42(23), 8630–8640 CrossRef CAS PubMed.
- S. Cheng, D. Xing, D. F. Call and B. E. Logan, Direct biological conversion of electrical current into methane by electromethanogenesis, Environ. Sci. Technol., 2009, 43(10), 3953–3958 CrossRef CAS PubMed.
- R. A. Rozendal, E. Leone, J. Keller and K. Rabaey, Efficient hydrogen peroxide generation from organic matter in a bioelectrochemical system, Electrochem. Commun., 2009, 11(9), 1752–1755 CrossRef CAS.
- K. Rabaey, S. Bützer, S. Brown, J. Keller and R. A. Rozendal, High current generation coupled to caustic production using a lamellar bioelectrochemical system, Environ. Sci. Technol., 2010, 44(11), 4315–4321 CrossRef CAS PubMed.
- H. Wang and Z. J. Ren, Bioelectrochemical metal recovery from wastewater: a review, Water Res., 2014, 66, 219–232 CrossRef CAS PubMed.
- E. Ntagia, P. Rodenas, A. Ter Heijne, C. J. N. Buisman and T. H. J. A. Sleutels, Hydrogen as electron donor for copper removal in bioelectrochemical systems Hydrogen as electron donor for copper removal in bioelectrochemical systems, Int. J. Hydrogen Energy, 2016, 1–7 Search PubMed.
- K. Rabaey and R. A. Rozendal, Microbial electrosynthesis - revisiting the electrical route for microbial production, Nat. Rev. Microbiol., 2010, 8(10), 706–716 CrossRef CAS PubMed.
- B. E. Logan and K. Rabaey, Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies, Science, 2012, 337(6095), 686–690 CrossRef CAS PubMed.
- T. H. J. A. Sleutels, A. Ter Heijne, C. J. N. Buisman, H. V. M. Hamelers and A. Ter Heijne, Bioelectrochemical systems: An outlook for practical applications, ChemSusChem, 2012, 5(6), 1012–1019 CrossRef CAS PubMed.
- C. I. Torres, H.-S. S. Lee and B. E. Rittmann, Carbonate species as OH- carriers for decreasing the pH gradient between cathode and anode in biological fuel cells, Environ. Sci. Technol., 2008, 42(23), 8773–8777 CrossRef CAS PubMed.
- S. Ishizaki, I. Fujiki, D. Sano and S. Okabe, External CO2 and water supplies for enhancing electrical power generation of air-cathode microbial fuel cells, Environ. Sci. Technol., 2014, 48(19), 11204–11210 CrossRef CAS PubMed.
- J. J. Fornero, M. Rosenbaum, M. A. Cotta and L. T. Angenent, Carbon dioxide addition to microbial fuel cell cathodes maintains sustainable catholyte pH and improves anolyte pH, alkalinity, and conductivity, Environ. Sci. Technol., 2010, 44(7), 2728–2734 CrossRef CAS PubMed.
- D. Ki, S. C. Popat and C. I. Torres, Reduced overpotentials in microbial electrolysis cells through improved design, operation, and electrochemical characterization, Chem. Eng. J., 2016, 287, 181–188 CrossRef CAS.
- Membrana, Liqui-Cel Membrane contactors Liquid Degassing & Gasification Solutions, 2010 Search PubMed.
- P. Kuntke, P. Zamora, M. Saakes, C. J. N. Buisman and H. V. Hamelers, Gas-permeable hydrophobic tubular membranes for ammonia recovery in Bio-Electrochemical Systems, Environ. Sci.: Water Res. Technol., 2016, 2, 261–265 CAS.
- T. H. J. A. Sleutels, R. Lodder, H. V. M. Hamelers and C. J. N. Buisman, Improved performance of porous bio-anodes in microbial electrolysis cells by enhancing mass and charge transport, Int. J. Hydrogen Energy, 2009, 34(24), 9655–9661 CrossRef CAS.
- A. J. B. Zehnder, B. A. Huser, T. D. Brock and K. Wuhrmann, Characterization of an acetate-decarboxylating, non-hydrogen-oxidizing methane bacterium, Arch. Microbiol., 1980, 124(1), 1–11 CrossRef CAS PubMed.
- T. H. J. A. Sleutels, A. Ter Heijne, C. J. N. Buisman and H. V. M. Hamelers, Steady-state performance and chemical efficiency of Microbial Electrolysis Cells, Int. J. Hydrogen Energy, 2013, 38(18), 7201–7208 CrossRef CAS.
- T. H. J. A. Sleutels, H. V. M. V. M. Hamelers, R. A. R. A. Rozendal and C. J. N. J. N. Buisman, Ion transport resistance in Microbial Electrolysis Cells with anion and cation exchange membranes, Int. J. Hydrogen Energy, 2009, 34(9), 3612–3620 CrossRef CAS.
- J. R. Kim, S. Cheng, S.-E. Oh and B. E. Logan, Power Generation Using Different Cation, Anion, and Ultrafiltration Membranes in Microbial Fuel Cells, Environ. Sci. Technol., 2007, 41(3), 1004–1009 CrossRef CAS PubMed.
- P. Kuntke, K. M. Śmiech, H. Bruning, G. Zeeman, M. Saakes, T. H. J. A. Sleutels, H. V. M. Hamelers, C. J. N. Buisman and K. M. Smiech, Ammonium recovery and energy production from urine by a microbial fuel cell, Water Res., 2012, 46(8), 2627–2636 CrossRef CAS PubMed.
- P. Kuntke, T. H. J. Sleutels, M. Saakes and C. J. N. Buisman, Hydrogen production and ammonium recovery from urine by a Microbial Electrolysis Cell, Int. J. Hydrogen Energy, 2014, 39(10), 4771–4778 CrossRef CAS.
- A. W. Jeremiasse, H. V. M. Hamelers, J. M. Kleijn and C. J. N. Buisman, Use of Biocompatible Buffers to Reduce the Concentration Overpotential for Hydrogen Evolution, Environ. Sci. Technol., 2009, 43(17), 6882–6887 CrossRef CAS PubMed.
- J. E. Dykstra, P. M. Biesheuvel, H. Bruning and A. Ter Heijne, Theory of ion transport with fast acid–base equilibrations in bioelectrochemical systems, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2014, 90(1), 013302 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |