Biodegradable dendritic polymersomes as modular, high-relaxivity MRI contrast agents†‡
Ali
Nazemi
a,
Francisco
Martínez
b,
Timothy J.
Scholl
b and
Elizabeth R.
Gillies
*ac
aDepartment of Chemistry, Western University, 1151 Richmond St., London, Canada. E-mail: egillie@uwo.ca; Fax: +001 519-661-3022
bDepartment of Medical Biophysics and the Robarts Research Institute, Western University, 100 Perth Dr, London, Canada
cDepartment of Chemical and Biochemical Engineering, Western University, 1151 Richmond St., London, Canada
First published on 6th August 2012
Abstract
A modular approach combining nanoscale components that each contribute to an enhancement in Gd(III) relaxivity is described here. Small molecule and dendritic alkyne derivatives of a Gd(III) complex were conjugated to the surfaces of biodegradable polymersomes resulting in ionic relaxivities of 10.6 mM−1 s−1 and 26.1 mM−1 s−1 respectively.
The early detection and diagnosis of disease is currently one of the major challenges in medicine. Clinical imaging plays a significant role in this process. Among the various imaging modalities, magnetic resonance imaging (MRI) has become a well-established and powerful tool, due to its excellent spatial resolution and soft tissue contrast. To aid in the differentiation between healthy and diseased tissues, small molecule Gd(III) chelates such as Magnevist® are used in approximately 50% of MRI scans. While the availability of these agents has enabled significant developments in MRI, they do suffer from some significant limitations. For example, they typically possess longitudinal relaxivities (r1) in the range of 3–5 mM−1 s−1, only a small fraction of the theoretically possible values.1 This results in the requirement for very large doses of these agents and also limits their applicability in molecular imaging.2 In addition, most of these agents are non-targeted and have very short circulation half-lives in the blood.
To address these limitations, Gd(III) complexes have been conjugated to a wide variety of macromolecular scaffolds including dendrimers,3,4 linear polymers,5 proteins,6 viral particles,7 micelles,8,9 liposomes,10,11 and polymersomes.12–14 This can result in improvements in r1 values due to the slower tumbling rates of macromolecules and the resulting increases in the rotational correlation times of the Gd(III).15,16 In addition, macromolecular systems can exhibit prolonged blood circulation times, enabling the targeting of tissues either passively or actively through the conjugation of targeting ligands. Among the available macromolecular assemblies, block copolymer vesicles, commonly referred to as polymersomes, have attracted significant attention due to their potential multifunctionality. They possess an aqueous core capable of encapsulating water-soluble species, a hydrophobic membrane that can encapsulate hydrophobes, and a surface to which specific targeting ligands or other species can be conjugated. Thus far, there are very few reports of polymersome-based MRI contrast agents. 12–14 Cheng et al. have investigated porous polymersomes containing Gd(III)-labeled dendrimers within their aqueous cores, and have obtained an r1 of 7.5 mM−1 s−1 (60 MHz, 40 °C) on a per Gd basis.12,13 More recently, Grüll et al. incorporated Gd(III)-labeled lipids into a polymersome membrane, resulting in an r1 of 22 mM−1 s−1 (20 MHz, 25 °C).14
Our group has developed approaches for the functionalization of polymersome surfaces with dendritic groups.17,18 It was shown that this is an effective method for tuning the surface chemistries of polymersomes in a single step, resulting in properties such as enhanced target binding and cell uptake.19,20 For the current work, it was proposed that polymersome-immobilized dendrons functionalized with Gd(III) complexes may serve as highly efficient MRI contrast agents. While conjugation of Gd(III) complexes to high generation dendrimers is known to enhance their relaxivities,3,4 immobilization on the polymersome surface should provide further enhancements, at the same time opening the possibility to exploit the multifunctional properties of polymersomes. We describe here the preparation of the dendron and polymersome components, and studies of their relaxivities. It is demonstrated how nanoscale components can be readily combined to provide additive enhancements in relaxivity.
Fig. 1 depicts the general approach for the preparation of the polymersome MRI contrast agents. Polycaprolactone-poly(ethylene oxide) (PCL-PEO) block copolymers were selected due to the biodegradability of the PCL block and the well demonstrated biocompatibility of PEO in various applications.21 Methoxy- (1) and azide-terminated (2) PCL-PEO were prepared as previously reported and were assembled into azide-functionalized vesicles (3).18 The z-average diameter of the polymersomes was 140 nm, as measured by dynamic light scattering (DLS). Gd(III)-functionalized dendron 4, having a focal point alkyne, was prepared starting from the third generation polyester dendron 8.17 As shown in Scheme 1, the peripheral amine groups of 8 were reacted with the commercially available diethylenetriaminepentaacetic acid (DTPA) isothiocyanate derivative 9. The resulting dendron 10 was then treated with GdCl3 to provide the target dendron 4.
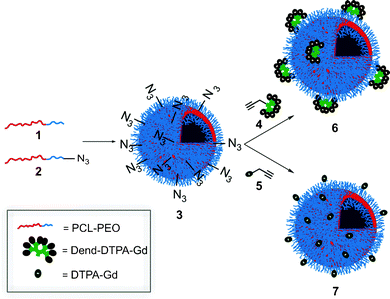 | ||
| Fig. 1 Schematic for the preparation of dendritic and non-dendritic Gd(III)-functionalized polymersomes. | ||
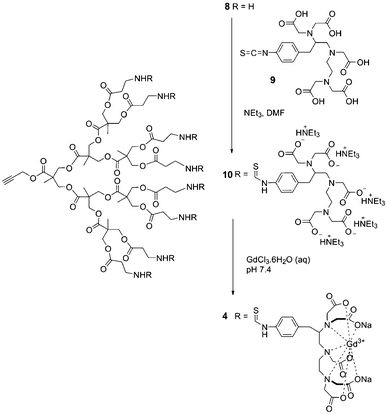 | ||
| Scheme 1 Synthesis of Gd(III)-functionalized dendron 4. | ||
As shown in Fig. 1, in order to determine the contribution of the dendron versus polymersome to the relaxivity, it was also desirable to prepare a non-dendritic alkyne derivative of the Gd(III) chelate (5). As shown in Scheme 2, this was accomplished by the reaction of propargyl amine with 9 to provide 11, followed by chelation of Gd(III).
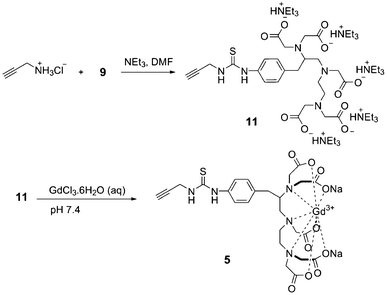 | ||
| Scheme 2 Synthesis of Gd(III) complex 5. | ||
Prior to Gd(III) insertion, compounds 10 and 11 were characterized by 1H and 13C NMR spectroscopy. In addition, infrared (IR) spectroscopy was informative for this class of compounds. For example, upon conversion of dendron 8 to 10, the absence of the characteristic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S stretch of the isothiocyanate functional group that was present in compound 9 confirmed the successful removal of excess 9 (ESI†). After insertion of Gd(III), NMR spectroscopic analysis was no longer possible due to the paramagnetic nature of the Gd(III) ion. However, inductively coupled plasma mass spectrometry (ICP-MS) was performed, confirming the successful insertion of Gd(III) into dendron 4 and compound 5. In addition, IR spectroscopy demonstrated that the peaks corresponding to the C
S stretch of the isothiocyanate functional group that was present in compound 9 confirmed the successful removal of excess 9 (ESI†). After insertion of Gd(III), NMR spectroscopic analysis was no longer possible due to the paramagnetic nature of the Gd(III) ion. However, inductively coupled plasma mass spectrometry (ICP-MS) was performed, confirming the successful insertion of Gd(III) into dendron 4 and compound 5. In addition, IR spectroscopy demonstrated that the peaks corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretches of the ligand shifted to significantly lower frequencies in compounds 4 and 5 relative to 10 and 11, respectively. This is also an indication of successful coordination of the carboxylate groups to Gd(III).22
O stretches of the ligand shifted to significantly lower frequencies in compounds 4 and 5 relative to 10 and 11, respectively. This is also an indication of successful coordination of the carboxylate groups to Gd(III).22
The next step was to conjugate 4 and 5 to the polymersome surfaces. This was accomplished by a Cu(I) mediated 3 + 2 “click” cycloaddition to provide the dendritic Gd(III)-functionalized polymersomes 6, and non-dendritic Gd(III)-functionalized polymersomes, 7, respectively. Unreacted 4 and 5 were removed by dialysis. ICP-MS measurements were performed on the products and the results indicated that 38% of the azide groups were functionalized in polymersomes 6 and 26% in polymersomes 7. The sizes and morphologies of the resulting polymersomes were evaluated by DLS and transmission electron microscopy (TEM). Small increases in the z-average diameters to 158 and 156 nm were found for vesicles 6 and 7, respectively (ESI†). TEM (ESI†) showed that the vesicular morphology was preserved and the contrast was enhanced upon incorporation of the Gd(III) in both dendritic and non-dendritic polymersomes.
The properties of the three newly developed contrast agents (4, 6, and 7) were then assessed in phosphate buffer (0.1 M, pH 7.4) at 298 K (Fig. 2) and 310 K (ESI†) between 0.01 and 42 MHz using a field cycling relaxometer. On a per Gd(III) basis, dendron 4 and polymersomes 6 and 7 exhibited r1 values of 12.1 ± 0.3, 26.1 ± 1.2, and 10.6 ± 0.4 mM−1 s−1, respectively (20 MHz, 298 K). In comparison with the clinical agent Magnevist® (Gd(III)-DTPA) which has a reported relaxivity of 4.6 mM−1 s−1 under the same conditions,23 this corresponds to 2.6-, 5.7-, and 2.3-fold increases in r1 for dendron 4, and polymersome 6 and 7, respectively. All of the systems exhibit an r1versus frequency curve shape that is characteristic of restricted tumbling motion of the Gd(III) complex.1
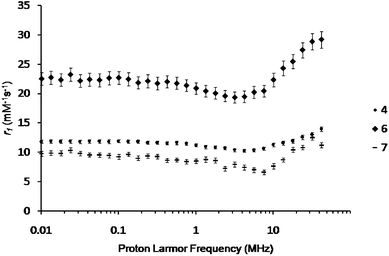 | ||
| Fig. 2 Longitudinal relaxivity (r1) of dendron 4, polymersome 6, and polymersome 7 in phosphate buffer (0.1 M, pH 7.4) as a function of field strength at 298 K. | ||
While polyester dendrons such as 4 have not previously been investigated as MRI contrast agents, the r1 value of 12.1 mM−1 s−1 is within the range expected for a third generation dendrimer.3,4 As with other dendrimers, this enhancement can likely be attributed to the crowded nature of the dendron periphery, which inhibits the free rotation of the Gd(III) complexes. The enhanced r1 value of 10.6 mM−1 s−1 obtained for the non-dendritic polymersomes 7 is also likely a result of the hindered motion of the Gd(III) complexes at the vesicle surface, as well as the slow tumbling rate of the entire vesicle system. This r1 value is lower than the value of 22 mM−1 s−1 at (20 MHz, 25 °C) obtained by Grüll et al. with lipid functionalized Gd(III) chelates incorporated into polymersomes.14 This is probably because their chelates were attached directly to the lipids, rather than through a long linker. The PEO chains in the current work introduce flexibility, which can decrease the rotational correlation time. PEO surrounding the chelate may also slow water exchange. However, r1 is higher than for the system reported by Cheng et al. which contained Gd(III) complexes within the polymersomes.12,13 This can be attributed to our selective attachment of the chelates to the periphery, where they are easily accessible to bulk water.
When both the dendritic component and the polymersome component are combined in polymersome 6, the resulting r1 of 26.1 mM−1 s−1 is the highest reported relaxivity for a polymersome system. This additive effect can result from the availability of chelates at the vesicle surface for water exchange, hindered motion of the Gd(III) complexes imparted by the dendron at a local level, and the large size and slow tumbling rate of the polymersome at the nanoscale level. Thus, this work elegantly demonstrates that different components can be combined through rational design to obtain additive effects on the relaxivity. An additional feature of the current system relative to those previously reported is the biodegradability imparted by the PCL block of the copolymer and the polyester dendron which is known to break down over a period of several days in physiological conditions.24 This should enable the release of low molecular weight Gd(III) complexes and polymer products from the body, an important consideration for MRI contrast agents.
In conclusion, through the synthesis of dendritic and non-dendritic Gd(III) chelates and their conjugation to polymersome surfaces, three new MRI contrast agents were developed. Using these systems, the effects of the dendritic and polymersome components on the relaxivities of the agents were elucidated. They were found to have an additive effect, resulting in the highest currently reported r1 for a polymersome system. In addition, this system possesses the advantage of being composed of PEO and biodegradable polyester components. Future work will be aimed at exploring the biodegradability and in vivo properties of the system as well as exploiting the multifunctional capabilities of polymersomes.
References
- P. Caravan, Chem. Soc. Rev., 2006, 35, 512 RSC.
- M. R. Prince, H. L. Zhang, G. H. Roditi, T. Leiner and W. Kucharczyk, J. Magn. Reson. Imaging, 2009, 30, 1298 CrossRef.
- S. Langereis, Q. G. de Lussanet, M. H. P. van Genderen, W. H. Backes and E. W. Meijer, Macromolecules, 2004, 37, 3084 CrossRef CAS.
- C. C. Cyran, Y. Fu, H. J. Raatschen, V. Rogut, B. Chaopathomkul, D. M. Shames, M. F. Wendland, B. M. Yeh and R. C. Brasch, J. Magn. Reson. Imaging, 2008, 27, 581 CrossRef.
- M. J. Allen, R. T. Raines and L. L. Kiessling, J. Am. Chem. Soc., 2006, 128, 6534 CrossRef CAS.
- P. Caravan, N. J. Cloutier, M. T. Greenfield, S. A. McDermid, S. U. Dunham, J. W. M. Bulte, J. C. Amedio, R. J. Looby, R. M. Supkowski, W. D. Horrocks, T. J. McMurry and R. B. Lauffer, J. Am. Chem. Soc., 2002, 124, 3152 CrossRef CAS.
- D. E. Prasuhn, R. M. Yeh, A. Obenaus, M. Manchester and M. G. Finn, Chem. Commun., 2007, 1269 RSC.
- A. Accardo, D. Tesauro, P. Roscigno, E. Gianolio, L. Paduano, G. D'Errico, C. Pedone and G. Morelli, J. Am. Chem. Soc., 2004, 126, 3097 CrossRef CAS.
- D. T. Schühle, M. Polášek, I. Lukeš, T. Chauvin, É. Tóth, J. Schatz, U. Hanefield, M. C. A. Stuart and J. A. Peters, Dalton Trans., 2010, 39, 185 RSC.
- D. T. Schühle, P. van Rijn, S. Laurent, L. Vander Elst, R. N. Muller, M. C. A. Stuart, J. Schatz and J. A. Peters, Chem. Commun., 2010, 46, 4399 RSC.
- F. Kielar, L. Tei, E. Terreno and M. Botta, J. Am. Chem. Soc., 2010, 132, 7836 CrossRef CAS.
- Z. L. Cheng and A. Tsourkas, Langmuir, 2008, 24, 8169 CrossRef CAS.
- Z. L. Cheng, D. L. J. Thorek and A. Tsourkas, Adv. Funct. Mater., 2009, 19, 3753 CrossRef CAS.
- H. Grüll, S. Langereis, L. Messager, D. D. Castelli, A. Sanino, E. Torres, E. Terreno and S. Aime, Soft Matter, 2010, 6, 4847 RSC.
- E. Strandberg and P. O. Westlund, J. Magn. Reson., Ser. A, 1996, 122, 179 CrossRef CAS.
- A. J. L. Villaraza, A. Bumb and M. W. Brechbiel, Chem. Rev., 2010, 110, 2921 CrossRef CAS.
- B. Li, A. L. Martin and E. R. Gillies, Chem. Commun., 2007, 5217 RSC.
- A. Nazemi, R. C. Amos, C. V. Bonduelle and E. R. Gillies, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 2546 CrossRef CAS.
- R. C. Amos, A. Nazemi, C. V. Bonduelle and E. R. Gillies, Soft Matter, 2012, 8, 5947 RSC.
- A. L. Martin, B. Li and E. R. Gillies, J. Am. Chem. Soc., 2009, 131, 734 CrossRef CAS.
- C. X. F. Lam, S. H. Teoh and D. W. Hutmacher, Polym. Int., 2007, 56, 718 CrossRef CAS.
- M. S. Konings, W. C. Dow, D. B. Love, K. N. Raymond, S. C. Quay and S. M. Rocklage, Inorg. Chem., 1990, 29, 1488 CrossRef CAS.
- S. Laurent, F. Botteman, L. V. Elst and R. N. Muller, Magn. Reson. Mater., Phys. Biol. Med., 2004, 16, 235 CAS.
- E. R. Gillies, E. Dy, J. M. J. Fréchet and F. C. Szoka, Mol. Pharmaceutics, 2005, 2, 129 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic procedures, IR, DLS, TEM, and additional relaxivity data. See DOI: 10.1039/c2ra20886e |
| ‡ Financial support from the Ontario Institute for Cancer Research, the Canada Research Chairs Program (E. R. G.) and the Government of Ontario (fellowship to A. N.) is acknowledged. |
| This journal is © The Royal Society of Chemistry 2012 |
