Open Access Article
Open Access ArticleSynthesis of nanostructured chitin–hematite composites under extreme biomimetic conditions†
Marcin
Wysokowski
a,
Mykhailo
Motylenko
b,
Juliane
Walter
c,
Grzegorz
Lota
d,
Jarosław
Wojciechowski
d,
Hartmut
Stöcker
c,
Roberta
Galli
e,
Allison L.
Stelling
f,
Cameliu
Himcinschi
g,
Elke
Niederschlag
h,
Enrico
Langer
i,
Vasilii V.
Bazhenov
c,
Tomasz
Szatkowski
a,
Jakub
Zdarta
a,
Iaroslav
Pertenko
c,
Zoran
Kljajić
j,
Tilmann
Leisegang
k,
Serguei L.
Molodtsov
clm,
Dirk C.
Meyer
c,
Teofil
Jesionowski
a and
Hermann
Ehrlich
*c
aInstitute of Chemical Technology and Engineering, Poznan University of Technology, 60965 Poznan, Poland
bInstitute of Materials Science, TU Bergakademie Freiberg, 09599 Freiberg, Germany
cInstitute of Experimental Physics, TU Bergakademie Freiberg, Leipziger 23, 09599 Freiberg, Germany. E-mail: hermann.ehrlich@physik.tu-freiberg.de; Tel: +49-393731392867
dInstitute of Chemistry and Technical Electrochemistry, Faculty of Chemical Technology, Poznan University of Technology, 60965 Poznan, Poland
eFaculty of Medicine Carl Gustav Carus, Department of Anesthesiology and Intensive Care Medicine, Clinical Sensoring and Monitoring, Technische Universität Dresden, 01069 Dresden, Germany
fDepartment of Mechanical Engineering and Materials Science, Duke University, 27708 Durham, NC, USA
gInstitute of Theoretical Physics, TU Bergakademie Freiberg, Leipziger 23, 09599 Freiberg, Germany
hInsitut für NE-Metallurgie und Reinstoffe, TU Bergakademie Freiberg, Leipziger Str. 34, 09599 Freiberg, Germany
iInstitut für Oberflächenphysik und Mikrostrukturphysik, TU Dresden, 01062 Dresden, Germany
jInstitute of Marine Biology, University of Montenegro, 85330 Kotor, Montenegro
kFraunhofer-Technologiezentrum Halbleitermaterialien THM, Am St.-Niclas-Schacht 13, 09599 Freiberg, Germany
lEuropean X-Ray Free-Electron Laser Facility (XFEL) GmbH, 22761 Hamburg, Germany
mITMO University, Kronoverskiy pr. 49, 197101 St. Petersburg, Russia
First published on 11th November 2014
Abstract
Chitin of poriferan origin is a unique and thermostable biological material. It also represents an example of a renewable materials source due to the high regeneration ability of Aplysina sponges under marine ranching conditions. Chitinous scaffolds isolated from the skeleton of the marine sponge Aplysina aerophoba were used as a template for the in vitro formation of Fe2O3 under conditions (pH ∼ 1.5, 90 °C) which are extreme for biological materials. Novel chitin–Fe2O3 three dimensional composites, which have been prepared for the first time using hydrothermal synthesis, were thoroughly characterized using numerous analytical methods including Raman spectroscopy, XPS, XRD, electron diffraction and HR-TEM. We demonstrate the growth of uniform Fe2O3 nanocrystals into the nanostructured chitin substrate and propose a possible mechanism of chitin–hematite interactions. Moreover, we show that composites made of sponge chitin–Fe2O3 hybrid materials with active carbon can be successfully used as electrode materials for electrochemical capacitors.
Introduction
Extreme Biomimetics is a recently established and powerful approach for the synthesis of advanced nanostructured inorganic–organic materials with complex morphology, hierarchical organization and polymorphism.1–5 This new scientific direction is a milestone for modern biological materials inspired chemistry: especially in aspects where there is massive interest in the combination of various inorganic structures (i.e. nanocrystals, nanoparticles) with biological macromolecules. These unique combinations can be used for synthesis of novel functional materials used in tissue engineering, drug delivery systems, photonics, biosensors, catalysts and electrochemistry.6–9 In contrast to traditional aspects of biomimetic synthesis of biominerals, Extreme Biomimetics is based on mineralization of various biomolecules under conditions which mimic extreme aquatic niches like hydrothermal vents, or hot springs. Therefore, the basic principle of this concept is to use biopolymers which are chemically and thermally stable under these specific conditions in vitro. Selection and application of such biomacromolecules plays a crucial role in the nucleation, thermodynamic and kinetic crystal growth; and can also be used as a soft template for inorganic structures.10,11 There are plenty of examples of biomineralization phenomena in hot springs and hydrothermal vents in Nature (see for review12–14). These niches with extreme conditions favor polysaccharides as templates for biomineralization, as most proteins and peptides are denatured by high temperature (90 °C) or low pH (∼1.5).12,13 The large number of reactive groups, differing chemical compositions, structures and functionalities characteristic for polysaccharides make them common nucleating and templating agents in living cells.15–18 It is reported that exopolysaccharides19,20 from various gram positive and gram negative bacteria are able to capture Fe3+ ions from solution and induce precipitation of hematite (Fe2O3) outside of the cell. This phenomenon protects the organisms from becoming encrusted with minerals.21One of the most abundant polysaccharide involved in biomineralization is chitin. The outstanding mechanical properties of chitin22 play a very important role in Nature, where it is the main structural material that conveys stiffness and mechanical stability to the hard structures of arthropod cuticles,23 mollusc shells,24,25 gastropod operculi,26 cuttlebones,27,28 diatoms,29 corals30 and sponges.31–41 Thus, chitin and its derivatives are heavily investigated polysaccharides with regard to in vitro biomimetic mineralization. This includes calcification,11,42–44 silicification4,45–47 as well as structural biotemplating for the formation of ZnO,2,48 ZrO2,1,3 TiO2,49 and magnetite.50 Chemically, chitin is a linear polysaccharide composed of oxygen-containing hexose rings with an acetamido group at the second carbon position, linked together by β-1,4-glycosidic bonds.51,52 From the materials science point of view, chitin has a number of interesting properties which make it suitable for the development of bone substitutes,36 drug delivery systems,53 biosensors, and adsorbents and wound dressing materials.36,51,54 Additionally, chitin has high thermal stability up to 360 °C.55–57 This thermostability is the key factor for the development of a new generation of biomaterials at high temperatures according to Extreme Biomimetics concepts.1–3
Hematite is the most thermochemically stable iron oxide under ambient conditions.58 Additionally, α-Fe2O3 is an environmental friendly material which is characterized by small band gap,59 as well as unique electrical60 and catalytic properties.59 All these properties make hematite suitable for wide spectrum of applications, including sensors,61,62 catalysts for water splitting59,63 and for chemical reactions, drug delivery systems,64 pigments and batteries. Numerous combinations of various biomacromolecules like chitosan,65,66 silk67 and cellulose68 with iron oxides are intensively studied. Recently, the development of a simple collagen/iron oxide material for potential applications in tissue engineering and imaging by crosslinking collagen to starch capped α-Fe2O3 nanoparticles was reported.69 These starch capped nanoparticles were found to be nontoxic to fibroblast cells and were thus used for developing novel collagen constructs.
Peng et al.50 proved that chitin isolated from butterfly wings can be used as a 3D structural template for the development of hematite replicas with magnetophotonic properties using a sol–gel method followed by calcination. However, to the best of our knowledge, chitin–hematite materials prepared using hydrothermal route have not been reported before. Thus, in this study we decided to develop a Fe2O3-containing hybrid material using three-dimensional tube-like fibrous α-chitin scaffolds for the first time. These were isolated from marine demosponge Aplysina aerophoba35 (Fig. 1), and served as a new organic template under hydrothermal synthesis conditions. We selected this very special organic matrix because three-dimensional chitin-based scaffolds isolated from sponges (Porifera) are principally the promising candidates for practical applications in tissue engineering, catalysis, enzyme immobilization, etc.36 Additionally, application of these morphologically defined skeletal networks from Nature overcomes challenges associated with the prefabrication of chitin into three-dimensional structures.
Experimental
The marine sponge Aplysina aerophoba (Aplysinidae: Verongida: Demospongiae: Porifera) was collected in the Adriatic Sea (Kotor Bay, Montenegro) in August 2008 by SCUBA diving. Due to the regeneration capacity of A. aerophoba, divers only cut the apical parts of the sponge body. The basic parts were remain intact and can regenerate under natural conditions. Sponge samples were put in ziplock bags underwater, brought back to the laboratory at Institute of Marine Biology (Kotor, Montenegro) and frozen less than 1 h after collection. The sponge fragments as collected were prepared, lyophilized and transported by the INTIB GmbH (Germany) to the Laboratory of Biomineralogy & Extreme Biomimetics Group, TU Bergakademie Freiberg (Germany). Iron(III) chloride (no. 157740) and was obtained from Sigma-Aldrich (Germany). The α-chitin standard from A. aerophoba was prepared according to a previously described method35 by INTIB GmbH (Germany).Hydrothermal synthesis of chitin–Fe2O3 composites
In a typical experiment, hydrothermal deposition of Fe2O3 on a chitinous template was performed by the forced hydrolysis of iron(III) chloride.70–72 In brief, 0.3 g of anhydrous FeCl3 was dissolved in 10 ml of ultra-pure water. Afterwards the solution was added to a mixture containing 90 ml of ultra-pure water and 0.75 ml of 1 M HCl. In the next step, sponge chitin fragment (1 × 0.5 cm) was added to the solution and the whole volume was transferred into a Teflon-lined vessel (200 ml) of the hydrothermal reactor (Parr, USA), and heated to 90 °C for 48 h. After this time, the chitin template covered with Fe2O3 nanocrystals was carefully isolated, washed with distilled water in an ultrasound bath (Elmasonic GmbH, Germany) for 20 min, brought up to pH 6.8 and dried at 90 °C for 48 h (Memmert incubator, Germany). Fragments of chitin–Fe2O3 were disrupted mechanically using liquid nitrogen and an agate mortar to obtain nanosized powder that was used for HR-TEM studies. As a control, Fe2O3 particles were also prepared within the same reaction system without the presence of any chitin templates.Scanning electron microscopy (SEM)
The surface morphology and microstructure of the chitinous scaffolds of sponge origin before and after Fe2O3 deposition were examined using SEM images recorded with an Ultra 55 microscope (Carl Zeiss AG, Germany). Before testing, the samples were coated with carbon over a period of 45 s using an Edwards S150B sputter coater.High resolution transmission electron microscopy (HRTEM)
The samples for the TEM investigation were prepared by the standard route, which includes the following steps: a drop of the water suspension containing the nanoparticulated sample was placed on the electron microscopy grid of copper (Plano GmbH, Wetzlar, Germany), covered with a perforated carbon film and afterwards dried in air.Different microstructure parameters such as crystallinity, crystallite size and shape, (local) preferred orientation of Fe2O3 crystallites in the local regions of chitin–Fe2O3 materials, were investigated and analysed by using of the selected area electron diffraction (SAED), high resolution TEM (HRTEM) and energy dispersive X-ray (EDX) methods. These investigations were done using analytical TEM JEM-2200FS of JEOL, which was operated with 200 kV acceleration voltage and equipped with a Cs-corrected illumination system, an ultra-high resolution (UHR) objective lens (Cs = 0.5) and an in-column filter. For the recording of micrographs and diffraction pattern a 2k × 2k CCD-camera by Gatan Inc. was used, meanwhile the EDX spectra and maps with energy dispersive X-ray analyser from JEOL were evaluated.
The analysis of diffraction pattern and Fast Fourier Transformed (FFT) HRTEM-images was done by using of DigitalMicrograph software of Gatan Inc. taking into account the inverse interlattice plane distances and angles between lattice planes to obtain the orientation of crystallites.
Raman spectroscopy
Raman spectra were recorded using a Raman spectrometer (RamanRxn1™, Kaiser Optical Systems Inc., USA) coupled to a light microscope (DM2500 P, Leica Microsystems GmbH, Germany), additional Raman measurements have been performed, for details see ESI.†X-ray diffraction
X-ray diffraction (XRD) was carried out on a Bruker D8 Advance in Bragg–Brentano geometry using Cu-Kα radiation provided by a secondary graphite monochromator. The samples were placed on rotating stainless steel holders without adding additional chemicals.X-ray photoelectron spectroscopy
XPS analyses were performed using a ESCALAB 250Xi from Thermo Scientific, with a monochromatic Al Kα X-ray source (1486.6 eV). The X-ray source has a spot size of 650 μm and operates at a power of 14.8 kV and 19.2 mA. The spectra were taken with a pass energy of 20 eV and an energy step width of 0.1 eV. The base pressure was 2 × 10−10 mbar but during the measurement the pressure increased to 3 × 10−7 mbar due to the ion gas flow from the flood gun, which was used for charge compensation.Electrochemical measurements
Electrochemical characterization was performed in a two electrode Swagelok® system. The composition of pellets was: 85 wt% of active materials, 10 wt% of polyvinylidene fluoride (PVDF Kynar Flex 2801) and 5 wt% of acetylene black. The active materials used were commercially available activated carbon Norit® DLC Supra 30 (S30) with a surface area of 1588 m2 g−1, chitin–Fe2O3 hybrid material (Ch–H) and a mixture of 80% activated carbon Norit® DLC Supra 30 with 20% of the chitin–Fe2O3 hybrid material (Ch–H (20%) + S30 (80%)). The masses of the electrodes were in the range of 7–9 mg and a geometric surface area of one electrode was 0.8 cm2. As an electrolyte, 6 mol l−1 KOH was used. The capacitance properties of the materials (expressed per mass of one electrode) were estimated by galvanostatic charge/discharge (100 mA g−1–1000 mA g−1), cycling voltammetry (CV; 1–100 mV s−1) and electrochemical impedance spectroscopy (100 kHz to 1 mHz) using VSP Biologic, France.Results and discussion
The presented SEM images (Fig. 2) indicate that using the α-chitin from the sponge A. aerophoba as a structural template during hydrothermal formation of iron oxide leads to the formation of chitin–Fe2O3 composites. They are homogeneously covered, with uniform, spherical nanoparticles of hematite as confirmed below using different analytical methods. It can also be observed that iron oxide nanocrystals are so tightly bonded to the chitinous substrate that they cannot be removed from its surface, even after the ultrasound-assisted washing procedure. The nanomorphology of the Fe2O3 crystals, which were grown on chitin, is also similar to those, which were prepared without template (see Fig. S1 ESI†). Therefore, it is assumed that chitin does not have an influence on principal oval morphology of hematite nanocrystals. However, as we will report below, chitin plays a specific role in the regulation of the size of hematite nanoparticles. | ||
| Fig. 2 SEM images of the surface of the isolated A. aerophoba chitinous scaffold before (a and b) and after (c and d) hydrothermal reaction with respect to obtaining the hematite crystals. | ||
The morphology of nanostructured chitin–Fe2O3-composite, which consists of chitin–nanofiber with oval nanoparticles of Fe2O3, is also quite visible using TEM (Fig. 3).
These nanoparticles are especially clearly visible in the dark field image (Fig. 3B). The size of the Fe2O3-particles ranges between 50 and 100 nm.
The analysis of the region of interest (ROI, Fig. 3C) by the combination of scanning TEM (STEM) and EDX-measurements and evaluation of EDX-mapping (Fig. 3D) showed the high concentrations of iron and oxygen in the nanoparticles. The increase in concentration of the corresponding elements toward the center of the nanoparticle means that the particles have a spherical shape. In addition, this shows the local point analysis (Fig. 3E) in the region of chitin, the increased concentration of potassium, which originate from the chitinous lamella.
We used several analytical methods (electron diffraction and HRTEM, XRD, Raman, XPS) to confirm the presence of hematite within chitin-based composite obtained in the study.
Detailed local analysis of individual Fe2O3 particles by HRTEM (FFT) and SAED has shown that these are mainly monocrystalline with different random orientations (Fig. 4). The indexed FFT from the HRTEM micrograph (Fig. 4B) shows a particle with a [152] zone axis. The contrast of HRTEM images is caused by nearby spherical shape of the particle. Another two particles have an [7![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ] and slightly tilted [
] and slightly tilted [![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[0 with combining macron]](https://www.rsc.org/images/entities/char_0030_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ] orientations (the selected area aperture overlaps two particles), which are indexed with yellow and pink text respectively (Fig. 4C).
] orientations (the selected area aperture overlaps two particles), which are indexed with yellow and pink text respectively (Fig. 4C).
 | ||
| Fig. 4 Detailed analysis of orientation of hydrothermal obtained Fe2O3-particles on sponge chitin (A) by evaluation of HRTEM micrograph (B) and SAED pattern (C). | ||
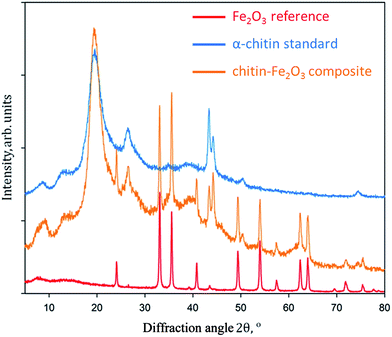
The XRD patterns of the prepared chitin–Fe2O3 composites, α-chitin standard from A. aerophoba, and α-Fe2O3 references are shown in Fig. 5. The obtained spectra for chitin–Fe2O3 perfectly match the α-Fe2O3 (hematite) reference (according to 33-664 JCPDS card). Rietveld refinement of these data reveals that the hematite reference sample, obtained without presence of chitin, exhibits crystallites with typical sizes of 74.7 ± 2.1 nm. The size of hematite crystallites on the chitin is 54.3 ± 5.2 nm, and thus our obtained data closely reflect the HRTEM results.
The size of the hematite nanoparticles grown upon the chitinous substrate fluctuates between 50–100 nm. This is in accordance with results from Raman spectroscopy (Fig. 6).
 | ||
| Fig. 6 Raman spectra of α-chitin standard from A. aerophoba, hematite and the obtained chitin–Fe2O3 composite. | ||
It has been reported73 that the Raman shift of peaks characteristic for hematite strongly depends on particle size. The Raman spectra for α-Fe2O3 reference nanoparticles, α-chitin standard from A. aerophoba and chitin–Fe2O3 composite materials are shown in Fig. 6 and S2 ESI,† respectively. The Raman spectrum of α-Fe2O3 nanoparticles show all the characteristic hematite bands which belong to the D3d6 space group: two A1g modes (224 cm−1 and 494 cm−1) and five Eg modes (at 245 cm−1, 289 cm−1, shoulder at 298 cm−1, 407 cm−1, 608 cm−1).73,74 The presence of several characteristic bands at 255, 323, 368, 395, 457, 501, 648, and 710 cm−1 which correspond to δ(CCC) ring deformation75 in the Raman spectrum of isolated chitin (Fig. S2 ESI†) additionally reveals that it is α-chitin polymorph.31,34
The Raman spectra of the obtained chitin–Fe2O3 composites show the presence of four very intense peaks characteristic of hematite (vertical lines). It is known76 that the position and width of the hematite nanoparticles peaks at 226 cm−1 and 412 cm−1 (Fig. S2 ESI†) are influenced by the nanoparticles' dimensions. According to this, from the obtained chitin–Fe2O3 spectra we can estimate a dimension of 80–100 nm for the particles, in agreement with SEM results. Because of the clearly visible deformations of –NH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bands in the Raman spectra of the chitin–hematite composite (Fig. 6), we suggest that these groups are interacting with Fe2O3.
O bands in the Raman spectra of the chitin–hematite composite (Fig. 6), we suggest that these groups are interacting with Fe2O3.
The thermal behavior of chitin and chitin–Fe2O3 composite was determined by thermogravimetry and differential thermal analysis performed in an oxidative (air) atmosphere (Fig. S3 ESI†). The TG curve of the α-chitin scaffold indicates that the main thermal degradation of the biopolymer starts at 200 °C and ends at 618 °C. The shape of the TG curve demonstrates that degradation in air occurs in two steps, and this result corresponds to thermal behaviors of α-chitin isolated from crab shells, previously published by Georgieva et al.57 Thermogravimetric analysis proves that thermal decomposition of chitin–Fe2O3 composite start at the same temperature, however it stops at 440 °C. Moreover, the shape of the degradation curve clearly indicates that it is one-step process. Therefore we suggest that incorporation of hematite nanoparticles into the chitin matrix inhibits the second stage of chitin degradation and results in an increase of thermal stability of the composite material in comparison to the pure chitin reference.
Important data about the role of chitin and formation mechanism of chitin–hematite composites were obtained using the XPS analysis (Fig. 7). The best information about interactions between chitin and iron oxide is provided by the O 1s peak from chitin–hematite composites. For example, the analysis of the results show subpeaks at 529.42 eV and 527.65 eV, which are ascribed to O atoms of chitin bound to Fe in α-Fe2O3 nanoparticles.77 Additionally, the peak at 531.70 eV is assigned to –OH groups of chitin. It is worth noting that the intensity of this peak decreases in spectra from the chitin–Fe2O3 composite.
 | ||
| Fig. 7 O 1s XPS spectrum for α-chitin from A. aerophoba (blue line), hematite (red line) and chitin–Fe2O3 composite (orange line). | ||
The Fe 2p peak of the chitin–hematite sample shows the presence of distinct subpeaks at 710.7 and 724 eV (Fig. S4a ESI†) and a satellite peak at around 718 eV.78 The presence these peaks indicates that the iron is almost completely in the Fe3+ state.50,79 Additionally, the XPS N 1s peak (Fig. S4b ESI†) was examined in detail to confirm the chemical state of nitrogen atoms present in the chitin and chitin–hematite materials. The observed profiles of the N 1s transition are virtually identical for both materials. They are both symmetrical with mixed Gaussian–Lorentzian profile shapes, and with a maximum at a binding energy of 398.8 eV. These obtained results using XPS spectroscopy indicate that –OH groups located in the chitin molecule plays a crucial role in the formation of chitin–hematite composites.
On the basis of the results presented in this work and previously reported studies realized with the chitinous butterfly wings50 and chitosan80,81 we propose a mechanism of formation and interactions between α-chitin scaffold and hematite nanoparticles within chitin–Fe2O3 composite, as illustrated in Fig. 8. The model proposed is based on the formation of an –O–Fe bond between chitin and hematite similar to the case described by Zhang et al.77 for PET–hematite composites. However, the presence of hydrogen bonds and the chelating effect82 between C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, NH and OH groups of the chitin and iron oxide is also included.
O, NH and OH groups of the chitin and iron oxide is also included.
 | ||
| Fig. 8 A schematic view on possible mechanism of chitin–hematite interactions under hydrothermal conditions. | ||
The question about the possible practical applications of the chitin–hematite composites obtained is of immense importance. Consequently, we investigated the electrochemical properties of this novel composite material in two-electrode cells using an alkaline electrolyte for the first time.
Voltammetric characteristics at 10 mV s−1 are shown in Fig. 9a and differences in capacitances between the initial materials and composite are quite well visible. The capacitance of raw chitin–Fe2O3 hybrid material (Ch–H) is negligible. Active carbon (S30) with high surface area shows a regular rectangle shape of the CV curve and good charge propagation. Considerable increase of the capacitance is noticed for the Ch–H/S30 composite. The increase can be the result of both redox reactions of the chitin–Fe2O3 hybrid material in an alkaline electrolyte, and the developed surface area of the active carbon component. Table S1 ESI† illustrates the capacitance data obtained from different electrochemical methods.
The use of activated carbon/Fe2O3–chitin composite in our work allows us to increase the capacitances of the electrochemical capacitor. This is due to the appearance of reduction and oxidation reactions on the electrodes. In this type of supercapacitor, the energy is stored in the electric double layer (active carbon) and from redox reactions (Fe2O3–chitin composite). Adsorption/desorption processes of ions derived from the electrolyte occur in the pores of carbonaceous material, while in the pores of the nanostructured hematite deintercalation/intercalation processes occur. Furthermore, during the process of oxidation and reduction on the electrode, consisting of Ch–H/S30 composite, a reversible reaction83 appears:
| Fe2O3 + OH− ↔ Fe2O3OH + e− | (1) |
The analysis of electrochemical impedance spectroscopy is presented in Fig. 9b. The results exhibit the improvement of capacitance and charge propagation using the composite as electrode materials for an electrochemical capacitor. Moreover, as shown in Fig. 9c, materials display the considerable stability during cycling measurements. After 5000 cycles of galvanostatic charge/discharge with the current regime of 1 A g−1 the capacitance of active carbon decreased only negligibly – less than one percent. In the case of chitin–Fe2O3 hybrid material/active carbon composite, the increase of capacitance after cyclability measurements is observable.
Probably, a redox bioactive film is built on the surface of the composite and an increase of (pseudo)capacitance is observed. Stability during cycling measurements is a very important parameter for practical applications of materials for electrochemical capacitors.
Conclusions
The results presented and discussed in this work have shown that the hydrothermal route for development of novel chitin–hematite composites is realistic. Chitin of poriferan origin is not only the a prospective thermostable biological material, but also represents an example of a renewable source due to high regeneration ability of Aplysina sponges under marine ranching conditions. It has been described that saccharides (for example, glucose) can be used in versatile fabrication of hierarchically nanostructured goethite with controlled morphologies from 1D unique architecture composed of arrayed nanoplates to 3D urchin-like superstructures.84 Moreover, this composite can be used as a precursor for the thermally-assisted (300 °C) formation of hematite. The morphology resembles that of the urchin-like glucose–goethite composite, and is characterized by high specific surface area. In accordance to this method, our study proves that formation of hematite occurs at a temperature of 90 °C and yields a composite polysaccharide (in this case chitin) with hematite nanoparticles. Therefore, the unique feature of this method is that we obtained hematite nanostructures in one-step method – additional calcination or thermal treatment is not needed. Composites of sponge chitin–Fe2O3 hybrid material with active carbon could be successfully use as electrode materials for electrochemical capacitors. Using suitable composite components with different mechanisms of energy storage can provide improvement to the electrochemical properties of electrode materials. Additionally, according to recently published papers, the development of this facile method for combining biocompatible and biodegradable polymers with iron oxide opens new possibilities for the development of iron oxide-based materials for applications in supercapacitors,60,85 sensors,61,86 catalysts59 and drug carriers for anticancer therapy.64 Therefore, we believe that this presented study will affect a wide range of research associated with hematite-based biomaterials.Acknowledgements
This work was partially supported by Research Grants for Doctoral Candidates and Young Academics and Scientists up to 6 months – DAAD Section 323-Project no. 50015537 and the following research grants: DFG Grant EH 394/3-1, PUT research grant 03/32/443/2014-DS-PB, BHMZ Programme of Dr.-Erich-Krüger-Foundation (Germany) at TU Bergakademie Freiberg, BMBF within the project CryPhys Concept (03 EK3029A).Notes and references
- H. Ehrlich, P. Simon, M. Motylenko, M. Wysokowski, V. V. Bazhenov, R. Galli, A. L. Stelling, D. Stawski, M. Ilan, H. Stöcker, B. Abendroth, R. Born, T. Jesionowski, K. J. Kurzydłowski and D. C. Meyer, J. Mater. Chem. B, 2013, 1, 5092–5099 RSC.
- M. Wysokowski, M. Motylenko, H. Stöcker, V. V. Bazhenov, E. Langer, A. Dobrowolska, K. Czaczyk, R. Galli, A. L. Stelling, T. Behm, Ł. Klapiszewski, D. Ambrożewicz, M. Nowacka, S. L. Molodtsov, B. Abendroth, D. C. Meyer, K. J. Kurzydłowski, T. Jesionowski and H. Ehrlich, J. Mater. Chem. B, 2013, 1, 6469–6476 RSC.
- M. Wysokowski, M. Motylenko, V. V. Bazhenov, D. Stawski, I. Petrenko, A. Ehrlich, T. Behm, Z. Kljajic, A. L. Stelling, T. Jesionowski and H. Ehrlich, Front. Mater. Sci., 2013, 7, 248–260 CrossRef.
- M. Wysokowski, T. Behm, R. Born, V. V Bazhenov, H. Meiβner, G. Richter, K. Szwarc-Rzepka, A. Makarova, D. Vyalikh, P. Schupp, T. Jesionowski and H. Ehrlich, Mater. Sci. Eng., C, 2013, 33, 3935–3941 CrossRef CAS PubMed.
- M. Wysokowski, A. Piasecki, V. V Bazhenov, D. Paukszta, R. Born, I. Petrenko and T. Jesionowski, Journal of Chitin and Chitosan Science, 2013, 1, 26–33 CrossRef PubMed.
- X. Yan, J. Li and H. Möhwald, Adv. Mater., 2012, 24, 2663–2667 CrossRef CAS PubMed.
- X. Yan, J. Blacklock, J. Li and H. Möhwald, ACS Nano, 2012, 6, 111–117 CrossRef CAS PubMed.
- E. Dujardin and S. Mann, Adv. Mater., 2002, 14, 775–788 CrossRef CAS.
- C. Sanchez, H. Arribart and M. M. G. Guille, Nat. Mater., 2005, 4, 277–288 CrossRef CAS.
- A.-W. Xu, Y. Ma and H. Cölfen, J. Mater. Chem., 2007, 17, 415–449 RSC.
- H. Ehrlich, Int. Geol. Rev., 2010, 52, 661–699 CrossRef.
- F. Jun, L. I. Jianghai and C. H. U. Fengyou, Acta Oceanol. Sin., 2009, 28, 87–95 Search PubMed.
- T. L. Cook and D. S. Stakes, Science, 1995, 267, 1975–1979 CrossRef CAS PubMed.
- A.-L. Reysenbach, Y. Liu, A. B. Banta, T. J. Beveridge, J. D. Kirshtein, S. Schouten, M. K. Tivey, K. L. Von Damm and M. A. Voytek, Nature, 2006, 442, 444–447 CrossRef CAS PubMed.
- M. Nidhin, K. J. Sreeram and B. U. Nair, Appl. Surf. Sci., 2012, 258, 5179–5184 CrossRef CAS PubMed.
- C. Ercole, P. Cacchio, A. L. Botta, V. Centi and A. Lepidi, Microsc. Microanal., 2007, 13, 42–50 CrossRef CAS PubMed.
- C. S. Chan, S. C. Fakra, D. C. Edwards, D. Emerson and J. F. Banfield, Geochim. Cosmochim. Acta, 2009, 73, 3807–3818 CrossRef CAS PubMed.
- R. Hedrich, S. Machill and E. Brunner, Carbohydr. Res., 2013, 365, 52–60 CrossRef CAS PubMed.
- P. U. P. A. Gilbert, M. Abrecht and B. H. Frazer, Rev. Mineral. Geochem., 2005, 59, 157–185 CrossRef CAS.
- B. Mamet, C. De Ridder, F. Boulvain and D. Gillan, Sediment. Geol., 2000, 137, 107–126 CrossRef.
- S. Poorni and K. Natarajan, Colloids Surf., B, 2014, 114, 186–192 CrossRef CAS PubMed.
- H.-O. Fabritius, C. Sachs, P. R. Triguero and D. Raabe, Adv. Mater., 2009, 21, 391–400 CrossRef CAS.
- S. Nikolov, M. Petrov, L. Lymperakis, M. Friák, C. Sachs, H.-O. Fabritius, D. Raabe and J. Neugebauer, Adv. Mater., 2010, 22, 519–526 CrossRef CAS PubMed.
- I. M. Weiss and V. Schönitzer, J. Struct. Biol., 2006, 153, 264–277 CrossRef CAS PubMed.
- T. Furuhashi, C. Schwarzinger, I. Miksik, M. Smrz and A. Beran, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2009, 154, 351–371 CrossRef PubMed.
- M. Poulicek, Biochem. Syst. Ecol., 1983, 11, 47–54 CrossRef CAS.
- K. M. Sherrard, Biol. Bull., 2000, 198, 404–414 CrossRef CAS.
- M. Florek, E. Fornal, P. Gómez-Romero, E. Zieba, W. Paszkowicz, J. Lekki, J. Nowak and A. Kuczumow, Mater. Sci. Eng., C, 2009, 29, 1220–1226 CrossRef CAS PubMed.
- E. Brunner, P. Richthammer, H. Ehrlich, S. Paasch, P. Simon, S. Ueberlein and K.-H. van Pée, Angew. Chem., Int. Ed., 2009, 48, 9724–9727 CrossRef CAS PubMed.
- M. Bo, G. Bavestrello, D. Kurek, S. Paasch, E. Brunner, R. Born, R. Galli, A. L. Stelling, V. N. Sivkov, O. V Petrova, D. Vyalikh, K. Kummer, S. L. Molodtsov, D. Nowak, J. Nowak and H. Ehrlich, Int. J. Biol. Macromol., 2012, 51, 129–137 CrossRef CAS PubMed.
- H. Ehrlich, M. Maldonado, K. Spindler, C. Eckert, T. Hanke, R. Born, P. Simon, S. Heinemann and H. Worch, J. Exp. Zool., Part B, 2007, 356, 347–356 CrossRef PubMed.
- H. Ehrlich, M. Krautter, T. Hanke, P. Simon, C. Knieb, S. Heinemann and H. Worch, J. Exp. Zool., Part B, 2007, 308B, 473–483 CrossRef CAS PubMed.
- E. Brunner, H. Ehrlich, P. Schupp, R. Hedrich, S. Hunoldt, M. Kammer, S. Machill, S. Paasch, V. V. Bazhenov, D. V. Kurek, T. Arnold, S. Brockmann, M. Ruhnow and R. Born, J. Struct. Biol., 2009, 168, 539–547 CrossRef CAS PubMed.
- M. Wysokowski, V. V Bazhenov, M. V Tsurkan, R. Galli, A. L. Stelling, H. Stöcker, S. Kaiser, E. Niederschlag, G. Gärtner, T. Behm, M. Ilan, A. Y. Petrenko, T. Jesionowski and H. Ehrlich, Int. J. Biol. Macromol., 2013, 62, 94–100 CrossRef CAS PubMed.
- H. Ehrlich, M. Ilan, M. Maldonado, G. Muricy, G. Bavestrello, Z. Kljajic, J. L. Carballo, S. Schiaparelli, A. Ereskovsky, P. Schupp, R. Born, H. Worch, V. Bazhenov, D. Kurek, V. Varlamov, D. Vyalikh, K. Kummer, V. V Sivkov, S. L. Molodtsov, H. Meissner, G. Richter, E. Steck, W. Richter, S. Hunoldt, M. Kammer, S. Paasch, V. Krasokhin, G. Patzke and E. Brunner, Int. J. Biol. Macromol., 2010, 47, 132–140 CrossRef CAS PubMed.
- H. Ehrlich, E. Steck, M. Ilan, M. Maldonado, G. Muricy, G. Bavestrello, Z. Kljajic, J. L. Carballo, S. Schiaparelli, A. Ereskovsky, P. Schupp, R. Born, H. Worch, V. V Bazhenov, D. Kurek, V. Varlamov, D. Vyalikh, K. Kummer, V. V Sivkov, S. L. Molodtsov, H. Meissner, G. Richter, S. Hunoldt, M. Kammer, S. Paasch, V. Krasokhin, G. Patzke, E. Brunner and W. Richter, Int. J. Biol. Macromol., 2010, 47, 141–145 CrossRef CAS PubMed.
- H. Ehrlich, P. Simon, W. Carrillo-Cabrera, V. V. Bazhenov, J. P. Botting, M. Ilan, A. V. Ereskovsky, G. Muricy, H. Worch, A. Mensch, R. Born, A. Springer, K. Kummer, D. V. Vyalikh, S. L. Molodtsov, D. Kurek, M. Kammer, S. Paasch and E. Brunner, Chem. Mater., 2010, 22, 1462–1471 CrossRef CAS.
- J. A. Cruz-Barraza, J. L. Carballo, A. Rocha-Olivares, H. Ehrlich and M. Hog, PLoS One, 2012, 7, e42049 CAS.
- H. Ehrlich, O. V Kaluzhnaya, M. V Tsurkan, A. Ereskovsky, K. R. Tabachnick, M. Ilan, A. Stelling, R. Galli, O. V Petrova, S. V Nekipelov, N. Sivkov, D. Vyalikh, R. Born, T. Behm, A. Ehrlich, L. I. Chernogor, S. Belikov, D. Janussen, V. V Bazhenov and G. Wörheide, Proc. R. Soc. Edinburgh, Sect. B: Biol. Sci., 2013, 280, 20130339 CrossRef PubMed.
- H. Ehrlich, O. V Kaluzhnaya, E. Brunner, M. V Tsurkan, A. Ereskovsky, M. Ilan, K. R. Tabachnick, V. V Bazhenov, S. Paasch, M. Kammer, R. Born, A. Stelling, R. Galli, S. Belikov, O. V Petrova, V. V Sivkov, D. Vyalikh, S. Hunoldt and G. Wörheide, J. Struct. Biol., 2013, 183, 474–483 CrossRef CAS PubMed.
- H. Ehrlich, J. K. Rigby, J. P. Botting, M. Tsurkan, C. Werner, P. Schwille, Z. Petrasek, A. Pisera, P. Simon, V. Sivkov, D. Vyalikh, L. S. Molodtsov, D. Kurek, M. Kammer, S. Hunoldt, R. Born, D. Stawski, A. Steinhof and T. Geisler-Wierwille, Sci. Rep., 2013, 3, 3497 CAS.
- N. H. Munro, D. W. Green and K. M. McGrath, Chem. Commun., 2013, 49, 3407–3409 RSC.
- N. H. Munro and K. M. McGrath, Dalton Trans., 2011, 40, 9269–9275 RSC.
- G. Falini and S. Fermani, Tissue Eng., 2004, 10, 1–6 CrossRef CAS PubMed.
- W. Ogasawara, W. Shenton, S. A. Davis and S. Mann, Chem. Mater., 2000, 12, 2835–2837 CrossRef CAS.
- K. Spinde, M. Kammer, K. Freyer, H. Ehrlich, J. N. Vournakis and E. Brunner, Chem. Mater., 2011, 23, 2973–2978 CrossRef CAS.
- B. Alonso and E. Belamie, Angew. Chem., Int. Ed., 2010, 49, 8201–8204 CrossRef CAS PubMed.
- Y. Chen, X. Zang, J. Gu, S. Zhu and H. Su, J. Mater. Chem., 2011, 21, 6140–6143 RSC.
- J. Chen, H. Su, X. You, J. Gao, W. M. Lau and D. Zhang, Mater. Res. Bull., 2014, 49, 560–565 CrossRef CAS PubMed.
- W. Peng, S. Zhu, W. Wang, W. Zhang, J. Gu, X. Hu, D. Zhang and Z. Chen, Adv. Funct. Mater., 2012, 22, 2072–2080 CrossRef CAS.
- A. C. A. Wan and B. C. U. Tai, Biotechnol. Adv., 2013, 31, 1776–1785 CrossRef CAS PubMed.
- R. Jayakumar, D. Menon, K. Manzoor, S. V. Nair and H. Tamura, Carbohydr. Polym., 2010, 82, 227–232 CrossRef CAS PubMed.
- A. Anitha, S. Sowmya, P. Kumar, S. Deepthi, K. P. Chennazhi, H. Ehrlich, M. Tsurkan and R. Jayakumar, Prog. Polym. Sci., 2014, 39, 1644–1667 CrossRef CAS PubMed.
- H. Tamura, T. Furuike, S. V. Nair and R. Jayakumar, Carbohydr. Polym., 2011, 84, 820–824 CrossRef CAS PubMed.
- D. Stawski, S. Rabiej, L. Herczyńska and Z. Draczyński, J. Therm. Anal. Calorim., 2008, 93, 489–494 CrossRef CAS.
- Y. Wang, Y. Chang, L. Yu, C. Zhang, X. Xu, Y. Xue, Z. Li and C. Xue, Carbohydr. Polym., 2013, 92, 90–97 CrossRef CAS PubMed.
- V. Georgieva, D. Zvezdova and L. Vlaev, J. Therm. Anal. Calorim., 2013, 111, 763–771 CrossRef CAS.
- A. M. Jubb, D. Verreault, R. Posner, L. J. Criscenti, L. E. Katz and H. C. Allen, J. Colloid Interface Sci., 2013, 400, 140–146 CrossRef CAS PubMed.
- J. Y. Kim, G. Magesh, D. H. Youn, J.-W. Jang, J. Kubota, K. Domen and J. S. Lee, Sci. Rep., 2013, 3, 2681 Search PubMed.
- X. Lu, Y. Zeng, M. Yu, T. Zhai, C. Liang, S. Xie, M.-S. Balogun and Y. Tong, Adv. Mater., 2014, 26, 3148–3155 CrossRef CAS PubMed.
- J. Gong, L. Wang, K. Zhao and D. Song, Electrochem. Commun., 2008, 10, 123–126 CrossRef CAS PubMed.
- G. Picasso, M. R. S. Kou, O. Vargasmachuca, J. Rojas, C. Zavala, A. Lopez and S. Irusta, Microporous Mesoporous Mater., 2014, 185, 79–85 CrossRef CAS PubMed.
- K. Sivula, R. Zboril, F. Le Formal, R. Robert, A. Weidenkaff, J. Tucek, J. Frydrych and M. Grätzel, J. Am. Chem. Soc., 2010, 132, 7436–7744 CrossRef CAS PubMed.
- M. Wu, X.-M. Xia, C. Cui, P. Yu, Y. Zhang, L. Liu, R.-X. Zhuo and S.-W. Huang, J. Mater. Chem. B, 2013, 1, 1687 RSC.
- J. Singh, M. Srivastava, J. Dutta and P. K. Dutta, Int. J. Biol. Macromol., 2011, 48, 170–176 CrossRef CAS PubMed.
- J. Huang and Z. Sun, Chin. J. Geochem., 2008, 27, 150–156 CrossRef CAS.
- X. Fei, Z. Shao and X. Chen, J. Mater. Chem. B, 2013, 1, 213 RSC.
- Y. Gu, X. Liu, T. Niu and J. Huang, Chem. Commun., 2010, 46, 6096–6098 RSC.
- M. Nidhin, M. Vedhanayagam, S. Sangeetha, M. S. Kiran, S. S. Nazeer, R. S. Jayasree, K. J. Sreeram and B. U. Nair, Sci. Rep., 2014, 4, 5968 Search PubMed.
- X. C. Jiang, a. B. Yu, W. R. Yang, Y. Ding, C. X. Xu and S. Lam, J. Nanopart. Res., 2009, 12, 877–893 CrossRef.
- S. Hamada and E. Matijević, J. Colloid Interface Sci., 1981, 84, 274–277 CrossRef CAS.
- W. Wang, J. Y. Howe and B. Gu, J. Phys. Chem. C, 2008, 112, 9203–9208 CAS.
- D. L. A. de Faria, S. V. Silva and M. T. de Oliveira, J. Raman Spectrosc., 1997, 28, 873–878 CrossRef CAS.
- F. Froment, A. Tournié and P. Colomban, J. Raman Spectrosc., 2008, 39, 560–568 CrossRef CAS.
- J. De Gelder, K. De Gussem, P. Vandenabeele and L. Moens, J. Raman Spectrosc., 2007, 38, 1133–1147 CrossRef CAS.
- I. V. Chernyshova, M. F. Hochella and A. S. Madden, Phys. Chem. Chem. Phys., 2007, 9, 1736–1750 RSC.
- H. Zhang, J. Song and C. Liu, Ind. Eng. Chem. Res., 2013, 52, 7403–7412 CrossRef CAS.
- V. Dhanasekaran, S. Anandhavelu, E. K. Polychroniadis and T. Mahalingam, Mater. Lett., 2014, 126, 288–290 CrossRef CAS PubMed.
- A. P. Grosvenor, B. A. Kobe, M. C. Biesinger and N. S. McIntyre, Surf. Interface Anal., 2004, 36, 1564–1574 CrossRef CAS.
- C. L. Huang, H. Y. Zhang, Z. Y. Sun and Z. M. Liu, Sci. China: Chem., 2010, 53, 1502–1508 CrossRef CAS PubMed.
- Y. Wang, B. Li, Y. Zhou, D. Jia and Y. Song, Polym. Adv. Technol., 2011, 22, 1681–1684 CrossRef CAS.
- J. R. B. Gomes, M. Jorge and P. Gomes, J. Chem. Thermodyn., 2014, 73, 121–129 CrossRef CAS PubMed.
- M. Zhu, Y. Wang, D. Meng, X. Qin and G. Diao, J. Phys. Chem. C, 2012, 116, 16276–16285 CAS.
- G. Tong, J. Guan and Q. Zhang, Mater. Chem. Phys., 2011, 127, 371–378 CrossRef CAS PubMed.
- S. Shivakumara, T. R. Penki and N. Munichandraiah, Mater. Lett., 2014, 131, 100–103 CrossRef CAS PubMed.
- S. Wang, Y. Wang, H. Zhang, X. Gao, J. Yang and Y. Wang, RSC Adv., 2014, 4, 30840–30849 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra10017d |
| This journal is © The Royal Society of Chemistry 2014 |