Integration of a plasmonic semiconductor with a metal–organic framework: a case of Ag/AgCl@ZIF-8 with enhanced visible light photocatalytic activity†
Abstract
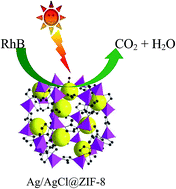
Efficient utilization of solar energy has received tremendous attention due to the increasing environmental and energy concerns. In this paper, a plasmonic photocatalyst integrated with metal–organic framework (MOF) material, Ag/AgCl@ZIF-8, was fabricated for the first time. The unique hetero-junction structure of Ag/AgCl@ZIF-8 greatly enhanced its photocatalytic activity. Selecting RhB as a model pollutant, 98% of RhB can be degraded after 16 min under solar radiation. The relationship between the photocatalytic activity and the structure of Ag/AgCl@ZIF-8 hybrid materials was discussed and the possible reaction mechanism was proposed. The high photocatalytic activity of the hybrid material under solar radiation revealed its great application potential in directly harvesting solar energy to solve the environmental pollution issues.

 Please wait while we load your content...
Please wait while we load your content...