Gas-permeable hydrophobic membranes enable transport of CO2 and NH3 to improve performance of bioelectrochemical systems
Abstract
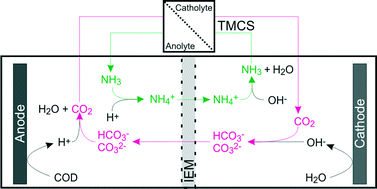
Application of bioelectrochemical systems (BESs), for example for the production of hydrogen from organic waste material, is limited by a high internal resistance, especially when ion exchange membranes are used. This leads to a limited current density and thus to large footprint and capital costs. Ion transport between anode and cathode compartment is one of the factors determining the internal resistance. The aim of this study was to reduce the resistance for ion transport in a microbial electrolysis cell (MEC) through the ion exchange membrane by shuttling of CO2 and NH3 between anode and cathode. The transport of these chemical species was enabled through the use of a hydrophobic TransMembraneChemiSorption module (TMCS) that was placed between anolyte and catholyte circulation outside the cell. The driving force for transport was the pH difference between both solutions. The transport of CO2 and NH3 resulted in an increase in current density from 2.1 to 4.1 A m−2 for a cation exchange membrane (CEM) and from 2.5 to 13.0 A m−2 for an anion exchange membrane (AEM) at 1 V applied voltage. The increase in current density was the result of a lower ion transport resistance through the membrane; this resistance was 60% lower for the CEM, as a result of NH3 recycling from cathode to anode, and 82% for the AEM, as a result of CO2 recycling from anode to cathode with TMCS, compared to experiments without TMCS.

 Please wait while we load your content...
Please wait while we load your content...