3D nitrogen-doped graphene/Co(OH)2-nanoplate composites for high-performance electrochemical pseudocapacitors†
Abstract
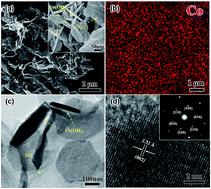
We report a simple hydrothermal synthesis of nanocomposites, constructed by 3D nitrogen-doped graphene (NG) networks with hexagonal Co(OH)2 nanoplates, which are optimized for applications as electrochemical pseudocapacitor materials. Single-crystalline Co(OH)2 plates are distributed homogeneously inside the conductive interconnected NG networks. The 71% Co(OH)2 weight content achieves a capacitance of 952 F g−1 at 1.0 A g−1, more than triple that of the pure NG and nearly four times that of Co(OH)2 plates; moreover, this value exceeds the recently reported values for 2D graphene/Co(OH)2 composites. Capacitance retention over 2000 cycles is still high as 95%. The improvements are attributed to the regular morphology of Co(OH)2 and the 3D porosity, which prevents the stacking of the Co(OH)2 plates as well as the composite, and the continuously connected pores and highly conductive NG networks, which facilitate electron and ion transport.

 Please wait while we load your content...
Please wait while we load your content...