Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Silicon(I) chemistry: the NHC-stabilised silicon(I) halides Si2X2(Idipp)2 (X = Br, I) and the disilicon(I)-iodido cation [Si2(I)(Idipp)2]+†
Marius I.
Arz
,
Daniel
Geiß
,
Martin
Straßmann
,
Gregor
Schnakenburg
and
Alexander C.
Filippou
*
Institut für Anorganische Chemie, Rheinische Friedrich-Wilhelms-Universität Bonn, Gerhard-Domagk-Straße 1, 53121 Bonn, Germany. E-mail: filippou@uni-bonn.de
First published on 17th August 2015
Abstract
An efficient method for the synthesis of the NHC-stabilised Si(I) halides Si2X2(Idipp)2 (2-X, X = Cl, Br, I; Idipp = C[N(C6H3-2,6-iPr2)CH]2) was developed, which involves the oxidation of Si2(Idipp)2 (1) with 1,2-dihaloethanes. Halogenation of 1 is a diastereoselective reaction leading exclusively to a racemic mixture of the RR and SS enantiomers of 2-X. Compounds 2-Br and 2-I were characterised by single-crystal X-ray crystallography and multinuclear NMR spectroscopy, and their electronic structures were analysed by quantum chemical methods. Dynamic NMR spectroscopy unraveled a fluxional process of 2-Br and 2-I in solution, which involved a hindered rotation of the NHC groups about the Si–CNHC bonds. Iodide abstraction from 2-I by [Li(Et2O)2.5][B(C6F5)4] selectively afforded the disilicon(I) salt [Si2(I)(Idipp)2][B(C6F5)4] (3). X-ray crystallography and variable-temperature NMR spectroscopy of 3 in combination with quantum chemical calculations shed light on the ground-state geometric and electronic structure of the [Si2(I)(Idipp)2]+ ion, which features a Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si bond between a trigonal planar coordinated SiII atom with a Si–I bond and a two-coordinate Si0 center carrying a lone pair of electrons. The dynamics of the [Si2(I)(Idipp)2]+ ion were studied in solution by variable-temperature NMR spectroscopy and they involve a topomerisation, which proceeds according to quantum theory via a disilaiodonium intermediate (“π-bonded” isomer) and exchanges the two heterotopic Si sites.
Si bond between a trigonal planar coordinated SiII atom with a Si–I bond and a two-coordinate Si0 center carrying a lone pair of electrons. The dynamics of the [Si2(I)(Idipp)2]+ ion were studied in solution by variable-temperature NMR spectroscopy and they involve a topomerisation, which proceeds according to quantum theory via a disilaiodonium intermediate (“π-bonded” isomer) and exchanges the two heterotopic Si sites.
Introduction
The molecular chemistry of silicon has witnessed remarkable progress in recent years following the discovery that N-heterocyclic carbenes (NHCs) are particularly suitable Lewis-bases for the thermodynamic and kinetic stabilisation of highly reactive, low-valent silicon species.1 Appealing examples substantiating this development include the Si(0) compounds Si2(NHC)2 (NHC = C[N(dipp)CH]2 (Idipp) and C[N(dipp)CH2]2 (SIdipp); dipp = C6H3-2,6-iPr2)2 and Si(bNHC) (bNHC = chelating bis-N-heterocyclic carbene),3 the SiI chloride Si2Cl2(Idipp)2,2a the NHC-stabilised SiII compounds SiX2 (X = Cl–I),4 Si(X)R (X = Cl, Br, H; R = aryl, amino, silyl),5 Si(R)(SiR![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) SiR2) (R = C6H2-2,4,6-iPr3)6 and 1-silacyclopenta-2,4-dienylidenes SiC4R4 (R = Ph, NEt2),7 and the NHC-trapped silyliumylidene cations [SiR]+ (R = I, aryl)4d,5e,8 and Si2+ ions.4d More recently, NHC-stabilised phosphasilenylidenes Si
SiR2) (R = C6H2-2,4,6-iPr3)6 and 1-silacyclopenta-2,4-dienylidenes SiC4R4 (R = Ph, NEt2),7 and the NHC-trapped silyliumylidene cations [SiR]+ (R = I, aryl)4d,5e,8 and Si2+ ions.4d More recently, NHC-stabilised phosphasilenylidenes Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PR (R = C6H2-2,4,6-tBu3)9 and disilavinylidenes Si
PR (R = C6H2-2,4,6-tBu3)9 and disilavinylidenes Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si(Br)R (R = C6H2-2,6-{CH(SiMe3)2}2-4-tBu) were also isolated, fortifying the binding capacity of NHCs.10 All these compounds offer new avenues of chemical exploration with potential applications in both molecular chemistry and materials science due to their functional versatility originating from the simultaneous presence of many reactive sites, such as the Si lone pairs, the silicon–halogen bonds or the displaceable NHC groups. In fact, the SiII halides SiX2(NHC) (X = Cl–I, NHC = Idipp and SIdipp) and SiCl(R)(NHC) (R = C6H3-2,6-Tip2, Tip = C6H2-2,4,6-iPr3; NHC = C[N(Me)CMe]2) were shown to be valuable starting materials, which paved the way to new classes of unsaturated silicon compounds, including silylidyne complexes, zwitterionic silylidene complexes, metallosilylenes and metallosilanones.11
Si(Br)R (R = C6H2-2,6-{CH(SiMe3)2}2-4-tBu) were also isolated, fortifying the binding capacity of NHCs.10 All these compounds offer new avenues of chemical exploration with potential applications in both molecular chemistry and materials science due to their functional versatility originating from the simultaneous presence of many reactive sites, such as the Si lone pairs, the silicon–halogen bonds or the displaceable NHC groups. In fact, the SiII halides SiX2(NHC) (X = Cl–I, NHC = Idipp and SIdipp) and SiCl(R)(NHC) (R = C6H3-2,6-Tip2, Tip = C6H2-2,4,6-iPr3; NHC = C[N(Me)CMe]2) were shown to be valuable starting materials, which paved the way to new classes of unsaturated silicon compounds, including silylidyne complexes, zwitterionic silylidene complexes, metallosilylenes and metallosilanones.11
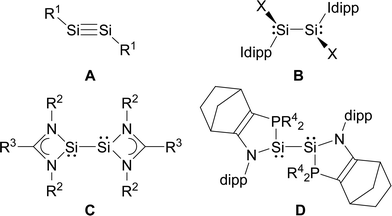
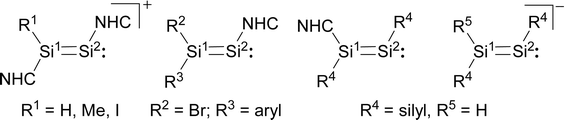
In comparison, the chemistry of NHC-stabilised silicon(I) halides (Chart 1, B) has not been explored so far. This can probably be attributed to the severely limited access to this very reactive class of compounds, as revealed by the very low yield synthesis (6.1%) of the only presently known example Si2Cl2(Idipp)2 (2-Cl) upon the reduction of SiCl4(Idipp) with C8K.2a Compounds B can be viewed as bis(NHC) adducts of the disilynes Si2R2 (A, Chart 1), the chemistry of which has flourished since the isolation of the first thermally stable compounds in 2004.12 The NHC-stabilised SiI halides B bear as their amidinato (C, Chart 1)13 or (phosphino)enamido-stabilised congeners (D, Chart 1),14 a reactive Si–Si single bond and a lone pair of electrons at each silicon atom, but contain beyond displaceable halide and NHC substituents, which offer additional dimensions of reactivity.15 In the present work, an efficient synthesis of NHC-stabilised SiI halides Si2X2(Idipp)2 (2-X, X = Cl, Br, I) is reported, facilitating the exploration of their reactivity. Moreover, iodide abstraction from 2-I is demonstrated to provide access to an unprecedented Si(I) salt containing the NHC-trapped [Si2I]+ cation.
Results and discussion
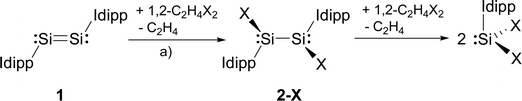
We presumed that halogenation of the Si–Si double bond of the disilicon(0) compound (Idipp)Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si(Idipp) (1)2a might be a suitable reaction for the synthesis of the target silicon(I) halides, analogous to the well-known olefin halogenation in carbon chemistry.16 The 1,2-dihaloethanes 1,2-C2H4X2 (X = Cl, Br, I) proved to be particularly suitable reagents to accomplish this transformation. In fact, the addition of a stock solution of 1,2-C2H4X2 to a dark red solution of 1 in THF or toluene at low temperature was accompanied by a rapid color change to red-orange and gas evolution (ethene), and selectively afforded the SiI halides 2-X (X = Cl, Br, I) in moderate to very good yields (2-Cl: 49%; 2-Br: 98%; 2-I: 61%) (Scheme 1).† Precise adherence to a 1
Si(Idipp) (1)2a might be a suitable reaction for the synthesis of the target silicon(I) halides, analogous to the well-known olefin halogenation in carbon chemistry.16 The 1,2-dihaloethanes 1,2-C2H4X2 (X = Cl, Br, I) proved to be particularly suitable reagents to accomplish this transformation. In fact, the addition of a stock solution of 1,2-C2H4X2 to a dark red solution of 1 in THF or toluene at low temperature was accompanied by a rapid color change to red-orange and gas evolution (ethene), and selectively afforded the SiI halides 2-X (X = Cl, Br, I) in moderate to very good yields (2-Cl: 49%; 2-Br: 98%; 2-I: 61%) (Scheme 1).† Precise adherence to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio of the reactants and a slow addition rate proved to be essential for a selective conversion of 1 to 2-X. This avoids further oxidation of 2-X by 1,2-C2H4X2 to the SiII halides SiX2(Idipp) (X = Cl, Br, I) (Scheme 1), which are difficult to separate from 2-X due to their similar solubilities.4a,b,d
1 stoichiometric ratio of the reactants and a slow addition rate proved to be essential for a selective conversion of 1 to 2-X. This avoids further oxidation of 2-X by 1,2-C2H4X2 to the SiII halides SiX2(Idipp) (X = Cl, Br, I) (Scheme 1), which are difficult to separate from 2-X due to their similar solubilities.4a,b,d
Halogenation of 1 by 1,2-C2H4X2 is a highly diastereoselective cis-addition leading exclusively to a racemic mixture of the RR and SS stereoisomers of 2-X (Scheme 1). No evidence for the formation of the meso diastereoisomer (trans-addition product) was found, which, according to quantum chemical calculations at the B97-D3/I level of theory,17 is thermodynamically less stable than the RR/SS stereoisomers by 57 kJ mol−1 (see ESI,† Section 5.1). In comparison, halogenation of the diphosphene RP![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PR (R = C(SiMe3)3) with Cl2 was reported to give exclusively the meso diastereomer.18,19
PR (R = C(SiMe3)3) with Cl2 was reported to give exclusively the meso diastereomer.18,19
An alternative approach to the SiI halides 2-X was also investigated, which involved comproportionation of the Si0 compound 1 with SiX2(Idipp). Whereas no reaction between 1 and SiBr2(Idipp) was observed at room temperature, heating a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixture of 1 and SiBr2(Idipp) in toluene at 85 °C afforded the SiI bromide 2-Br, as confirmed by NMR spectroscopy. However, conversion to the comproportionation product competed with the slow decomposition of 2-Br occurring under the same conditions (vide infra), leading finally to a mixture of 2-Br, SiBr2(Idipp) and Idipp. Whereas Idipp could easily be removed, separation of 2-Br from SiBr2(Idipp) proved to be difficult due to their similar solubility preventing the isolation of 2-Br in high-yield and pure form.
2 mixture of 1 and SiBr2(Idipp) in toluene at 85 °C afforded the SiI bromide 2-Br, as confirmed by NMR spectroscopy. However, conversion to the comproportionation product competed with the slow decomposition of 2-Br occurring under the same conditions (vide infra), leading finally to a mixture of 2-Br, SiBr2(Idipp) and Idipp. Whereas Idipp could easily be removed, separation of 2-Br from SiBr2(Idipp) proved to be difficult due to their similar solubility preventing the isolation of 2-Br in high-yield and pure form.
The silicon(I) halides 2-X were isolated as vermillion, extremely air-sensitive solids, which immediately decolourised when in contact with air, but can be stored indefinitely at room temperature under an atmosphere of argon. Compounds 2-Br and 2-I are thermally quite robust in the solid-state and decompose upon heating at a temperature (190 °C) similar to that previously reported for 2-Cl (184 °C).2a However, in toluene solution, 2-Br starts to decompose at a much lower temperature (at 85 °C, ca. 10% decomposition within 2 h), and the decomposition leads to Idipp, SiBr2(Idipp) and an unknown toluene-insoluble solid (see Fig. S5 in the ESI†).
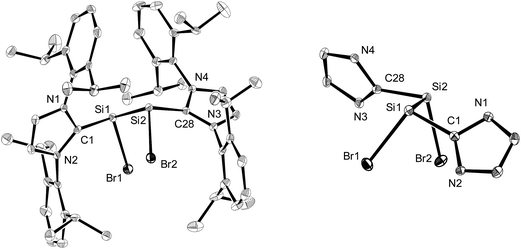
Compounds 2-Br and 2-I are the first molecular silicon(I) bromide and iodide to be reported and were comprehensively characterised by single-crystal X-ray crystallography, NMR spectroscopy and quantum chemical calculations.†
The molecular structures of the n-hexane semisolvates 2-Br·0.5(n-C6H14) and 2-I·0.5(n-C6H14) were determined by single-crystal X-ray diffraction (Fig. 1, Table 1 and Fig. S23 in the ESI†). All compounds 2-X (X = Cl–I) feature two stereogenic trigonal pyramidal silicon centers of the same configuration and display similar bonding parameters (Table 1). The halogen substituents of 2-X adopt a synclinal conformation and the sterically more demanding Idipp groups adopt an antiperiplanar conformation, as indicated by the X–Si–Si–X torsion angles (2-Cl: −46.5(1)°,2a2-Br: −46.81(4)°, 2-I: 50.46(3)°) and CNHC–Si–Si–CNHC torsion angles (2-Cl: −162.9(3)°,2a2-Br: 161.5(1)°, 2-I: −160.31(9)°), respectively (Fig. 1). The degree of silicon pyramidalisation (DP) of the SiI halides ranges from 57–64% and is considerably smaller than that of the SiII halides SiX2(Idipp) (DP = 70–78%) (Table 1).20 This suggests a lower s-character of the lone pair orbitals in 2-X compared to those in SiX2(Idipp), which was confirmed by comparative NBO analyses (see Tables S6 and S7 in the ESI†). Furthermore, these analyses indicate a higher s-character of the Si hybrid orbitals employed in the bonding to the NHC groups in 2-X, providing a rationale for the observed shortening of the Si–CNHC bonds of 2-Xversus those in SiX2(Idipp) (Table 1). These trends follow the predictions of Bent's rule made for a replacement of one halogen atom in SiX2(Idipp) by the more electropositive substituent SiX(Idipp).21 The Si–Si bond lengths of 2-X (2-Cl: 2.393(3) Å,2a2-Br: 2.385(1) Å, 2-I: 2.3909(9) Å) are slightly longer than that in α-Si (2.352 Å)22 and lie in-between those of the amidinato-substituted SiI compounds C (2.413(2) Å and 2.489(2) Å)13 and the (phosphino)enamido containing compound D (2.331(1) Å) (Chart 1).14 Remarkably, a plot of the Si–Si bond length of the SiI compounds B, C and Dversus the sum of the bond angles at silicon revealed a good linear correlation, with the Si–Si bond length decreasing upon an increase in the sum of the bond angles (decreasing pyramidalisation) at the silicon atoms (see Fig. S25 in the ESI†).
| 1 | 2-Cl | 2-Br | 2-I | SiCl2(L)b | SiBr2(L)c | SiI2(L)d | |
|---|---|---|---|---|---|---|---|
| a The data were obtained from ref. 2a. b The data were obtained from ref. 4a; the arithmetic mean value of the bonding parameters of two independent molecules in the unit cell of SiCl2(Idipp) is reported. c The data were obtained from ref. 4b. d The data were obtained from ref. 4d; the arithmetic mean value of the bonding parameters of the three independent molecules in the unit cell of SiI2(Idipp) is given. e NMR chemical shifts are given in ppm in C6D6 at 298 K. f The uncertainty (u) of the sum of angles is given in parenthesis and was calculated from the individual uncertainties (ui) by error propagation using the formula u = (∑(ui)2)1/2. g The standard deviation (σ) of the unweighted arithmetic mean values xu is given in parenthesis and was calculated using the formula σ2 = ∑(xi − xu)2/n2 − n, where xi is the respective individual value and n is the total number of individual values. | |||||||
| Si–Si | 2.229(1) | 2.393(3) | 2.385(1) | 2.3909(9) | |||
| Si–CNHC | 1.927(2) | 1.939(6) | 1.940(3) | 1.943(2) | 1.985(4) | 1.989(3) | 1.984(7)g |
| 1.929(7) | 1.936(3) | 1.939(2) | |||||
| Si–X | 2.161(3) | 2.3602(8) | 2.6036(6) | 2.159(2) | 2.3607(8) | 2.573(6)g | |
| 2.168(3) | 2.3677(9) | 2.5919(6) | 2.174(2) | 2.3379(8) | 2.577(1)g | ||
| CNHC–Si–Si | 93.37(5) | 98.8(2) | 97.87(9) | 97.04(7) | |||
| 98.7(2) | 96.74(9) | 97.49(7) | |||||
| ∑Si | 308.8(3)f | 303.9(1)f | 304.4(1)f | 290.7 | 292.7(1)f | 297(1)g | |
| 307.4(3)f | 302.3(1)f | 304.0(1)f | |||||
| DP | 57 | 62 | 62 | 78 | 75 | 70 | |
| 58 | 64 | 63 | |||||
| δ(29Si)e | 224.5 | 38.4 | 34.9 | 18.7 | 19.06 | 10.9 | −9.7 |
| δ(CNHC)e | 196.3 | 180.0 | 177.1 | 174.4 | 168.5 | 164.5 | 158.4 |
In the 29Si{1H} NMR spectra in C6D6, the SiI halides display a characteristic singlet signal (2-Cl: δ = 38.4 ppm, 2-Br: δ = 34.9 ppm, 2-I: δ = 18.7 ppm), which appears at a lower field than that of the corresponding Si(II) halides SiX2(Idipp) (X = Cl: δ = 19.2 ppm,4a X = Br: δ = 10.9 ppm,4b X = I: δ = −9.7 ppm4d) (Table 1).† In both series of compounds, the 29Si NMR signals shift progressively to a higher field upon Cl → Br → I substitution, and the same trend is observed for the 13C NMR signals of the Si-bonded CNHC atoms (Table 1). The 1H and 13C{1H} NMR spectra of 2-Br and 2-I display a single set of signals for the homotopic Idipp groups originating from the time-averaged C2-symmetry of the RR/SS stereoisomers (the C2 axis perpendicularly intersects the Si–Si bond).† The peripheral N-bonded dipp substituents are locked in an orthogonal conformation versus the N-heterocyclic rings (Fig. 1), which, in combination with the presence of stereogenic Si centers, gives rise to two different sets of 1H/13C NMR signals for the ortho- and meta-positioned groups, respectively (see Fig. S1, S2, S6 and S8 in the ESI†). Whereas all the 1H (300.1 MHz) and 13C (75.47 MHz) NMR signals of 2-Br were sharp at 298 K, several signals of 2-I were broadened under the same conditions, suggesting a dynamic behavior (see Fig. S6–S8 in the ESI†). Variable-temperature 1H NMR spectroscopy of 2-Br and 2-I in the temperature range 203–333 K revealed a hindered rotation of the NHC groups about the Si–CNHC bonds, which leads to a duplication of the signals of the dipp substituents and the N-heterocyclic C4,5-H ring protons in the slow-exchange limit spectra (see Fig. S4 and S10 in the ESI†). Analysis of the full coalescence behavior of the two singlet signals observed for the N-heterocyclic C4,5-H ring protons in the temperature range 203–333 K (see Fig. S4 and S10 in the ESI†) allowed an estimation of the standard Gibbs energy of activation for the hindered Si–CNHC rotation (2-Br: ΔG≠ = 46 kJ mol−1, Tc (coalescence temperature) = 228 K; 2-I: ΔG≠ = 51 kJ mol−1, Tc = 248 K).†
Compounds 2-X contain many reactive sites for further functionalisation with the most appealing ones being the displacable halide and Idipp groups, which are not available in the silicon(I) congeners C and D (Chart 1). First reactivity studies were carried out focusing on the abstraction of the halide groups. In fact, the addition of one equivalent of [Li(Et2O)2.5][B(C6F5)4] to a solution of 2-I in fluorobenzene at ambient temperature was accompanied by a colour change from bright to dark red and precipitation of LiI. Iodide abstraction from 2-I selectively afforded the disilicon(I) salt [Si2(I)(Idipp)2][B(C6F5)4] (3), as evidenced by NMR spectroscopy of the crude reaction mixture (Scheme 2). The salt was isolated after a work-up and crystallisation from a fluorobenzene/n-hexane mixture as dark red crystals of the fluorobenzene monosolvate (3·(C6H5F)) in 62% yield, and was comprehensively characterised.†
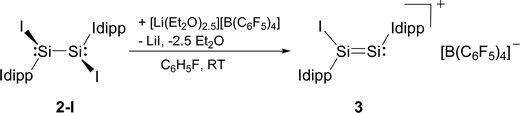 | ||
| Scheme 2 Synthesis of the disilicon(I) iodido salt 3 by iodide abstraction from 2-I. Formal charges are not included for simplicity. | ||
Compound 3·(C6H5F) is an extremely air-sensitive solid, which is instantly degraded by air to a colourless powder. It is stable in THF-d8 solution for several days under strict exclusion of air, and decomposes upon heating in a sealed glass capillary tube under vacuum at 208 °C.
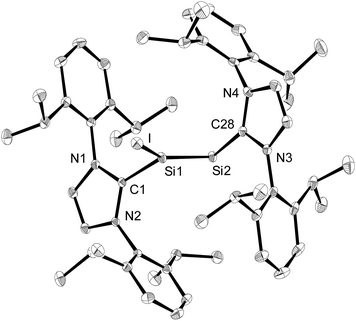
The solid-state structure of 3·(C6H5F) was determined by single-crystal X-ray diffraction and it is composed of well separated [Si2(I)(Idipp)2]+ and [B(C6F5)4]− ions.† The closest Si⋯F interionic contacts (6.371(3) Å) are significantly longer than the sum of the van der Waals radii of silicon and fluorine (3.6 Å).23 The cations [Si2(I)(Idipp)2]+ feature a trigonal planar coordinated Si1 atom (sum of the bond angles at Si1 = 359.7(1)°) and a two-coordinate Si2 atom with V-shaped geometry (Fig. 2). The two silicon atoms form a planar core with the end-on bonded iodine atom and the two CIdipp atoms (C1 and C28). The Si–Si bond of 3·(C6H5F) is considerably shorter (2.1739(9) Å) than the Si–Si single bond of 2-I (2.3909(9) Å), and also shorter than the Si–Si double bond of 1 (2.229(1) Å),2a but lies in the reported range of Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si bond lengths.24 The presence of a Si–Si double bond was further confirmed by the electronic structure analysis of [Si2(I)(Idipp)2]+ (vide infra). The bulky NHC groups are trans-arranged at the Si–Si double bond (torsion angles: C1–Si–Si2–C28 = −178.5(1)° and I–Si1–Si2–C28 = −6.71(9)°) and orthogonally oriented with respect to the planar core of the cation.25 The angle at the two-coordinate Si atom is quite narrow (Si1–Si2–C28 = 96.61(7)°) and compares well with those observed in 1 (Si–Si–CNHC = 93.37(5)°),2a the NHC-stabilised phosphasilenylidene (Idipp)Si
Si bond lengths.24 The presence of a Si–Si double bond was further confirmed by the electronic structure analysis of [Si2(I)(Idipp)2]+ (vide infra). The bulky NHC groups are trans-arranged at the Si–Si double bond (torsion angles: C1–Si–Si2–C28 = −178.5(1)° and I–Si1–Si2–C28 = −6.71(9)°) and orthogonally oriented with respect to the planar core of the cation.25 The angle at the two-coordinate Si atom is quite narrow (Si1–Si2–C28 = 96.61(7)°) and compares well with those observed in 1 (Si–Si–CNHC = 93.37(5)°),2a the NHC-stabilised phosphasilenylidene (Idipp)Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PR (R = C6H2-2,4,6-tBu3; P–Si–CNHC = 96.90(6)°)9 and the NHC-stabilised disilavinylidene (SIdipp)Si
PR (R = C6H2-2,4,6-tBu3; P–Si–CNHC = 96.90(6)°)9 and the NHC-stabilised disilavinylidene (SIdipp)Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si(Br)R (R = C6H2-2,6-{CH(SiMe3)2}2-4-tBu; Si–Si–CNHC = 97.6(1)°).10 A rationale for the narrow angle at Si2 is provided by the NBO analysis of [Si2(I)(Idipp)2]+, which indicates the presence of a stereochemically active lone-pair in an orbital of high s-character (77%) and Si2 hybrid orbitals of high p-character employed for the σ-bonding to the Si1 atom and the NHC group (87 and 89%, respectively, see Table 4). The Si1–I bond (2.4654(7) Å) compares well with that of the iodotriaryldisilene Tip(I)Si
Si(Br)R (R = C6H2-2,6-{CH(SiMe3)2}2-4-tBu; Si–Si–CNHC = 97.6(1)°).10 A rationale for the narrow angle at Si2 is provided by the NBO analysis of [Si2(I)(Idipp)2]+, which indicates the presence of a stereochemically active lone-pair in an orbital of high s-character (77%) and Si2 hybrid orbitals of high p-character employed for the σ-bonding to the Si1 atom and the NHC group (87 and 89%, respectively, see Table 4). The Si1–I bond (2.4654(7) Å) compares well with that of the iodotriaryldisilene Tip(I)Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) SiTip2 (2.4520(7) Å, Tip = C6H2-2,4,6-iPr3),26 but is considerably shorter than the Si–I bond lengths of 2-I (Si1–I1: 2.6036(6) Å; Si2–I2 2.5919(6) Å) (Table 1). This trend can be rationalised according to comparative NBO analyses of 3 and 2-I with the increased s-character of the Si hybrid orbital employed in the Si–I bond of 3 (20%) compared to that in 2-I (4%), and this is also reflected in the Si–I Wiberg bond indexes (3: WBI (Si–I) = 0.89; 2-I: WBI (Si–I) = 0.78) (see Table 4 and Table S8 in the ESI†). The Si–CNHC bond lengths of 3·(C6H5F) (1.901(2) and 1.931(2) Å) have similar values to those of 2-I (1.943(2) Å and 1.939(2) Å) and 1 (1.927(2) Å) (Table 1).2a
SiTip2 (2.4520(7) Å, Tip = C6H2-2,4,6-iPr3),26 but is considerably shorter than the Si–I bond lengths of 2-I (Si1–I1: 2.6036(6) Å; Si2–I2 2.5919(6) Å) (Table 1). This trend can be rationalised according to comparative NBO analyses of 3 and 2-I with the increased s-character of the Si hybrid orbital employed in the Si–I bond of 3 (20%) compared to that in 2-I (4%), and this is also reflected in the Si–I Wiberg bond indexes (3: WBI (Si–I) = 0.89; 2-I: WBI (Si–I) = 0.78) (see Table 4 and Table S8 in the ESI†). The Si–CNHC bond lengths of 3·(C6H5F) (1.901(2) and 1.931(2) Å) have similar values to those of 2-I (1.943(2) Å and 1.939(2) Å) and 1 (1.927(2) Å) (Table 1).2a
Notably, a comparison of [Si2(I)(Idipp)2]+ with the related cations [Si2(H)(Idipp)2]+ and [Si2(Me)(Idipp)2]+, the NHC-stabilised disilavinylidenes, the NHC-stabilised disilynes and the disilenide anions (Fig. 3) reveals a similar electronic structure of these molecules leading to common structural features, such as a planar core, similar Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si bond lengths and similar bond angles at the two-coordinate Si atom (Table 2).10,27–29
Si bond lengths and similar bond angles at the two-coordinate Si atom (Table 2).10,27–29
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si(NHC)]+ (NHC = C[N(dipp)CH]2 (Idipp), R1 = I, H, Me), the neutral NHC-stabilised disilavinylidenes R2R3Si
Si(NHC)]+ (NHC = C[N(dipp)CH]2 (Idipp), R1 = I, H, Me), the neutral NHC-stabilised disilavinylidenes R2R3Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si(NHC) (NHC = C[N(dipp)CH2]2 (SIdipp); R2 = Br, R3 = C6H2-2,6-{CH(SiMe3)2}2-4-tBu (Tbb)), the NHC-stabilised disilynes (NHC)R4Si
Si(NHC) (NHC = C[N(dipp)CH2]2 (SIdipp); R2 = Br, R3 = C6H2-2,6-{CH(SiMe3)2}2-4-tBu (Tbb)), the NHC-stabilised disilynes (NHC)R4Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) SiR4 (NHC = C[N(Me)CMe]2 (IMe4), R4 = SiiPr{CH(SiMe3)2}2) and the disilenide anions [R4HSi
SiR4 (NHC = C[N(Me)CMe]2 (IMe4), R4 = SiiPr{CH(SiMe3)2}2) and the disilenide anions [R4HSi![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) SiR4]−
SiR4]−
| d(Si–Si) [Å] | ∠Si1–Si2–R [°] | Ref. | |
|---|---|---|---|
[(Idipp)(I)Si1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si2(Idipp)]+ Si2(Idipp)]+ |
2.1739(9) | 96.61(7) | This paper |
[(Idipp)(H)Si1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si2(Idipp)]+ Si2(Idipp)]+ |
2.1873(8) | 95.34(6) | 27 |
[(Idipp)(Me)Si1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si2(Idipp)]+ Si2(Idipp)]+ |
2.1909(8) | 95.13(6) | 27 |
| Tbb(Br)Si1 = Si2(SIdipp) | 2.167(2) | 97.6(1) | 10 |
(IMe4)R4Si1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si2R4 Si2R4 |
2.1989(6) | 120.35(2) | 28 |
[R4(H)Si1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si2R4]− Si2R4]− |
2.2034(9) | 102.69(3) | 29 |
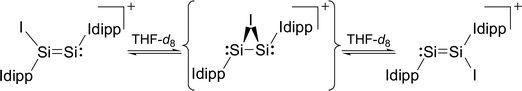
Variable-temperature 1H, 29Si and 13C NMR studies of 3·(C6H5F) in THF-d8 revealed an interesting dynamic process leading to an exchange of the heterotopic Si sites. The degenerate isomerisation (topomerisation)30 is suggested by quantum chemical calculations to proceed via a NHC-stabilised disilaiodonium ion (Scheme 3).31
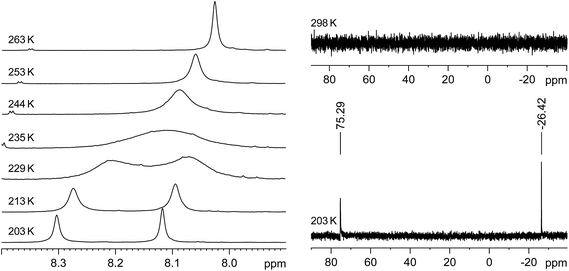
Thus, two well separated 29Si NMR signals at δ = −26.4 ppm and +75.3 ppm are observed in the slow exchange limit 29Si{1H} NMR spectrum of 3·(C6H5F) at 203 K (Fig. 4, right), which are assigned by B97-D3/IGLOIII/ZORA-def2-TZVP (iodine atom)/ZORA/COSMO(THF) calculations to the three-coordinate, I-bonded silicon (Si1) and the two-coordinate silicon (Si2) nuclei, respectively.32 In comparison, no 29Si NMR signals could be detected at 298 K even after a long accumulation time (intermediate time regime) (Fig. 4, right). Similarly, the slow-exchange limit 1H NMR spectrum of 3·(C6H5F) at 203 K displays a double set of resonance signals for the chemically different Idipp groups (see Fig. S11 and S12 in the ESI†). Most distinctive are the two singlet signals for the N-heterocyclic C4,5-H ring protons, which, upon increasing temperature coalesce at Tc = 235 K, and then merge into one sharp signal in the fast-exchange limit 1H NMR spectrum at 298 K (see Fig. 4, left and Fig. S20 in the ESI†). Likewise, the 13C{1H} NMR spectrum of 3·(C6H5F) at 203 K shows a double set of signals for the inequivalent Idipp groups (for example, two singlets for the Si-bonded CNHC nuclei at δ = 153.6 and 172.2 ppm), which merge into one set of signals at 298 K (see Fig. S14–S17 in the ESI†). The number and relative intensity of the signals in the slow-exchange limit 1H and 13C{1H} NMR spectra of 3·(C6H5F) are compatible with the results of the single-crystal X-ray diffraction and show an averaged Cs-symmetric structure of the cation [Si2(I)(Idipp)2]+ with fast rotating NHC substituents about the respective Si–CNHC bonds.33
The rate constants of the dynamic process were determined by full line-shape analyses of the signals of the N-heterocyclic C4,5-H ring protons in the temperature range of 203–263 K (Fig. 4, left and Fig. S20 in the ESI†). An Eyring plot of ln(k/T) against 1/T (k = rate constant, T = temperature) afforded a linear relationship (see Section 3 in the ESI†). The activation parameters of the dynamic process were obtained from the slope and the intercept of the corresponding regression line (R2 = 0.9966) and were found to be ΔH≠ = 47.3 (±0.7) kJ mol−1, ΔS≠ = 1.39 (±3.0) J K−1 mol−1 and ΔG≠ (Tc = 235 K) = 47.0 (±1.4) kJ mol−1.†
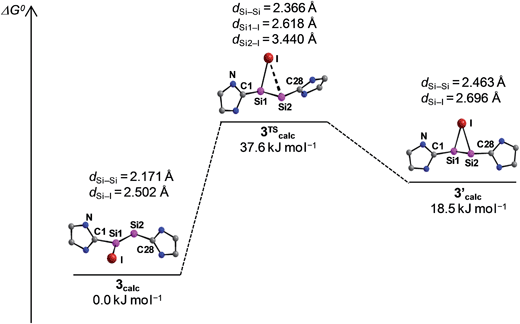
The potential energy hypersurface (PES) of the cation [Si2(I)(Idipp)2]+ was studied by quantum chemical calculations at the B97-D3/I level of theory17 in order to analyse the topomerisation process of 3·(C6H5F) observed in solution. Geometry optimization of [Si2(I)(Idipp)2]+ afforded a “σ-bonded” minimum structure (3calc) with an excellent agreement between the calculated and the experimental bond lengths obtained for 3·(C6H5F) by single-crystal X-ray crystallography (Fig. 2 and Table 3).
| Si1–Si2 [Å] | Si1–C1 [Å] | Si2–C28 [Å] | Si–I [Å] | C1–Si1–Si2 [°] | C1–Si1–I [°] | Si1–Si2–C28 [°] | I–Si1–Si2 [°] | φ NHC1 [°] | φ NHC2 [°] | |
|---|---|---|---|---|---|---|---|---|---|---|
| a The dihedral angles φNHC1 and φNHC2 are the respective angles between the least-square plane of the atoms C1, Si1, Si2 and C28 and the respective NHC central ring planes. b The corresponding angles C28–Si2–I and I–Si2–Si1 are 76.12° and 49.48° (3TScalc) and 98.24° and 62.84° (3′calc). | ||||||||||
| 3·(C6H5F)exp | 2.1739(9) | 1.901(2) | 1.931(2) | 2.4654(7) | 112.83(7) | 104.56(7) | 96.61(7) | 142.27(3) | 96.69(7) | 95.78(7) |
| 3calc | 2.171 | 1.903 | 1.923 | 2.502 | 112.06 | 103.58 | 96.96 | 144.35 | 89.10 | 89.68 |
| 3TScalc | 2.366 | 1.950 | 1.936 | 2.618 | 95.39 | 104.66b | 97.44 | 87.14b | 91.98 | 45.56 |
| 3.440 | ||||||||||
| 3′calc | 2.463 | 1.977 | 1.975 | 2.696 | 101.49 | 98.24b | 101.79 | 62.84b | 78.45 | 81.02 |
Furthermore, a “π-bonded” C2-symmetric minimum structure (3′calc) was located on the PES, which is less stable by 18.5 kJ mol−1 than the “σ-bonded” isomer of [Si2(I)(Idipp)2]+ (3calc) (Fig. 5). The two minimum structures are connected via a transition state (3TScalc), which lies at an energy 37.6 kJ mol−1 higher than the overall minimum structure 3calc (Fig. 5). The transition state has an imaginary frequency of −92 cm−1, and connects the two minimum structures via a rocking vibrational mode of the iodine atom. The calculated barrier of 37.6 kJ mol−1 compares acceptably well with that obtained from the variable-temperature NMR studies (vide supra). The most striking bonding parameters of 3′calc are the elongated Si–Si single bond (2.463 Å), which is considerably longer than the Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si bond of 3calc (2.171 Å), as well as the Si–I bonds (2.696 Å), which are longer than that of 3calc (Si–I: 2.502 Å). These bonding parameters suggest that 3′calc can be better described as a NHC-stabilised disilaiodonium ion34 rather than a Si2(Idipp)2 (1) π-complex of I+. Notably, the structure of 3′calc is reminiscent of those of the symmetrical 1,2-bridged halonium ions, which have been extensively studied in organic chemistry.35
Si bond of 3calc (2.171 Å), as well as the Si–I bonds (2.696 Å), which are longer than that of 3calc (Si–I: 2.502 Å). These bonding parameters suggest that 3′calc can be better described as a NHC-stabilised disilaiodonium ion34 rather than a Si2(Idipp)2 (1) π-complex of I+. Notably, the structure of 3′calc is reminiscent of those of the symmetrical 1,2-bridged halonium ions, which have been extensively studied in organic chemistry.35
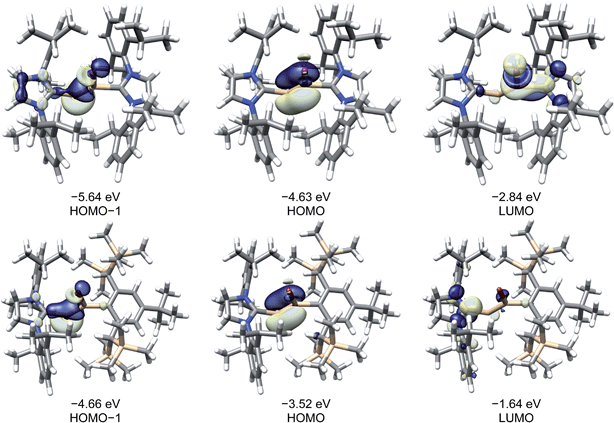
A comparison of the frontier Kohn–Sham orbitals of the ion [Si2(I)(Idipp)2]+ with those of the NHC-stabilised disilavinylidene (SIdipp)Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si(Br)R (R = C6H2-2,6-{CH(SiMe3)2}2-4-tBu)10 or the model compound (IMe4)RSi
Si(Br)R (R = C6H2-2,6-{CH(SiMe3)2}2-4-tBu)10 or the model compound (IMe4)RSi![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) SiR (IMe4 = C[N(Me)CMe]2, R = SiMe3)28 revealed the same symmetry properties, shape and approximate energy of the frontier orbitals, confirming the isolobal analogy of these molecules, which can be traced back to the electronic relationship of the fragments Si(NHC) and (SiR)− (R = singly bonded substituent). In all cases, the HOMO is the Si
SiR (IMe4 = C[N(Me)CMe]2, R = SiMe3)28 revealed the same symmetry properties, shape and approximate energy of the frontier orbitals, confirming the isolobal analogy of these molecules, which can be traced back to the electronic relationship of the fragments Si(NHC) and (SiR)− (R = singly bonded substituent). In all cases, the HOMO is the Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si π-bonding orbital, which is followed by the lone-pair orbital at the two-coordinate Si atom (HOMO−1) (Fig. 6). The electronic structure of the “σ-bonded” isomer of [Si2(I)(Idipp)2]+ (3calc) was analysed by the natural bond orbital (NBO) method and natural resonance theory (NRT) (see Table 4), and the results were compared with those of the “π-bonded” isomer of [Si2(I)(Idipp)2]+ (3′calc) (see Table S10 in the ESI†) and 2-Icalc (see Table S8 in the ESI†). NBO analysis of the wavefunction of 3calc suggests a high localization of the orbitals describing the Si
Si π-bonding orbital, which is followed by the lone-pair orbital at the two-coordinate Si atom (HOMO−1) (Fig. 6). The electronic structure of the “σ-bonded” isomer of [Si2(I)(Idipp)2]+ (3calc) was analysed by the natural bond orbital (NBO) method and natural resonance theory (NRT) (see Table 4), and the results were compared with those of the “π-bonded” isomer of [Si2(I)(Idipp)2]+ (3′calc) (see Table S10 in the ESI†) and 2-Icalc (see Table S8 in the ESI†). NBO analysis of the wavefunction of 3calc suggests a high localization of the orbitals describing the Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si, Si–CNHC, and Si–I bonds (Table 4). For example, the Si–Si σ-bond NBO is occupied by 1.90 electrons and the Si–Si π-bond NBO is occupied by 1.89 electrons. Whereas the Si–Si σ-bond is slightly polarised towards the Si1 atom and is formed from the overlap of a Si1 natural hybrid orbital (NHO) with high s-character (59%) and a Si2 NHO with high p-character (87%), the Si–Si π-bond NBO is less polarised and is formed from pure Si p-orbitals. The moderate polarisation of the Si
Si, Si–CNHC, and Si–I bonds (Table 4). For example, the Si–Si σ-bond NBO is occupied by 1.90 electrons and the Si–Si π-bond NBO is occupied by 1.89 electrons. Whereas the Si–Si σ-bond is slightly polarised towards the Si1 atom and is formed from the overlap of a Si1 natural hybrid orbital (NHO) with high s-character (59%) and a Si2 NHO with high p-character (87%), the Si–Si π-bond NBO is less polarised and is formed from pure Si p-orbitals. The moderate polarisation of the Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si bond of 3calc and the high occupancies of its NBO lead to a high Wiberg bond index (WBI) of 1.81, which is twice as large as the WBI of the Si–Si single bond of 3′calc (0.89) and 2-I (0.96). These findings verify the presence of a Si
Si bond of 3calc and the high occupancies of its NBO lead to a high Wiberg bond index (WBI) of 1.81, which is twice as large as the WBI of the Si–Si single bond of 3′calc (0.89) and 2-I (0.96). These findings verify the presence of a Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si bond in the “σ-bonded” isomer (3calc) and a Si–Si single bond in the “π-bonded” isomer (3′calc) of [Si2(I)(Idipp)2]+ or in 2-I, and are further confirmed by the NRT Si–Si bond orders, which, in the case of 3calc, is twice as large (NRT-BO = 1.95, Table 4) as that of 3′calc (0.95) or 2-I (0.93) (see Tables S8 and S10 in the ESI†). In the “σ-bonded” isomer 3calc, the two-coordinate silicon atom (Si2) carries a lone pair of electrons in an NHO with high s-character (77%, Table 4), as was found for the NHC-stabilised disilavinylidene.10 In comparison, both Si atoms in the “π-bonded” isomer 3′calc carry a lone pair of electrons in NHO orbitals with high s-character (79%, see Table S10 in the ESI†), providing additional evidence for the different structures of the “σ-bonded” and “π-bonded” isomers of [Si2(I)(Idipp)2]+.
Si bond in the “σ-bonded” isomer (3calc) and a Si–Si single bond in the “π-bonded” isomer (3′calc) of [Si2(I)(Idipp)2]+ or in 2-I, and are further confirmed by the NRT Si–Si bond orders, which, in the case of 3calc, is twice as large (NRT-BO = 1.95, Table 4) as that of 3′calc (0.95) or 2-I (0.93) (see Tables S8 and S10 in the ESI†). In the “σ-bonded” isomer 3calc, the two-coordinate silicon atom (Si2) carries a lone pair of electrons in an NHO with high s-character (77%, Table 4), as was found for the NHC-stabilised disilavinylidene.10 In comparison, both Si atoms in the “π-bonded” isomer 3′calc carry a lone pair of electrons in NHO orbitals with high s-character (79%, see Table S10 in the ESI†), providing additional evidence for the different structures of the “σ-bonded” and “π-bonded” isomers of [Si2(I)(Idipp)2]+.
| NBO analysis | NPA partial chargesb | NRT analysisc | ||||||
|---|---|---|---|---|---|---|---|---|
| occ.a | pol.a [%] | hyb.a | WBIa | tot/cov/ionica | ||||
| a occ.: occupancy, pol.: polarization, hyb.: hybridization, WBI: Wiberg bond index, tot/cov/ionic: total bond order/covalent bond order/ionic bond order. b Partial charges obtained by natural population analysis (NPA). c A local NRT analysis was carried out including the Si1, Si2, I, N, C1 and C28 atoms. | ||||||||
| σ(Si1–Si2) | 1.90 | 62.0 (Si1) | sp0.69 (Si1) | 1.81 | Si1 | 0.30 | Si1–Si2 | 1.95/1.55/0.41 |
| 38.1 (Si2) | sp7.03 (Si2) | |||||||
| π(Si1–Si2) | 1.89 | 58.1 (Si1) | p (Si1) | Si2 | 0.18 | |||
| 41.9 (Si2) | p (Si2) | |||||||
| σ(Si1–C1) | 1.95 | 24.3 (Si1) | sp3.85 (Si1) | 0.72 | C1 | 0.05 | Si1–C1 | 1.00/0.47/0.53 |
| 75.8 (C1) | sp1.39 (C1) | ∑(NHC1) | 0.41 | |||||
| σ(Si2–C28) | 1.93 | 21.8 (Si2) | sp8.50 (Si2) | 0.76 | C28 | 0.06 | Si2–C28 | 1.03/0.43/0.61 |
| 78.3 (C28) | sp1.28 (C28) | ∑(NHC2) | 0.28 | |||||
| σ(Si1–I) | 1.96 | 34.5 (Si1) | sp3.87 (Si1) | 0.89 | I | −0.18 | Si1–I | 0.93/0.64/0.30 |
| 65.5 (I) | sp5.62 (I) | |||||||
| n(Si2) | 1.77 | sp0.29 | ∑(Si2I) | 0.30 | ||||
Finally, a natural population analysis of 3calc indicates a considerable charge flow from the NHC to the disilaiodonium ion [Si2I]+, as evidenced by the overall NPA charges of the NHCs (Si1-bonded: q(∑(NHC)) = 0.41; Si2-bonded: q(∑(NHC)) = 0.28) (Table 4).
Conclusions
An efficient method for the synthesis of silicon(I) halides Si2X2(Idipp)2 (2-X, X = Cl, Br, I) was developed, which involved a diastereoselective halogenation of Si2(Idipp)2 (1) with 1,2-dihaloethanes. This allowed the isolation of the first silicon(I) bromide (2-Br) and silicon(I) iodide (2-I) in high yield, enabling first reactivity studies of 2-I. The geometric and electronic structures of 2-Br and 2-I were comprehensively studied by experimental and theoretical methods. Iodide abstraction from 2-I selectively afforded the unprecedented disilicon(I) iodido salt [Si2(I)(Idipp)2][B(C6F5)4] (3), the geometric and electronic structure of which is isolobal to that of a NHC-stabilised disilavinylidene recently reported by our group. The topomerisation of the cation [Si2(I)(Idipp)2]+, leading to an exchange of the two heterotopic Si sites, was studied by variable-temperature NMR spectroscopy and the underlying dynamic process was analysed by quantum chemical calculations. The calculations suggest the intermediate formation of a C2-symmetric π-bonded isomer with homotopic Si sites reminiscent of the symmetrical 1,2-bridged halonium ions in organic chemistry. The present results corroborate the ability of N-heterocyclic carbenes to stabilise low-valent main-group element centers with unusual bonding features. Further studies addressing the reactivity of the NHC-stabilised Si(I) halides 2-X and the SiI salt 3 are currently underway.Acknowledgements
We thank the Deutsche Forschungsgemeinschaft (SFB813, “Chemistry at Spin Centers”) for the financial support for this work. We also thank Dr S. Nozinovic, K. Prochnicki and H. Spitz for recording the solution NMR spectra and K. Kühnel-Lysek, A. Martens and Dr S. Rings for the elemental analyses.Notes and references
- For recent reviews see: (a) H. W. Roeksy, J. Organomet. Chem., 2013, 730, 57 CrossRef; (b) R. S. Ghadwal, R. Azhakar and H. W. Roesky, Acc. Chem. Res., 2013, 46, 444 CrossRef CAS PubMed; (c) Y. Wang and G. H. Robinson, Inorg. Chem., 2014, 53, 11815 CrossRef CAS PubMed; (d) E. Rivard, Struct. Bonding, 2014, 156, 203 CrossRef CAS.
- (a) Y. Wang, Y. Xie, P. Wei, R. B. King, H. F. Schaefer III, P. v. R. Schleyer and G. H. Robinson, Science, 2008, 321, 1069 CrossRef CAS PubMed; (b) O. Chernov, Novel Molecular Si(II) Precursors for the Synthesis of the First Compounds with Metal–Silicon Triple Bonds, PhD thesis, University of Bonn, 2012.
- Y. Xiong, S. Yao, S. Inoue, J. D. Epping and M. Driess, Angew. Chem., Int. Ed., 2013, 52, 7147 ( Angew. Chem. , 2013 , 125 , 7287 ) CrossRef CAS PubMed.
- (a) R. S. Ghadwal, H. W. Roesky, S. Merkel, J. Henn and D. Stalke, Angew. Chem., Int. Ed., 2009, 48, 5683 ( Angew. Chem. , 2009 , 121 , 5793 ) CrossRef CAS PubMed; (b) A. C. Filippou, O. Chernov and G. Schnakenburg, Angew. Chem., Int. Ed., 2009, 48, 5687 ( Angew. Chem. , 2009 , 121 , 5797 ) CrossRef CAS PubMed; (c) A. C. Filippou, O. Chernov and G. Schnakenburg, Chem.–Eur. J., 2011, 17, 13574 CrossRef CAS PubMed; (d) A. C. Filippou, Y. N. Lebedev, O. Chernov, M. Straßmann and G. Schnakenburg, Angew. Chem., Int. Ed., 2013, 52, 6974 ( Angew. Chem. , 2013 , 125 , 7112 ) CrossRef CAS PubMed.
- (a) A. C. Filippou, O. Chernov, B. Blom, K. W. Stumpf and G. Schnakenburg, Chem.–Eur. J., 2010, 16, 2866 CrossRef CAS PubMed; (b) H. Cui and C. Cui, Dalton Trans., 2011, 40, 11937 RSC; (c) S. M. I. Al-Rafia, R. McDonald, M. J. Ferguson and E. Rivard, Chem.–Eur. J., 2012, 18, 13810 CrossRef CAS PubMed; (d) S. Inoue and C. Eisenhut, J. Am. Chem. Soc., 2013, 135, 18315 CrossRef CAS PubMed; (e) T. Agou, N. Hayakawa, T. Sasamori, T. Matsuo, D. Hashizume and N. Tokitoh, Chem.–Eur. J., 2014, 20, 9246 CrossRef CAS PubMed.
- M. J. Cowley, V. Huch, H. S. Rzepa and D. Scheschkewitz, Nat. Chem., 2013, 5, 876 CrossRef CAS PubMed.
- (a) Y. Gao, J. Zhang, H. Hu and C. Cui, Organometallics, 2010, 29, 3063 CrossRef CAS; (b) Y. N. Lebedev, U. Das, O. Chernov, G. Schnakenburg and A. C. Filippou, Chem.–Eur. J., 2014, 20, 9280 CrossRef CAS PubMed.
- S. U. Ahmad, T. Szilvási and S. Inoue, Chem. Commun., 2014, 50, 12619 RSC.
- D. Geiß, M. I. Arz, M. Straßmann, G. Schnakenburg and A. C. Filippou, Angew. Chem., Int. Ed., 2015, 54, 2739 ( Angew. Chem. , 2015 , 127 , 2777 ) CrossRef PubMed.
- P. Ghana, M. I. Arz, U. Das, G. Schnakenburg and A. C. Filippou, Angew. Chem., Int. Ed., 2015, DOI: 10.1002/anie.201504494, Angew. Chem., 2015, DOI: 10.1002/ange.201504494.
- (a) A. C. Filippou, O. Chernov, K. W. Stumpf and G. Schnakenburg, Angew. Chem., Int. Ed., 2010, 49, 3296 ( Angew. Chem. , 2010 , 122 , 3368 ) CrossRef CAS PubMed; (b) A. C. Filippou, O. Chernov and G. Schnakenburg, Angew. Chem., Int. Ed., 2011, 50, 1122 ( Angew. Chem. , 2011 , 123 , 1154 ) CrossRef CAS PubMed; (c) A. C. Filippou, B. Baars, O. Chernov, Y. N. Lebedev and G. Schnakenburg, Angew. Chem., Int. Ed., 2014, 53, 565 ( Angew. Chem. , 2014 , 126 , 576 ) CrossRef CAS PubMed.
- (a) A. Sekiguchi, R. Kinjo and M. Ichinohe, Science, 2004, 305, 1755 CrossRef CAS PubMed; (b) N. Wiberg, S. K. Vasisht, G. Fischer and P. Mayer, Z. Anorg. Allg. Chem., 2004, 630, 1823 CrossRef CAS; (c) T. Sasamori, K. Hironaka, Y. Sugiyama, N. Takagi, S. Nagase, Y. Hosoi, Y. Furukawa and N. Tokitoh, J. Am. Chem. Soc., 2008, 130, 13856 CrossRef CAS PubMed; (d) Y. Murata, M. Ichinohe and A. Sekiguchi, J. Am. Chem. Soc., 2010, 132, 16768 CrossRef CAS PubMed; (e) S. Ishida, R. Sugawara, Y. Misawa and T. Iwamoto, Angew. Chem., Int. Ed., 2013, 52, 12869 ( Angew. Chem. , 2013 , 125 , 13107 ) CrossRef CAS PubMed. For a review see: (f) M. Asay and A. Sekiguchi, Bull. Chem. Soc. Jpn., 2012, 85, 1245 CrossRef CAS.
- (a) S. S. Sen, A. Jana, H. W. Roesky and C. Schulzke, Angew. Chem., Int. Ed., 2009, 48, 8536 ( Angew. Chem. , 2009 , 121 , 8688 ) CrossRef CAS PubMed; (b) C. Jones, S. J. Bonyhady, N. Holzmann, G. Frenking and A. Stasch, Inorg. Chem., 2011, 50, 12315 CrossRef CAS PubMed.
- D. Gau, R. Rodriguez, T. Kato, N. Saffon-Merceron, A. de Cózar, F. P. Cossío and A. Baceiredo, Angew. Chem., Int. Ed., 2011, 50, 1092 ( Angew. Chem. , 2011 , 123 , 1124 ) CrossRef CAS PubMed.
- For a recent review on the reactivity of compounds C and D, see: (a) S. S. Sen, S. Khan, S. Nagendran and H. W. Roesky, Acc. Chem. Res., 2012, 45, 578 CrossRef CAS PubMed. For further reactivity studies after 2012 see: (b) H.-X. Yeong, S.-H. Zhang, H.-W. Xi, J.-D. Guo, K. H. Lim, S. Nagase and C.-W. So, Chem.–Eur. J., 2012, 18, 2685 CrossRef CAS PubMed; (c) S.-H. Zhang, H.-W. Xi, K. H. Lim, Q. Meng, M.-B. Huang and C.-W. So, Chem.–Eur. J., 2012, 18, 4258 CrossRef CAS PubMed; (d) S.-H. Zhang, H.-W. Xi, K. H. Lim and C.-W. So, Angew. Chem., Int. Ed., 2013, 52, 12364 ( Angew. Chem. , 2013 , 125 , 12590 ) CrossRef CAS PubMed; (e) H.-X. Yeong, H.-W. Xi, Y. Li, K. H. Lim and C.-W. So, Chem.–Eur. J., 2013, 19, 11786 CrossRef CAS PubMed.
- (a) J. Clayden, N. Greeves, S. Warren and P. Wothers, Organic Chemistry, Oxford University Press, Oxford, 2001 Search PubMed; (b) M. B. Smith and J. March, Advanced Organic Chemistry, Wiley-Interscience, New Jersey, 6th edn, 2007 Search PubMed.
- The following abbreviations were used to designate the levels of theory used for the quantum chemical calculations: B97–D3/I = B97-D3/def2-TZVP(Si, N and carbene C atoms)/def2-TZVP-ZORA(iodine atom)/def2-SVP(peripherical C atoms and all H atoms)/RIJCOSX/COSMO(THF); B97-D3/II = B97-D3/def2-TZVP(Si, N and carbene C atoms)/def2-SVP(peripherical C atoms and all H atoms)/RIJ/COSMO(THF).
- J. Escudie, C. Couret, H. Ranaivonjatovo, J. Satge and J. Jaud, Phosphorus Sulfur Relat. Elem., 1983, 17, 221 CrossRef CAS.
- Remarkably, both diastereomers were reported for the isolobal 1,2-dihalodiphosphanes P2X2R2 depending on the substituents. For example, P2I2Mes2 (Mes = C6H2-2,4,6-Me3) adopts a meso form in the solid state, whereas for P2I2(η5-C5Me5)2 the (R,R) stereoisomer was found: (a) D. C. Pestana and P. P. Power, Inorg. Chem., 1991, 30, 528 CrossRef CAS; (b) H.-G. Stammler, P. Jutzi, B. Neumann and N. Brusdeilins, Acta Crystallogr., Sect. C: Struct. Chem., 1995, 51, 1139 CrossRef.
- A degree of pyramidalisation (DP) value of 0% describes a trigonal planar coordination of the silicon atom with the sum of the bond angles equal to 360°. A DP value of 100% corresponds to a trigonal pyramidal coordination of the silicon atom with the sum of the bond angles equal to 270°.
- H. A. Bent, Chem. Rev., 1961, 61, 275 CrossRef CAS.
- A. F. Holleman and E. Wiberg, Inorganic Chemistry, Academic Press, San Diego/London, 2001, Lehrbuch der Anorganischen Chemie, 101. verbesserte und stark erweiterte Auflage, deGruyter, Berlin, 2001.
- S. S. Batsanov, Inorg. Mater., 2001, 37, 871 CrossRef CAS.
- Si
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si bond lengths of disilenes have been reported to range from 2.118(1)–2.2700(5) Å: T. Iwamoto and S. Ishida, Struct. Bonding, 2014, 156, 125 CrossRef CAS.
Si bond lengths of disilenes have been reported to range from 2.118(1)–2.2700(5) Å: T. Iwamoto and S. Ishida, Struct. Bonding, 2014, 156, 125 CrossRef CAS. - The dihedral angles between the least-square planes of the NHC central rings and the C1–Si1–Si2–C28 plane are 96.69(7)° and 95.78(7)°, respectively.
- M. Hartmann, A. Haji-Abdi, K. Abersfelder, P. R. Haycock, A. J. P. White and D. Scheschkewitz, Dalton Trans., 2010, 39, 9288 RSC.
- The syntheses and molecular structures of [Si2(H)(Idipp)2][B(ArF)4] and [Si2(Me)(Idipp)2][B(ArF)4] have been reported: A. C. Filippou, O. Schiemann, M. Straßmann, A. Meyer, G. Schnakenburg and M. I. Arz, presented in part at The 17th International Symposium on Silicon Chemistry (ISOS XVII), Berlin, 3–8 August, 2014 Search PubMed.
- T. Yamaguchi, A. Sekiguchi and M. Driess, J. Am. Chem. Soc., 2010, 132, 14061 CrossRef CAS PubMed.
- R. Kinjo, M. Ichinohe and A. Sekiguchi, J. Am. Chem. Soc., 2007, 129, 26 CrossRef CAS PubMed.
- G. Binsch, E. L. Eliel and H. Kessler, Angew. Chem., Int. Ed. Engl., 1971, 10, 570 ( Angew. Chem. , 1971 , 83 , 618 ) CrossRef CAS.
- A similar dynamic process was suggested for the copper complex [CuCl(1)] without unambiguous experimental verification: M. Chen, Y. Wang, Y. Xie, P. Wei, R. J. Gilliard, Jr., N. A. Schwartz, H. F. Schaefer III, P. v. R. Schleyer and G. H. Robinson, Chem.–Eur. J., 2014, 20, 9208 CrossRef CAS PubMed.
- W. Kutzelnigg, U. Fleischer and M. Schindler, in NMR Basic Principles and Progress, ed. P. Diehl, E. Fluck, H. Günther, R. Kosfeld and J. Seelig, Springer-Verlag, Berlin/Heidelberg, 1991, vol. 23, pp. 165–262 Search PubMed.
- Only one set of signals was observed for the ortho- and meta-positioned nuclei of the N-bonded dipp substituents of each Idipp group. In the case of frozen Si–CNHC rotations, instead, a double set of signals would be expected, given the fixed orthogonal orientation of dipp substituents versus the respective N-heterocyclic rings and the orthogonal orientation of the N-heterocyclic rings versus the planar core of the cation [Si2(I)(Idipp)2]+.
- Disilahalonium ions [Si2X]+ (X = F, Cl) have been observed by mass spectrometry. However, their structures are not currently known. (a) W. D. Reents, Jr., A. M. Mujsce, V. E. Bondybey and M. L. Mandich, J. Chem. Phys., 1987, 86, 5568 CrossRef; (b) D. Schröder and H. Schwarz, Russ. Chem. Bull., 2001, 50, 2087 CrossRef.
- (a) G. A. Olah, Halonium Ions (Reactive intermediates in organic chemistry), Wiley-Interscience, New York, 1975 Search PubMed; (b) G. A. Olah, G. K. S. Prakash and V. V. Krishnamurthy, J. Org. Chem., 1983, 48, 5116 CrossRef CAS; (c) M.-F. Ruasse, Acc. Chem. Res., 1990, 23, 87 CrossRef CAS; (d) G. Bellucci, R. Bianchini, C. Chiappe, R. S. Brown and H. Slebocka-Tilk, J. Am. Chem. Soc., 1991, 113, 8012 CrossRef CAS; (e) B. K. Ohta, R. E. Hough and J. W. Schubert, Org. Lett., 2007, 9, 2317 CrossRef CAS PubMed; (f) B. K. Ohta, Pure Appl. Chem., 2013, 85, 1959 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Syntheses and analytical data of 2-Cl, 2-Br, 2-I and 3, illustrations of the 1H, 13C and 29Si NMR spectra of 2-Br, 2-I and 3, results of the analysis of the dynamic process of 3 in solution and details of the quantum chemical calculations of 2-Br, 2-I, SiBr2(Idipp) and the cation in 3. CCDC 1414787–1414789. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5sc02681d. NHC = N-heterocyclic carbene; Idipp = C[N(dipp)CH]2, dipp = C6H3-2,6-iPr2. |
| This journal is © The Royal Society of Chemistry 2015 |