The occurrence of methyl, ethyl, propyl, and butyl parabens in the urban rivers and stormwaters of Sydney, Australia†
Wendy A.
Evans
a,
Peter J.
Davies
*b and
Christopher
McRae
c
aMacquarie University, NSW 2109, Australia. E-mail: wendy.evans@students.mq.edu.au
bMacquarie University, NSW 2109, Australia. E-mail: peter.davies@mq.edu.au; Tel: +61 2 98507220
cMacquarie University, NSW 2109, Australia. E-mail: christopher.mcrae@mq.edu.au
First published on 8th June 2016
Abstract
Parabens, commonly used preservatives, are emerging pollutants that are known to enter waterways through the wastewater system where they can pose a threat to aquatic organisms. Less is known about their presence and contribution to urban waterways in cities with separated stormwater and wastewater systems, such as Sydney, Australia. We measured the occurrence of methyl-(MeP), ethyl-(EtP), propyl-(PrP) and butyl-(BuP) parabens in urban river and stormwater samples across a range of land uses, using solid-phase microextraction with gas chromatography-mass spectrometry for the analysis. MeP in modified stormwater channels was more frequently detected and found at higher mean levels (6.29 μg L−1) than in urban rivers (3.62 μg L−1). Waterways in residential catchments had greater mean total paraben load (26.87 μg L−1) when compared to parkland catchments (12.71 μg L−1) and bushland catchments (2.10 μg L−1). EtP had the highest peak concentrations across the study area (max = 305.55 μg L−1) associated with industrial land uses and areas historically associated with poor water quality. The levels of EtP were relatively high when compared to international studies in cities with combined stormwater and wastewater systems. The results also suggest overflows from the sewer during heavy rain are likely not as significant when compared to the contribution from urban runoff. The study did not reveal the source of the EtP and further studies are recommended to identify this and the potential environmental impact.
Water impactThis is the first study describing the geographic distribution of four parabens in the urban waterways of Sydney. Unlike many studied cities, Sydney has separated stormwater and wastewater systems giving it international relevance. Differences in paraben concentrations were identified between stormwater, rivers, and in relation to land uses that have implications for urban water management and highlight areas for further investigation. |
1.0 Introduction
Parabens are the alkyl esters of p-hydroxybenzoic acid. Common esters include methyl-(MeP), ethyl-(EtP), propyl-(PrP), and butyl-(BuP) parabens and their sodium salts.1 These compounds have been added as an antimicrobial and preservative agent since the 1920s in a wide range of personal care products (PCPs) such as shampoos, deodorants, shower gels, sunscreens, and cosmetics, but they are also found in pharmaceuticals, food, paper products, and packaging.2–8 Their popularity and wide spread use is largely due to their cost effectiveness, ease of use, and perceived overall safety.9–11In 2004 Darbre, et al., identified a potential risk associated with parabens and human health due to their mild oestrogenic properties and presence in breast cancer tissue.12 While a causal link between breast cancer and parabens is still under investigation,13,14 Charles & Darbre (2013) identified the growth of breast cancer MCF-7 cells in vitro when found in combination with parabens.15 In 2011 Meeker, et al., reported a link with sperm damage in men.16 These studies have served as the catalyst for the European Union to place a limit on parabens in food products17 and Denmark to also limit parabens on the import, sale and use of in PCP and in products used by children under the age of three.18
Parabens have also been implicated in endocrine disruption in other organisms11,19 including adverse effects on the male reproductive system such as reduced sperm count in rodents20,21 and imposex and fertility problems in fish.22,23 Additionally Terasaki, et al. (2009) reported that parabens can be toxic to aquatic organisms such as daphnia.24 Toxicological research on acute and long term low-level exposure, however, is sparse. Although the oestrogenic activity of parabens is relatively weak when compared to that of the naturally occurring steroid and oestrogen hormone 17β-oestradiol,25 they also readily react with free chlorine in tap water, raw wastewater and where chlorine is used in water treatment.24,61–63 The resulting chlorinated by-products have shown higher acute toxicity responses in Daphnia,24 and this affect should be considered where parabens are entering the aquatic environment, although more study in this area is required.25
Parabens can enter the environment through a variety of means including via wastewater.4,26–32 They have also been detected in house dust,33,66 soil,34 rivers and lakes,26–29,31,32,35–43 and in marine sediments.44 The presence of parabens in surface waters has largely been attributed to leaks, overflows, and discharges of wastewater. Other sources include industrial discharges, leachate from landfill, and surface runoff. The extent of these pathways is yet to be fully described. Concentrations in surface waters are typically low and, as an emerging pollutant, significant research has been directed to extraction techniques.4,25,32,45
In this study the presence and concentration of MeP, EtP, PrP, and BuP are examined across urban waterways including rivers and stormwater systems in Sydney, Australia. Sydney is unique when compared to many international urban study areas in that the stormwater and wastewater systems are independent of each other.46 The study also aims to identify if there are correlations between the type and concentration of parabens related to land use and catchment area.
2.0 Materials and methods
2.1 Study area and site selection
The urban water environments and stormwater drainage systems within the Sydney metropolitan area were the focus of this study (Table 2, Appendix 1). Sydney has a separated wastewater and stormwater system:46,47 the stormwater system is designed to convey urban runoff and drains to local waterways and the ocean. Stormwater assets in Sydney are owned and managed primarily by local government. The wastewater system is owned and operated by Sydney Water Corporation and treats more than 1.3 billion liters of wastewater each day.48 The wastewater system has over 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km of wastewater pipes with effluent treated via inland and mostly coastal sewerage treatment plants.49 Some mixing of the stormwater and wastewater systems occurs via infiltration and exfiltration from the extensive pipe network and directly into and from waterways. Designed wastewater overflows are discharged into urban waterways during periods of heavy rain and form part of the environmental pollution licence Sydney Water Corporation has with the NSW Government.50
000 km of wastewater pipes with effluent treated via inland and mostly coastal sewerage treatment plants.49 Some mixing of the stormwater and wastewater systems occurs via infiltration and exfiltration from the extensive pipe network and directly into and from waterways. Designed wastewater overflows are discharged into urban waterways during periods of heavy rain and form part of the environmental pollution licence Sydney Water Corporation has with the NSW Government.50
Sample sites were selected to cover catchments with various land uses, as delineated geographically by the Australian Bureau of Statistics (refer to Tables 2 and 3 for a summary of sample sites and their land use types).51 These uses comprised of: parkland, including bushland, reserves, and national parks; residential, including areas of low to high density housing; commercial uses including CBDs, educational facilities, and shopping complexes; industrial sites that included factories associated with manufacturing (incorporating sites with bio-retention systems and rain gardens), waste management services (landfills), processing, and construction; and post-treated sewage treatment plant (STP) discharge.
Sampling sites were also classified by the physical type. These included: stormwater, which incorporated locations from, or directly downstream of pipes, drains, and channels specifically designed to convey excess urban surface runoff associated with rainfall; rivers, which ranged from natural unmodified bushland catchments to highly urban rivers with modified banks; and sites upstream and downstream from STP discharge points. Only post treated urban wastewater was sampled as access to STP inlet waste water was not possible.
2.2 Sample collection
Water samples (n = 72) were collected between July and September 2014. Samples were taken from waterway edges/banks as far into the flow as possible, from bridges, directly from stormwater discharge points and storage tanks. The sample collection method followed that used by Peng (2008).29 Samples were collected in clean (non-sterilised, so as to reduce potential contamination from detergents) 40 mL amber glass bottles to reduce the likelihood of photodegradation.64 Sampling occurred from banks or bridges at a water depth of <1 m as parabens tend to concentrate in the top of the water column.29 Before sample collection, each bottle was pre-rinsed with sample water three times. However, unlike Peng (2008), microbial biodegradation suppressant, sodium azide, was not added to the samples as any present parabens were unlikely to undergo biodegradation due to their own antimicrobial properties.9 Samples were stored at 4 °C and the sample storage and hold times followed EPA (2007) guidelines52 for the testing of personal care products in water.34 samples were taken from rivers, 34 from stormwater systems and 4 from post-treated STP discharge points. Sampling from tidal rivers was undertaken during the ebbing period in order to reduce the dilution effect of tidal influx. The coordinates of each sample site were recorded using GPS.
2.3 Materials
Four esters of 4-hydroxybenzoic acid were used in this study. Methyl paraben (C8H8O3) analytical standard and ethyl (C9H10O3), propyl (C10H12O3) and butyl (C11H14O3) paraben secondary standards, were purchased from Sigma-Aldrich (Sydney, NSW, Australia). Methanol (CH₃OH) and ethyl acetate (C4H8O2) were from Honeywell, sodium chloride (NaCl) was from Univar. Glassware, including unsterilized 60 mL amber glass headspace injector vials and un-bonded PTFE/Silicone Septa, were purchased from Velocity Scientific (Perth, WA, Australia). SPME holder and fibers coated with polyacrylate (PA; 85 μm film thickness) were obtained from Supelco (via Sigma-Aldrich, Sydney, Australia). Prior to use the fibers were prepared as per the manufacturer's instructions. Standard solutions (between 2–50 μg L−1) were prepared in a mixture of methanol and RO water and stored at 4 °C in a fridge.The GPS (Garmin) was set to WGS 84 map datum and data was removed using MapSource software version 6.16.3. All spatial analysis was undertaken using ArcGIS 10.2 software. The map layers (shape files) included the Sydney Special (hydrography and framework) from Geoscience Australia (2004),53 and NSW Land Use Mesh Blocks from the Australian Bureau of Statistics (2011).51
2.4 Extraction and analysis
The pH of each sample was recorded using a Hanna pH 211 microprocessor to ensure the samples were within the operating range of the SPME fibers. The extraction technique used was modeled on that used by Canosa et al. (2006). Final micro-extractions were accomplished using a manual solid-phase microextraction (SPME) holder and PA fibers in 40 mL volume glass vessels containing a magnetic Teflon coated stir bar and filled with 10 mL of water sample. Sodium chloride content was added at a rate of 150 mg mL−1 to assist diffusion of the analytes to the fibre.4 However, unlike the method derived by Canosa et al. (2006), derivatisation for detection enhancement using MTBSTFA was not undertaken as the tert-butyldimethylsilyl derivative would likely decrease the volatility of the analytes and increase the risk of error, especially in this case where derivatisation would have been completed on fibre. Prior to the analysis of samples, the GC-MS was evaluated using a Grob Test Mix (Restek Cat.#: 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). The test mix showed excellent peak shape (asymmetry was <1.2 on all peaks of interest) and recoveries for the alcohol and phenol components of the mix, including for chlorophenol, which is much more acidic than the paraben analytes, and no loss or tailing was observed for this compound. For these reasons derivatisation was deemed unnecessary.
000). The test mix showed excellent peak shape (asymmetry was <1.2 on all peaks of interest) and recoveries for the alcohol and phenol components of the mix, including for chlorophenol, which is much more acidic than the paraben analytes, and no loss or tailing was observed for this compound. For these reasons derivatisation was deemed unnecessary.
SPME extractions were carried out manually in the direct sampling mode, at room temperature (21.7 °C, 70% humidity), using a PA fiber. During extraction the fibers were fully submerged in the samples. After an exposure period of 10 minutes, which was determined by the authors to be the optimum exposure time for analytes to be absorbed across the PA fiber, the SPME fiber was retracted into the needle of the holder syringe, water drops attached to the metallic needle were removed using a soft paper tissue.
The analysis technique developed by the authors was undertaken using a gas chromatography-mass spectrometry (GC-MS) on a Shimadzu GC-17A gas chromatograph fitted with a Restek Rtx-5Sil capillary column (30 m × 0.25 mm, 0.25 μm film thickness) interfaced to a Shimadzu QP-5000 mass selective detector. A constant carrier (99.999% helium – velocity of 40 cm s−1) was used, the injector was held at 270 °C, and the transfer line held at 300 °C.
Samples were introduced by thermal desorption into a split/splitless injector fitted with a SPME specific injector liner (0.75 mm × 5 mm × 95 mm). The injector was operated in splitless mode with a sampling time of 5 minutes. The mass spectrometer was run in selected ion monitoring (SIM) mode monitoring ions m/z 121 and m/z 149 over a 0.2 second sampling interval. Ion m/z 149 was used to monitor the presence of phthalates which elute in the same region as parabens. Ion m/z 121 was used for both qualitative and quantitative purposes and m/z 149 was used to qualitatively identify phthalates. Identification of the paraben analytes was confirmed by co-elution with the internal surrogates (monitored at m/z 127), thus allowing for the monitoring of two ions.
The oven program used was 70 °C for 5 minutes, then ramped at 30 °C per minute to 140 °C, and then ramped at 10 °C per minute to 220 °C before a final ramp at 30 °C per minute to 300 °C and held for 5 minutes. Total run time was 23 minutes. Fibers were desorbed between runs.
Quantification was accomplished using an internal standard method, using a 13C labelled analogue containing methyl 4-hydroxybenzoate-(ring-13C6), ethyl 4-hydroxybenzoate-(ring-13C6), propyl 4-hydroxybenzoate-(ring-13C6), and butyl 4-hydroxybenzoate-(ring-13C6), as an internal surrogate. Calibration was achieved against absolute standard solutions of the analytes in RO water. A 6-point calibration curve (2.00–100.0 μg L−1) was created for each paraben ester. The detection limits (LOD) of the method ranged from 1.00–2.00 μg L−1 for the analytes. The calibration showed a linear regression with correlation coefficients (R2) ranging from 0.946 to 0.996. The repeatability (RSD, n = 3) was between 6.07–12.0% (see Table 1).
| Linearity, repeatability and quantification limits of the method – 10 μg L−1 | ||||
|---|---|---|---|---|
| Compound | MeP | EtP | PrP | BuP |
| Spiked RO samples have been prepared using standards made up to 10, 20 and 100 parts per billion (μg L−1). Target and result shown. | ||||
| Correlation coefficient (R2) | 0.947 | 0.993 | 0.996 | 0.985 |
| Repeatability (RSD%) | 12.0 | 6.57 | 6.07 | 11.8 |
| Limit of detection (μg L−1) | 1.00 | 2.00 | 1.00 | 1.00 |
| Spiked sample 1 (10 μg L−1) | 9.3 | 8.7 | 8.8 | 8.0 |
| Spiked sample 2 (20 μg L−1) | 20.8 | 21.5 | 21.4 | 22.3 |
| Spiked sample 3 (100 μg L−1) | 99.9 | 99.8 | 99.8 | 99.7 |
2.5 QA/QC
Extraction blanks (10 mL of RO water) and spiked blanks (between 2.00–10 μg L−1 of each analyte in 10 mL of RO water) were included in runs of samples. RO water was tested as a sample before use and no parabens were found within the detection limits of the GC-MS. The GC septum was changed every 20 injections to reduce the chance of leaks and fibers were desorbed between runs. A number of samples (n = 5) were re-processed and no significant difference was found between the runs. To reduce the likelihood of sample contamination, personal care items (e.g. moisturizers and detergents that contained parabens) were avoided during sample collection, preparation, and analysis.3.0 Results
At least two parabens were found at all sites with the exception of a bushland catchment in the parkland land use category which was used as a reference point. Levels of MeP across the 72 sample sites ranged between 4.62 μg L−1 and 13.78 μg L−1 with a mean of 5.11 μg L−1 and a median of 5.75 μg L−1 (Table 2). MeP was the least frequently detected analyte (n = 50) (Table 3). EtP ranged between 2.75 μg L−1 and 305.55 μg L−1 with a mean of 13.81 μg L−1 and a median of 5.17 μg L−1. EtP was the second most frequently detected paraben found at 68 sites and included the 9 highest readings of parabens at any of the sites (between 13.78–305.55 μg L−1) which were associated with industrial areas. PrP (n = 59) ranged from 2.42 μg L−1 to 8.29 μg L−1 with a mean of 2.79 μg L−1 and a median of 2.87 μg L−1 and was persistent at low levels in the sample area. BuP was found at levels between 3.86 μg L−1 and 8.47 μg L−1 with a mean of 4.31 μg L−1 and a median of 4.36 μg L−1 and was detected the most frequently (n = 69).| Site | Latitude | Longitude | Type | pH | Land use | Detected parabens μg L−1 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| MeP | EtP | PrP | BuP | Total load | ||||||
| n.d. Not detected at sample site. | ||||||||||
| 001 | −33.7874 | 151.1116 | Stormwater | 6.13 | Residential | 7.31 | 5.65 | 5.61 | 4.41 | 22.99 |
| 002 | −33.7941 | 151.1098 | River | 6.34 | Residential | 6.38 | n.d. | 5.60 | 4.34 | 16.31 |
| 003 | −33.7228 | 151.1461 | Stormwater | 7.35 | Residential | 7.66 | 5.98 | n.d. | 4.42 | 18.06 |
| 004 | −33.8917 | 151.2819 | Stormwater | 7.95 | Residential | 7.95 | 5.72 | 5.59 | 4.40 | 23.67 |
| 005 | −33.8964 | 151.2740 | Stormwater | 7.25 | Residential | 7.81 | n.d. | 5.70 | 4.39 | 17.91 |
| 006 | −33.8981 | 151.2756 | Stormwater | 7.16 | Residential | 7.86 | 5.74 | 5.60 | 4.41 | 23.61 |
| 007 | −33.8992 | 151.2725 | Stormwater | 8.02 | Residential | 7.43 | 5.76 | 5.62 | 4.36 | 23.17 |
| 008 | −33.8971 | 151.0853 | Stormwater | 8.16 | Industrial | 9.52 | 58.50 | 5.20 | 5.11 | 78.33 |
| 009 | −33.9043 | 151.1104 | Stormwater | 9.27 | Residential | 10.82 | 54.57 | 6.22 | 5.53 | 77.15 |
| 010 | −33.9142 | 151.1237 | River | 7.82 | Residential | 12.51 | 35.17 | 5.12 | 5.92 | 58.72 |
| 011 | −33.9230 | 151.1427 | River | 7.40 | Residential | 9.51 | 27.28 | 3.83 | 5.20 | 45.81 |
| 012 | −33.9279 | 151.1589 | River | 6.70 | Industrial | 7.14 | 14.07 | 3.19 | 4.50 | 28.90 |
| 013 | −33.8891 | 151.0770 | Stormwater | 7.50 | Industrial | n.d. | 6.32 | 2.69 | 4.04 | 13.05 |
| 014 | −33.8995 | 151.0786 | Stormwater | 8.00 | Industrial | n.d. | 7.82 | 2.60 | 4.06 | 14.48 |
| 015 | −33.9152 | 151.1204 | River | 9.67 | Industrial | 6.60 | 8.33 | 2.87 | 4.40 | 22.20 |
| 016 | −33.9262 | 151.0971 | Stormwater | 8.00 | Industrial | 5.78 | 5.16 | 2.42 | 4.10 | 17.45 |
| 017 | −33.9382 | 151.1028 | Stormwater | 9.67 | Industrial | n.d. | 3.66 | 2.46 | 3.89 | 10.01 |
| 018a | −33.9297 | 151.1382 | River | 8.03 | Industrial | n.d. | 5.18 | 8.29 | 4.36 | 17.83 |
| 018b | −33.9297 | 151.1382 | River | 7.96 | Industrial | 6.17 | 4.52 | 2.90 | 4.13 | 17.71 |
| 019 | −33.8400 | 151.1436 | River | 7.63 | Residential | n.d. | 5.57 | 3.01 | 4.20 | 12.77 |
| 020 | −33.8325 | 151.1344 | River | 8.44 | Residential | n.d. | 5.98 | 2.75 | 4.31 | 13.04 |
| 021 | −33.8209 | 151.0903 | River | 7.91 | Residential | n.d. | 6.93 | 2.68 | 8.47 | 18.07 |
| 022 | −33.8149 | 151.0881 | Stormwater | 8.75 | Parkland | n.d. | 4.61 | 2.42 | 4.08 | 11.11 |
| 023 | −33.8158 | 151.0783 | River | 7.81 | Parkland | n.d. | 4.95 | 2.76 | 4.19 | 11.90 |
| 024 | −33.8170 | 151.0788 | River | 6.88 | Parkland | n.d. | 4.70 | 2.45 | 4.19 | 11.34 |
| 025 | −33.8140 | 151.0312 | River | 7.07 | Industrial | n.d. | 4.89 | 2.58 | 3.94 | 11.42 |
| 026 | −33.8176 | 151.0408 | River | 7.23 | Commercial | n.d. | 5.24 | 2.75 | 4.18 | 12.17 |
| 027 | −33.8177 | 151.0409 | River | 7.86 | Commercial | n.d. | 4.38 | n.d. | 3.89 | 8.27 |
| 028 | −33.8108 | 151.0037 | Stormwater | 7.87 | Commercial | 7.75 | 3.51 | 2.55 | 4.01 | 17.82 |
| 029 | −33.8431 | 151.0164 | River | 7.44 | Industrial | n.d. | 4.30 | 3.21 | 4.59 | 12.11 |
| 030 | −33.8323 | 151.0163 | Stormwater | 7.17 | Commercial | 6.69 | 3.87 | 3.42 | 4.89 | 18.87 |
| 031 | −33.8356 | 151.0231 | Stormwater | 7.32 | Industrial | 5.68 | 305.55 | 3.14 | 4.53 | 318.89 |
| 032 | −33.8248 | 151.0517 | River | 7.56 | Commercial | 4.62 | 13.78 | 3.12 | 4.88 | 26.40 |
| 033a | −33.9640 | 151.2535 | Stormwater | 8.47 | Residential | 13.76 | 4.78 | 2.65 | 4.58 | 25.77 |
| 033b | −33.9640 | 151.2535 | Stormwater | 8.30 | Residential | 9.68 | 4.56 | 3.09 | 4.69 | 22.01 |
| 034a | −33.9652 | 151.2518 | Stormwater | 7.92 | Residential | 6.93 | 6.44 | 3.44 | 5.30 | 22.10 |
| 034b | −33.9652 | 151.2518 | Stormwater | 8.00 | Residential | 11.76 | 5.86 | 3.32 | 5.26 | 26.20 |
| 035 | −33.9463 | 151.2582 | Stormwater | 7.66 | Residential | 5.49 | 4.96 | 3.18 | 4.68 | 18.30 |
| 036 | −33.9538 | 151.2577 | Stormwater | 7.05 | Residential | 7.56 | 3.04 | 2.59 | 4.12 | 17.31 |
| 037 | −33.9193 | 151.2596 | Stormwater | 7.60 | Residential | 5.57 | 6.26 | 3.23 | 5.23 | 20.29 |
| 038 | −33.6070 | 150.8252 | River | 7.83 | Residential | n.d. | 4.06 | 2.91 | 4.57 | 11.54 |
| 039 | −33.6066 | 150.8250 | Stormwater | 5.79 | Residential | 13.78 | 5.15 | 3.72 | 5.43 | 28.08 |
| 040 | −33.5999 | 150.8334 | STP discharge | 7.15 | STP | 12.28 | 4.95 | 3.15 | 4.69 | 25.07 |
| 041 | −33.5767 | 150.7101 | River | 6.90 | Commercial | 7.93 | 6.29 | 3.15 | 5.09 | 22.46 |
| 042 | −33.5749 | 150.7165 | STP discharge | 6.98 | STP | 4.83 | 4.76 | 2.91 | 4.82 | 17.32 |
| 043 | −33.5727 | 150.7312 | STP discharge | 7.08 | STP | 6.41 | 4.23 | 2.64 | 4.21 | 17.49 |
| 044 | −33.7025 | 151.0805 | River | 7.95 | Parkland | n.d. | 4.54 | 2.74 | 4.63 | 11.91 |
| 045 | −33.7028 | 151.0802 | River | 7.87 | Parkland | n.d. | 5.45 | 3.04 | 4.64 | 13.14 |
| 046 | −33.7011 | 151.0809 | Stormwater | 7.79 | Parkland | n.d. | 5.19 | 2.68 | 4.32 | 12.19 |
| 047 | −33.7010 | 151.0808 | STP discharge | 7.67 | STP | 6.93 | 4.88 | 2.78 | 4.42 | 19.01 |
| 048 | −33.7912 | 151.1159 | Stormwater | 7.59 | Residential | 6.20 | 3.87 | 2.51 | 4.10 | 16.67 |
| 049 | −33.7689 | 151.1227 | River | 7.90 | Parkland | 8.50 | 4.94 | 3.06 | 4.46 | 20.97 |
| 050 | −33.7699 | 151.1239 | Stormwater | 7.80 | Residential | n.d. | 3.63 | n.d. | 4.00 | 7.64 |
| 051 | −33.7698 | 151.1219 | River | 7.71 | Parkland | 5.37 | 5.53 | 3.09 | 4.61 | 18.59 |
| 052 | −33.7651 | 151.1319 | River | 7.56 | Residential | n.d. | 5.94 | n.d. | 4.25 | 10.19 |
| 053 | −33.7926 | 151.1570 | River | 7.34 | Parkland | 5.73 | 3.41 | n.d. | 3.97 | 13.11 |
| 054 | −33.8011 | 151.1439 | River | 7.06 | Parkland | 5.55 | 6.32 | 2.86 | 4.33 | 19.07 |
| 055 | −33.6967 | 151.1118 | River | 7.00 | Industrial | 8.10 | 5.31 | 2.92 | 4.43 | 20.75 |
| 056 | −33.6224 | 151.1509 | Stormwater | 7.20 | Residential | 5.08 | 4.26 | n.d. | 3.95 | 13.29 |
| 057 | −33.7199 | 151.0826 | River | 7.25 | Industrial | 4.79 | 3.68 | 2.87 | 3.97 | 15.31 |
| 058 | −33.7638 | 151.0890 | Stormwater | 7.25 | Residential | 8.12 | 4.27 | n.d. | 4.36 | 16.75 |
| 059 | −33.7595 | 151.1015 | Stormwater | 7.26 | Residential | 6.02 | 3.42 | n.d. | 4.27 | 13.71 |
| 060a | −33.7840 | 151.0851 | Stormwater | 7.36 | Residential | 5.17 | 3.04 | n.d. | n.d. | 8.21 |
| 060b | −33.7840 | 151.0851 | Stormwater | 7.49 | Residential | 5.27 | n.d. | n.d. | 3.86 | 9.13 |
| 061a | −33.7341 | 151.0873 | Stormwater | 7.34 | Residential | 6.44 | 6.10 | 2.91 | 4.69 | 20.14 |
| 061b | −33.7341 | 151.0873 | Stormwater | 7.58 | Residential | 4.91 | 169.19 | 2.89 | 4.31 | 181.31 |
| 062 | −33.6415 | 151.1357 | River | 7.33 | Industrial | 6.45 | 16.38 | 2.64 | 4.17 | x29.64 |
| 063 | −33.7715 | 151.1104 | River | 7.15 | Commercial | 5.09 | 2.75 | n.d. | n.d. | 7.84 |
| 064a | −33.6988 | 151.2374 | River | 7.00 | Landfill | 7.04 | 7.85 | 2.67 | 4.12 | 21.68 |
| 064b | −33.6988 | 151.2374 | River | 7.26 | Landfill | 5.84 | 6.25 | 2.87 | 3.92 | 18.88 |
| 065 | −33.7015 | 151.2379 | River | 7.56 | Parkland | n.d. | 5.29 | n.d. | 3.87 | 9.16 |
| 066 | −33.7073 | 151.2314 | River | 6.04 | Parkland | n.d. | n.d. | n.d. | n.d. | n.d. |
| Detection frequencies (n) of four parabens according to sample type and land use | ||||
|---|---|---|---|---|
| Sample type | MeP | EtP | PrP | BuP |
| Stormwater (n = 34) | 28 | 32 | 27 | 33 |
| River water (n = 34) | 18 | 32 | 28 | 32 |
| Land use type | ||||
| Commercial (n = 7) | 5 | 7 | 5 | 6 |
| Industrial (n = 15) | 9 | 15 | 15 | 15 |
| Parkland (n = 14) | 6 | 13 | 11 | 13 |
| Residential (n = 32) | 26 | 29 | 24 | 31 |
| STP (n = 4) | 4 | 4 | 4 | 4 |
| Total (n = 72) | 50 | 68 | 59 | 69 |
The Cooks River in the inner west region of Sydney accounted for five of the 10 most contaminated sites, which was unsurprising given the river's association with poor water quality.54 The two most contaminated sites of significance were site 031 Duck River Auburn and site 061b the Dawson Avenue raingarden in Thornleigh (Table 2). The Duck River site is downstream of an industrial area that also includes a waste transfer station. The Dawson Avenue raingarden drains a small retail shopping complex in a suburban catchment.
A comparison of the summarized results of this study with a random sample of the international published literature is presented in Table 4. Generally the concentrations of parabens found in this study are higher. This is particularly relevant when comparing concentrations of parabens in urban rivers and more urbanised catchments.
| Comparison of results to international studies | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Year | Country | Waterway | Type | MeP | EtP | PrP | BuP |
| n.d.: Not detected – under detection limits of the method n.a.: Not analysed * Denotes samples of influent and effluent from the same wastewater treatment site (a). | ||||||||
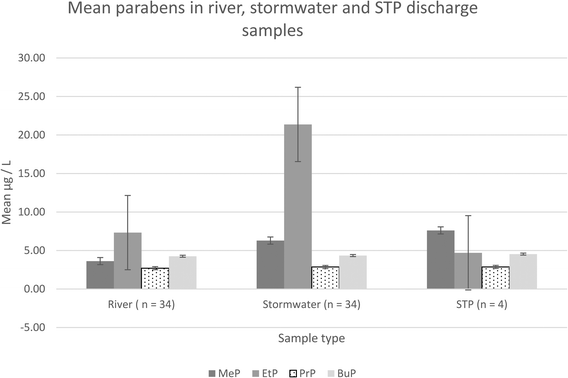
| Mean results from this Sydney study | 2014 | Australia | River | Urban/industrial | 3.63 | 7.33 | 2.70 | 4.26 |
| 2014 | Australia | Stormwater | Urban/industrial | 6.29 | 21.37 | 2.87 | 4.35 | |
| 2014 | Australia | Effluent | Urban | 7.61 | 4.70 | 2.87 | 4.54 | |
| Leusch, et al.43 | 2013 | Australia | Influent | Urban | n.a. | n.a. | 0.011 | n.a. |
| Peng, et al.29 | 2008 | China | River | Urban | 1.062 | n.a. | 3.142 | n.d. |
| Peng, et al.29 | 2008 | China | River | Urban | 0.213 | n.a. | 0.693 | n.d. |
| Terasaki, et al.57 | 2012 | Japan | River | Urban | 0.0037 | n.d. | 0.022 | 0.012 |
| Gonzalez-Marino, et al.65 | 2009 | Spain | River | Urban | 0.0034 | 0.0030 | 0.069 | 0.007 |
| Gonzalez-Marino, et al.65 | 2009 | Spain | River | Urban | 0.009 | 0.0012 | 0.0059 | 0.001 |
| Villaverde-de-saa, et al.55 | 2010 | Spain | River | Urban | 0.054 | 0.029 | 0.105 | 0.0064 |
| Ramírez, et al.56 | 2012 | Spain | River | Urban | 0.042 | 0.0011 | n.d. | n.d. |
| Canosa, et al.4 | 2006 | Spain | Sewer | Urban/medical | 1.48 | 0.10 | 1.22 | 0.019 |
| Canosa, et al.4 | 2006 | Spain | Influent (a)* | Urban | 2.92 | 0.21 | 0.81 | 0.086 |
| Canosa, et al.4 | 2006 | Spain | Effluent (a)* | Urban | n.d. | n.d. | n.d. | n.d. |
| Canosa, et al.4 | 2006 | Spain | Influent (b)* | Urban | 0.43 | 0.052 | 0.23 | 0.020 |
| Canosa, et al.4 | 2006 | Spain | Effluent (b)* | Urban | n.d. | n.d. | 0.064 | n.d. |
| Lee, et al.26 | 2005 | Canada | Influent (c)* | Urban/industrial | 1.47 | 0.27 | 2.43 | 0.26 |
| Lee, et al.26 | 2005 | Canada | Effluent (c)* | Urban/industrial | 0.04 | <0.01 | 0.04 | <0.01 |
| Lee, et al.26 | 2005 | Canada | Influent (d)* | Urban/industrial | 0.63 | 0.12 | 0.86 | 0.12 |
| Lee, et al.26 | 2005 | Canada | Effluent (d)* | Urban/industrial | 0.02 | <0.01 | <0.01 | <0.01 |
| Villaverde-de-saa, et al.55 | 2010 | Spain | Influent | Urban | 6.81 | 0.48 | 1.227 | 0.088 |
| Ramírez, et al.56 | 2012 | Spain | Influent | Urban | 0.696 | 0.048 | 0.0053 | 0.052 |
| Ramírez, et al.56 | 2012 | Spain | Influent | Industrial | 14.243 | 5.927 | 23.593 | 0.681 |
| Gonzalez-Marino, et al.25 | 2011 | Spain | Influent | Urban | 4.20 | 0.880 | 1.40 | 0.014 |
| Terasaki, et al.57 | 2012 | Japan | Influent | Urban | 2.40 | 0.57 | 2.60 | 4.45 |
3.1 Statistical analysis
Data was analyzed according to sample type (river/stormwater) and the land use categories mentioned in Table 3 using one-way ANOVAs with Welch's Correction for variance in MiniTab Version 17. Two-sample t-tests were then conducted on certain populations using MiniTab and MS Excel 365 for post hoc analysis.Concentrations of MeP are not only higher in stormwater than in river water, but they also occur more frequently (see Table 3). In stormwater samples MeP is likely to be in greater concentrations than BuP and PrP, and levels of BuP are also likely to be higher than PrP, while in river water samples both EtP and BuP are likely to be present at higher levels than PrP. These results also show a difference in paraben distribution between river and stormwater samples particularly in relation to MeP and EtP (Fig. 1).
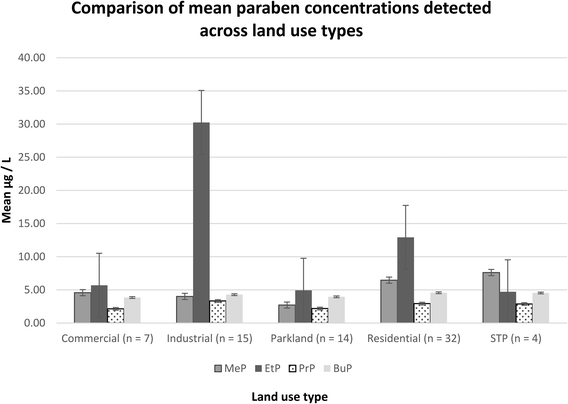
A one way ANOVA (p = 0.042) identified differences in the total paraben concentration between residential and parkland land uses. post hoc t-tests showed that residential samples (M = 26.87, SD = 31.6) had higher concentrations of parabens when compared to parkland (M = 12.71, SD = 5.42) land use types; t(35) = 2.44, p = 0.020. Additionally a one way ANOVA (p = 0.031) and further t-tests showed that the concentrations of MeP in the samples from residential areas (M = 6.47, SD = 3.95) were significantly higher than those found in parkland (M = 2.10, SD = 3.20); t(42) = 3.42, p = 0.001. These results show that residential waterways tested have greater concentrations of MeP, as well as a greater total concentration of parabens than those in parkland catchment areas.
Within parkland land uses a one way ANOVA (p = 0.001) and post hoc two-sample t-tests indicated that concentrations of BuP (M = 3.94, SD = 1.27) were higher than PrP (M = 2.09, SD 1.28); t(21) = −3.56, p = 0.002, and that EtP (M = 4.58, SD 1.6) was higher than PrP (M = 2.09, SD = 1.28); t(20) = 4.2, p < 0.001. Within residential land use categories, a one way ANOVA (p < 0.001) followed by two sample t-tests showed levels of BuP (M = 4.56, SD 1.19) to be greater than PrP (M = 2.93, SD = 2.05); t(49) = 3.90, p < 0.001 and that MeP (M = 6.47, SD = 3.95), was higher than both PrP (M = 2.93, SD = 2.05); t(46) = 4.50, p < 0.001, and BuP (M = 4.56, SD 1.19); t(36) = 2.61, p = 0.013. These results found that within parkland land use areas EtP and BuP are both at greater concentrations than PrP. Within residential land use areas, MeP not EtP, is found at greater levels than BuP and PrP. The statistically significant differences in the distribution of MeP, EtP, PrP and BuP within Sydney's residential and parkland waters may point to multiple pathways for which parabens are entering the urban environment that are dependent on land use.
4.0 Discussion
The principal aim of this pilot study was to characterise the presence of parabens in urban waterways across Sydney. The results show that at least two of the four parabens (BuP, PrP, and/or EtP and/or MeP) are present across all sites and all land use types with the exception of internally drained bushland (site 066 that served as a reference location). The study reported comparatively high concentrations of parabens, particularly EtP, across the urban waterways of Sydney. These findings were higher when compared to urban waterway studies from other cities.The higher concentration of EtP found in many of the urban locations in this study, relative to the other parabens, could be explained by a number of reasons. The high variability in paraben concentration across the study may suggest the need for more samples across the selected land use types, although the number of samples (72) is relatively high compared to other studies. Seasonal variation related to recent weather conditions can influence the amount of exfiltration from the sewerage system and therefor the composition of parabens in urban waterways. As sampling was undertaken in dry conditions, MeP related to PCP in the wastewater stream may have been under represented.
The urban drainage system (such as materials used in the drainage system) or practices by the community and industry may contribute to the higher proportion and concentration of EtP. The contributing factor leading to the presence of EtP, in particular, is unknown and requires additional research.
The analysis techniques, sample collection, storage and processing methods used in this study differed from other studies. For these reasons, additional study on the relative proportion and concentration of parabens across a range of urban land uses is recommended. Furthermore, repeat sampling of sites and examination of samples using an independent analytical approach is suggested.
Sydney has a separated sewer and stormwater system in contrast to the combined sewer/stormwater systems that exist in many other countries.46 This may account for a difference in the concentrations and relative presence of the four parabens studied when compared to international studies (Table 4) although further comparative study would be required to validate this assumption.
Based on previous research it was expected that MeP would be the most dominant paraben as it is most frequently used in PCPs.9 This was not the case. Across the study area MeP occurred less frequently and generally at much lower concentrations when compared to international sewer influent results (Table 4). This may reflect the shorter half-life of MeP when compared to other parabens,25 photodegradation64 of MeP in the environment or in sample (although care was taken to reduce the likelihood of this occurring), or that exfiltration from the sewer to stormwater and local waterways is not occurring at levels anticipated and/or contributing to total load of parabens linked to urban runoff.
Sampling up- and down-stream of the North Richmond (sites 042 and 043) and West Hornsby (sites 045 to 047) sewerage treatment plants (STPs) reported overall levels of parabens generally higher upstream (that is influenced by runoff) than below the discharge point. The exception was MeP which was lower upstream (and this would be expected given the association with MeP with PCPs). Without testing influent and determining the effectiveness of the STP in removing parabens, the reasons for the lower concentrations are unclear and may simply relate to the impact of dilution of parabens in the stream from the wastewater discharge. Both STPs are small scale tertiary treatment systems with North Richmond discharging approximately 0.9 ML per day and West Hornsby 11.9 ML per day (Sydney Water 2014a).49
The analysis identified statistically significant correlations between land use types and paraben concentrations. This study found that there was a significantly greater concentration of MeP as well as overall total paraben load in residential catchments when compared to catchments dominated by parkland, bushland, national parks and reserves. It is likely that sewage infiltration in the residential areas is a contributing factor to the total paraben load found in urban waterways, although it is not the only factor.
The highest concentration of parabens was reported at the Duck River site (031) a tributary of the Parramatta River draining an industrial area. The Cooks River, widely known in Sydney as being polluted58–60 reported the five of the top 10 highest parabens readings. The peak level of parabens along the Cooks River occurred at Ford Park (site 008) that drains an industrial area. A similar pattern of paraben concentration was also found along the Parramatta River with the peak concentrations occurring around Silverwater, a former industrial area. Ordinarily runoff from industrial premises is drained to the sewer via a trade waste agreement and not discharged to the stormwater system. The results may suggest illegal discharges, poor site management or the historical release of chemicals containing parabens that is transported during surface flow.
A high concentration of EtP was also found in the rain garden treating a small commercial catchment (061b). This could be the result of surface processes, the leaching of a product linked to the rain garden or an anomalous result. From a water sensitive urban design perspective there is need to identify the source and if the rain garden or biofiltration system would be able to capture and remove this pollutant.
5.0 Conclusions
The concentrations of parabens reported in this study are higher than that reported by the Australian study by Leusch, et al. (2013) and other international studies on urban waterways.43 The concentration of EtP is of particular interest as it frequently occurred at higher than median levels reported in all sewerage influent studies. The source of EtP is unknown. Significant statistical correlations between paraben concentrations were detected when comparing residential versus parkland. The differences between paraben concentrations in river and stormwater samples also highlight the need for further study in this area.As a study of an emergent pollutant, the results identify that catchment processes in the urban area of Sydney are contributing to paraben concentrations often over and above what would be expected from leaks from the sewer. Higher results were associated with some industrial land uses however this was not statistically significant. While sewerage may contribute to part of the total concentration of parabens in urban waterways, the relatively high levels of EtP and lower levels and detection rates of MeP suggest other processes are important and worthy of additional enquiry.
Acknowledgements
Bryan Gilfedder and Dominic May for assistance in the field. Hornsby Shire Council for their assistance with stormwater sampling.References
- R. S. Tavares, M. C. Martins, P. J. Oliveira, J. Ramalho-Santos and F. P. Peixoto, Reprod. Toxicol., 2008, 27, 1 CrossRef PubMed
.
- R. L. Elder, J. Am. Coll. Toxicol., 1984, 3, 147 CrossRef
.
- S. C. Rastogi, A. Schouten, N. De Kruijf and J. W. Weijland, Contact Dermatitis, 1995, 32, 28 CrossRef CAS PubMed
.
- P. Canosa, I. Rodríguez, E. Rubí, M. H. Bollaín and R. Cela, J. Chromatogr. A, 2006, 1124(1–2), 3 CrossRef CAS PubMed
.
- F. A. Andersen, Int. J. Toxicol., 2008, 27, 1 Search PubMed
.
- C. Liao, F. Liu and K. Kannan, Environ. Sci. Technol., 2013, 47(8), 3918 CrossRef CAS PubMed
.
- C. Liao, L. Chen and K. Kannan, Environ. Int., 2013, 57-58, 68 CrossRef CAS PubMed
.
- C. Liao and K. Kannan, Concentrations and composition profiles of parabens in currency bills and paper products including sanitary wipes, Sci. Total Environ., 2014, 475, 8–15 CrossRef CAS PubMed
.
- M. G. Soni, S. L. Taylor, N. A. Greenberg and G. A. Burdock, Food Chem. Toxicol., 2002, 40(10), 1335 CrossRef CAS PubMed
.
- H. Y. Shen, H. L. Jiang, H. L. Mao, G. Pan and Y. F. Cao, J. Sep. Sci., 2007, 30, 48 CrossRef CAS PubMed
.
- P. D. Darbre and P. W. Harvey, J. Appl. Toxicol., 2008, 28(5), 561 CrossRef CAS PubMed
.
- P. D. Darbre, A. Aljarrah, W. R. Miller, N. G. Coldham, M. J. Sauer and G. S. Pope, J. Appl. Toxicol., 2004, 24, 5 CrossRef CAS PubMed
.
- J. R. Byford, L. E. Shaw, M. G. Drew, G. S. Pope, M. J. Sauer and P. D. Darbre, J. Steroid Biochem. Mol. Biol., 2002, 80, 49 CrossRef CAS PubMed
.
- P. D. Darbre, Best Pract. Res., Clin. Endocrinol. Metab., 2006, 20, 121 CrossRef CAS PubMed
.
- A. K. Charles and P. D. Darbre, J. Appl. Toxicol., 2013, 33, 390 CrossRef CAS PubMed
.
- J. D. Meeker, T. Yang, X. Ye, A. M. Calafat and R. Hauser, Environ. Health Perspect., 2011, 119, 252 CrossRef CAS PubMed
.
- European Commission, 2014, http://europa.eu/rapid/press-release_IP-14-1051_en.htm, (accessed August 2014)
.
- DME EPA (Danish Ministry for the Environment- Environmental Protection Agency), 2013, http://eng.mst.dk/media/mst/Attachments/Engelskparabenbekendtgrelse.pdf, (accessed May 2014)
.
- M. G. Soni, I. G. Carabin and G. A. Buradock, Food Chem. Toxicol., 2005, 43, 985 CrossRef CAS PubMed
.
- S. Oishi, Toxicol. Ind. Health, 2001, 17, 31 CrossRef CAS PubMed
.
- S. Oishi, Effects of butyl paraben on the male reproductive system in mice, Arch. Toxicol., 2002, 76, 423–429 CrossRef CAS PubMed
.
- B. Alslev, B. Korsgaard and P. Bjerregaard, Aquat. Toxicol., 2005, 72, 295 CrossRef CAS PubMed
.
- P. Bjerregaard, D. N. Anderson, K. L. Pedersen, S. N. Pedersen and B. Korsgaard, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2003, 136, 309 CrossRef PubMed
.
- M. Terasaki, M. Makino and N. Tatarazako, J. Appl. Toxicol., 2009, 29, 242 CrossRef CAS PubMed
.
- I. González-Mariño, J. B. Quintana, I. Rodríguez and R. Cela, Water Res., 2011, 45(20), 6770 CrossRef PubMed
.
- H. B. Lee, T. E. Peart and M. L. Svoboda, J. Chromatogr. A, 2005, 1094(1–2), 122 CrossRef CAS PubMed
.
- A. L. Gregory and E. P. Mark, Environ. Sci. Technol., 2006, 40, 687 CrossRef
.
- B. Kasprzyk-Hordern, R. M. Dinsdale and A. J. Guwy, Water Res., 2008, 42, 3498 CrossRef CAS PubMed
.
- X. Peng, Y. Yu, C. Tang, J. Tan, Q. Huang and Z. Wang, Sci. Total Environ., 2008, 397, 158 CrossRef CAS PubMed
.
-
I. Bazin, A. Gadal, E. Touraud and B. Roig, in Xenobiotics in the urban water cycle: mass flows, environmental processes, mitigation and treatment strategies, ed. D. Fatta-Kassinos, K. Bester and K. Kümmerer, Dordrecht, Netherlands, 2010, ch. 14, vol. 16, pp. 245–257 Search PubMed
.
- N. Jonkers, A. Sousa, S. Galante-Oliveira, C. M. Barroso, H.-P. Kohler and W. Giger, Environ. Sci. Pollut. Res., 2010, 17, 834 CrossRef CAS PubMed
.
- A. Ibn Hadj Hassine, I. Bazin, K. Um, A. Bartegi and C. Gonzalez, Eur. J. Water Qual., 2011, 42, 91 CrossRef
.
- L. Wang, C. Liao, F. Liu, Q. Wu, Y. Guo, H.-B. Moon, H. Nakata and K. Kannan, Environ. Sci. Technol., 2012, 46(21), 11584 CrossRef CAS PubMed
.
- C. Liao, S. Lee, H.-B. Moon, N. Yamashita and K. Kannan, Environ. Sci. Technol., 2013, 47, 10895 CrossRef CAS PubMed
.
- H. M. Kuch and K. Ballschmitter, Environ. Sci. Technol., 2001, 35, 3201 CrossRef CAS PubMed
.
- D. Ashton, M. Hilton and K. V. Thomas, Sci. Total Environ., 2004, 333, 167 CrossRef CAS PubMed
.
- T. Benijts, W. Lambert and A. De Leenheer, Anal. Chem., 2004, 76, 704 CrossRef CAS PubMed
.
- T. Furuichi, K. Kannan, J. P. Giesy and S. Masunaga, Water Res., 2004, 38, 4491 CrossRef CAS PubMed
.
- S. Wiegel, A. Aulinger, R. Brockmeyer, H. Harms, J. Löffler, H. Reincke, R. Schmidt, B. Stachel, W. von Tümpling and A. Wanke, Chemosphere, 2004, 57, 107 CrossRef CAS PubMed
.
- P. Labadie and H. Budzinski, Environ. Sci. Technol., 2005, 39, 5113 CrossRef CAS PubMed
.
- N. Lindqvist, T. Tuhkanen and L. Kronberg, Water Res., 2005, 39, 2219 CrossRef CAS PubMed
.
- J. M. Brausch and G. M. Rand, Chemosphere, 2011, 82(11), 1518 CrossRef CAS PubMed
.
- F. D. Leusch, S. J. Khan, S. Laingam, E. Prochazka, S. Froscio, T. Trinh, H. F. Chapman and A. Humpage, Water Res., 2013, 49, 300 CrossRef PubMed
.
- L. Núñez, E. Turiel, A. Martin-Esteban and J. L. Tadeo, Talanta, 2010, 80(5), 1782 CrossRef PubMed
.
- B. Albero, R. A. Pérez, C. Sánchez-Brunete and J. L. Tadeo, J. Hazard. Mater., 2012, 239–240, 48 CrossRef CAS PubMed
.
- P. J. Davies and I. A. Wright, Land Use Policy, 2014, 36, 450 CrossRef
.
-
J. A. Tarr and F. C. McMichael, Civil Engineering - American Society of Civil Engineers, 1977, vol. 47, issue no. (10), p. 82 Search PubMed
.
- Sydney Water, 2014, http://www.sydneywaternews.com.au/2014/01/12/sydney-waters-efforts-to-manage-leaks/, (accessed August 2014)
.
- Sydney Water, 2014, http://www.sydneywater.com.au/SW/water-the-environment/how-we-manage-sydney-s-water/wastewater-network/wastewater-treatment-plants/index.htm, (accessed August 2014)
.
- Sydney Water, 2013, http://www.sydneywaternews.com.au/2013/12/16/our-work-on-wastewater-overflows-in-the-georges-river/, (accessed August 2014)
.
- Australian Bureau of Statistics, 2011, http://www.abs.gov.au/AUSSTATS/abs@.nsf/Details Page/1270.0.55.001July%202011?OpenDocument, (accessed July 2014)
.
- EPA, 2007, http://water.epa.gov/scitech/methods/cwa/bioindicators/upload/2008_01_03_ methods_method_1694.pdf, (accessed October 2015)
.
- Geoscience Australia, 2004, http://www.ga.gov.au/metadata-gateway/metadata/record/ 63742, (accessed June 2014)
.
- City of Canterbury, 2015, http://www.canterbury.nsw.gov.au/Environment/Cooks-River Cooks River, (accessed October 2015)
.
- E. Villaverde-de-Saa, I. González-Mariño, J. Quintana, R. Rodil, I. Rodriguez and R. Cela, Anal. Bioanal. Chem., 2010, 397(6), 2259 CrossRef PubMed
.
- N. Ramírez, F. Borrull and R. Marcé, J. Sep. Sci., 2012, 35(4), 580 CrossRef PubMed
.
- M. Terasaki, Y. Takemura and M. Makino, Environ. Chem. Lett., 2012, 10(4), 401 CrossRef CAS
.
-
S. Khan, 2011, http://newsroom.unsw.edu.au/news/science-technology/sydney-river-open-sewer, (accessed August 2014)
.
- Cooks River Alliance, 2014, http://cooksriver.org.au/cooksriver/overview/Cooks River: Overview, (accessed August 2014)
.
- Sydney Water, 2012, http://www.sydneywaternews.com.au/2012/02/15/cooks-river-sydney-waters-commitment-to-river-health/, (accessed August 2014)
.
- P. Canosa, I. Rodríguez, E. Rubí, N. Negreira and R. Cela, Anal. Chim. Acta, 2006, 575, 106–113 CrossRef CAS PubMed
.
- M. Terasaki, R. Kamata, F. Shiraishi and M. Makino, Environ. Toxicol. Chem., 2009, 28, 204–208 CAS
.
- M. Terasaki and M. Makino, Int. J. Environ. Anal. Chem., 2008, 88, 911–922 CrossRef CAS
.
- X. Feng, Y. Chen, Y. Fang, X. Wang, Z. Wang, T. Tao and Y. Zuo, Sci. Total Environ., 2014, 472, 130–136 CrossRef CAS PubMed
.
- I. Gonzalez-Marino, J. B. Quintana, I. Rodriguez and R. Cela, Rapid Commun. Mass Spectrom., 2009, 23(12), 1756–1766 CrossRef CAS PubMed
.
- P. Canosa, I. Rodriguez, E. Rubi and R. Cela, Anal. Chem., 2007, 79(4), 1675–1681 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ew00240k |
| This journal is © The Royal Society of Chemistry 2016 |