Effect of N-substitution in naphthalenediimides on the electrochemical performance of organic rechargeable batteries†
Dong Jun
Kim
a,
Sang Hyun
Je
b,
Srinivasan
Sampath
b,
Jang Wook
Choi
*b and
Ali
Coskun
*bc
aDepartment of Materials Science and Engineering, Korea Advanced Institute of Science and Technology (KAIST), 373-1 Guseong Dong, Yuseong Gu, Daejeon 305-701, Republic of Korea
bGraduate School of Energy, Environment, Water and Sustainability (WCU), Korea Advanced Institute of Science and Technology (KAIST), 373-1 Guseong Dong, Yuseong Gu, Daejeon 305-701, Republic of Korea
cDepartment of Chemistry and Nano Century KAIST Institute, Korea Advanced Institute of Science and Technology (KAIST), 373-1 Guseong Dong, Yuseong Gu, Daejeon 305-701, Republic of Korea. E-mail: coskun@kaist.ac.kr; Fax: (+82)-42-350-1710; Tel: (+82)-42-350-1724
First published on 26th June 2012
Abstract
We have demonstrated that even small structural variations on the imide nitrogens of naphthalenediimides bearing identical Li-ion binding sites can cause dramatic effects in the performance of organic rechargeable batteries. In particular, naphthalenedimide dilithium salt showed excellent cycling with a capacity of 130 mA h g−1 at potentials as high as 2.5 V vs. Li/Li+.
Lithium ion batteries1 (LIBs) are currently expanding their territory into large-scale applications represented by electrical vehicles and utility grids.2,3 These large-scale applications are expected to play important roles in addressing urgent and global-scale energy and environmental issues.4,5 The emergence of the large-scale LIBs, however, imposes more challenging standards in the performance of various electrochemical aspects, including energy density, rate capability, cycle life, and safety. Furthermore, the battery scale-up makes cheap and abundant materials far more preferable as electrode raw materials, and simultaneously raises the recyclability issue of battery materials after their lifetime.6 Therefore, it is very important to find alternative cathode materials that exhibit decent electrochemical properties and are also produced from cheap, abundant, environmentally friendly, and recyclable raw materials.7,8
In this direction, redox-active organic molecules have started to gain considerable attention as they can be synthesised from environmentally friendly resources and can also be recycled easily into useful compounds after their lifetime.7,8 Their synthetic versatility and modularity to tune electrochemical properties are also conspicuous advantages.9 Historically, organic materials for battery applications were demonstrated9 in the early 1970s, but their electrochemical performance was far inferior to that of inorganic material-based counterparts. Recently, Poizot and co-workers7 proposed the concept of sustainable LIBs, wherein organic redox-active materials were obtained from renewable starting materials. The electrochemical performance of organic electrodes has also made significant progress, particularly in specific capacity and cycle life.10,11 Among various redox-active materials, those incorporating carbonyl groups – namely, quinones,12,13 anhydrides,14,15 imides16,17 and polyketones18,19 – have become extremely popular on account of their ability to reversibly bind Li ions through an enolisation mechanism. Poizot et al.19 recently described the synthesis of a pyromelliticdiimide dilithium salt with significantly improved stability and cyclability in LIBs, thus indicating the importance of substituents on the nitrogens in diimides. Therefore, it is very important to understand the effect of substituents in these types of molecules in order to correlate molecular structure with the battery performance. Although naphthalenediimides (NDs) have higher molecular weight than pyromellitic diimides, their ability to associate with two Li ions at potentials as high as 2.5 V vs. Li/Li+ makes them more suitable as cathode materials. Polyimides based on NDs have recently been investigated16,17 as organic cathode materials, the relationship between molecular structure and device cycling behavior in these systems, however, is still in question. Herein, we have investigated the effect of N-substitution on the LIB performance in NDs in order to establish a structure–property relationship. We have synthesised (Fig. 1a) three different ND derivatives, in which the imide nitrogens are functionalised with –H (NDH), –Me (NDMe) and –Li (NDLi) groups. While LIBs based on NDH gave the highest capacity, those based on NDLi gave the most stable cycling. By contrast, those based on NDMe gradually decreased their capacities over cycling. Our results clearly indicate that small structural modifications on the nitrogen moiety in NDs have a drastic effect on the key battery performance. The mechanism of Li insertion/deinsertion is shown in Fig. 1b.
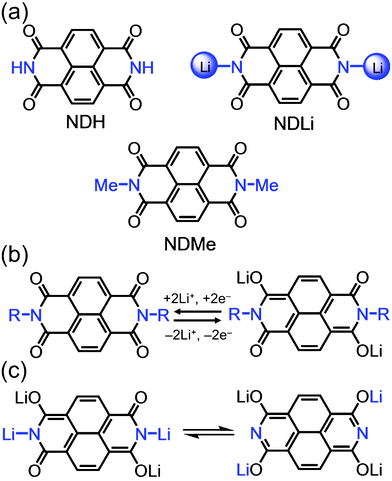 | ||
| Fig. 1 (a) Structural formulae of the three naphthalenediimides (ND) derivatives, in which the imide nitrogens are functionalised with –H (NDH), –Li (NDLi) and –Me (NDMe) groups. (b) Electrochemical redox mechanism of Li insertion/deinsertion in NDs. (c) Possible mechanism of amide tautomerisation in the lithiated state of NDLi. | ||
NDH20 and NDMe21 were prepared by following procedures reported in the literature. NDLi was synthesised (see ESI†) by reacting NDH with lithium hydride in anhydrous DMF under an Ar atmosphere for 16 h. The two coordinating DMF molecules were removed by annealing the sample at 200 °C under an argon atmosphere for 2 h, which resulted in the formation of NDLi as a brown powder in 96% yield. NDLi was characterized using both 1H and 13C NMR spectroscopy and mass spectrometry. The removal of the two coordinating DMF molecules was further evidenced by 1H NMR spectroscopy (results are shown in Fig. S2 in the ESI†) and thermal analysis (results are shown in Fig. S3 in the ESI†).
Electrochemical characterizations were carried out by preparing 2032 coin cells and employing cyclic voltammetry (CV) and galvanostatic measurements. Detailed electrode preparation and characterization procedures are described in the ESI†. As shown in Fig. 2, the small structural changes at the N-site results in dramatic effects on the electrochemical properties of LIBs. These effects can be summarised as follows:
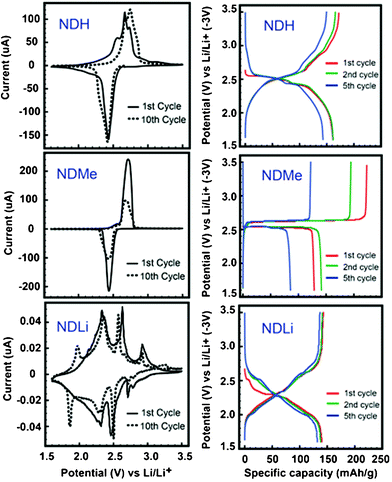 | ||
| Fig. 2 CV and galvanostatic profiles of the three ND derivatives. The CV was measured at a scan rate of 150 μA s−1, and the C-rate of galvanostatic tests was 0.05 C. | ||
1) The N-substitution significantly affects the potential profiles of the compounds. In the cases of NDH and NDMe, both samples showed plateaus near 2.5 V vs. Li/Li+ for both lithiation and delithiation processes. All the potentials addressed hereafter are with respect to that of Li/Li+. However, even between these two samples, the potential profiles turned out to be different. In their galvanostatic data, NDH exhibited plateaus in a broader range between 2.41 and 2.64 V, however, NDMe showed well-defined flat plateaus at 2.53 (lithiation) and 2.63 V (delithiation). By contrast, NDLi exhibited plateaus in a much wider range between 2.0 and 2.65 V in its galvanostatic data. Its CV data also indicates lithiation and delithiation processes over a wider voltage range. Furthermore, a larger number of the CV peaks in the voltage range imply that lithiation and delithiation take place through multiple steps at different potentials, which indicates the possibility of reversible sequential structural changes during the Li insertion/deinsertion.
2) The N-substitution also largely affects the capacity retention of the samples. As indicated by the galvanostatic profiles as well as the CV data, while all of the three samples showed similar lithiation capacities of around 140 mA h g−1 in their first cycles, NDH and NDLi exhibited superior capacity retention in the period of five cycles compared to that of NDMe. In the case of NDMe, the capacity decay was evident, even in the first couple of cycles and continued severely thereafter. Even between NDH and NDLi, NDLi showed better cycling performance over extended cycles.
3) The coulombic efficiencies (CEs, delithiation capacity/lithiation capacity) of these samples showed a consistent trend with capacity retention. NDH and NDLi exhibited CEs close to 100%, whereas NDMe exhibited an anomalously high CE of 142% in their first cycles. This result of CE greater than 100% suggests that NDMe suffers from severe side reactions. Although the dissolution of small organic molecules in the electrolyte in LIBs is a common occurrence,18 polyimides based on NDMe, which are insoluble in the electrolyte, have also exhibited16 constant decay in their capacities, thus indicating that side reactions during the charge/discharge cycles are more probable.
The cycling data measured at a 0.5 C-rate for both lithiation and delithiation (Fig. 3a) display a clear difference between the samples and thus between the N-substitutions. Amongst the three samples, NDLi exhibited the best cycling performance such that it retained 87.6% of its initial capacity (131 mA h g−1) after 100 cycles. NDH showed an intermediate performance of 45% capacity retention compared to its initial capacity (∼170 mA h g−1) after the same number of cycles. By contrast, NDMe exhibited severe capacity decay, as its original capacity (167 mA h g−1) dropped rapidly to 20 mA h g−1 even after 40 cycles.
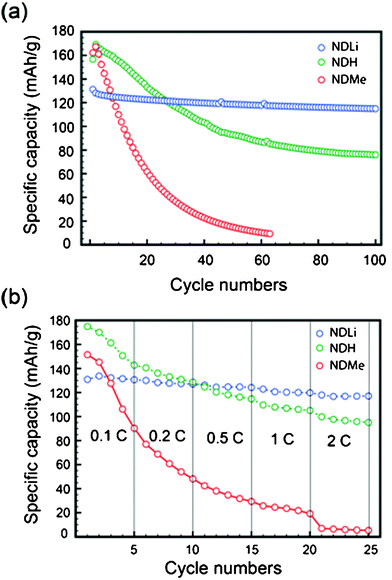 | ||
| Fig. 3 (a) The cycling performance of the three ND derivatives measured at 0.5 C for both lithiation and delithiation. (b) The delithiation capacities at various C-rates. The C-rate was same for lithiation and delithiation. | ||
The rate capability test was also performed for the three samples by employing different C-rates (Fig. 3a). NDH and NDMe exhibited capacity decays upon the increase of the C-rate, which are likely to be associated with their intrinsic capacity decays as observed in Fig. 3a. As shown in the cycling data, NDLi exhibited superior rate performance. As the current rate increased 20-fold from 0.1 to 2 C, its original capacity of 131 mA h g−1 only dropped to 117 mA h g−1, displaying 89% capacity retention. This excellent rate capability implies that the electrochemical reactions of NDLi with Li ions can occur based on kinetically efficient processes.
The superior cycling and rate performance of NDLi can be explained by the sequential rearrangements of chemical bonds of the active molecules during the lithiation. We speculate that following the insertion of two Li ions via the enolisation mechanism (Fig. 1b), NDLi can undergo Li-triggered amide tautomerisation (Fig. 1c), which results in the migration of Li ions to the oxygens, thus forming an aromatic structure.22 We believe that the aromatic character of this lithiated state could be one of the factors for the structural stability of the NDLi. We also believe that a similar mechanism can occur in the case of NDH, however, it results in the formation of an imidic acid intermediate which can possibly interact with the electrolyte. It is also important to note that NDMe cannot undergo tautomerisation to stabilise its lithiated state, which could cause the severe capacity decay. These findings suggest that although the dissolution of small organic molecules is surely an important problem, there are other important structural effects, which could lead to a poor battery performance.
In conclusion, although it has been known that Li ions bind to the carbonyl groups of NDs via the enolisation mechanism, we revealed for the first time that even for the organic compounds with the same number of carbonyl groups at identical positions, the N-substitution can dramatically affect key battery performance. The superior electrochemical performance of NDLi could be attributed to its aromatization in its lithiated state. While polyimide formation is the most straightforward way of preparing polymers from dianhydrides, our results suggest that polymerization of these materials from the aromatic core rather than the anhydride moieties could yield highly stable polymeric battery materials. These effects of the N-substitution would suggest a useful design principle in the development of next generation organic battery materials.
Acknowledgements
We acknowledge the National Research Foundation of Korea Grant funded by the Korean Government (MEST) for financial support through the Secondary Battery Program (NRF-2010-0029042) and the World Class University Program (R-31-2008-000-10055-0) for financial support.References
- A. Yoshino, Angew. Chem., Int. Ed., 2012, 51, 5798–5800 CrossRef CAS.
- R. A. Huggins, Advanced Batteries, Springer, 2008 Search PubMed.
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS.
- Z. G. Yang, J. L. Zhang, M. C. W. Kintner-Meyer, X. C. Lu, D. W. Choi, J. P. Lemmon and J. Liu, Chem. Rev., 2011, 111, 3577–3613 CrossRef CAS.
- J. M. Tarascon, Phil. Trans. R. Soc. A, 2010, 368, 3227–3241 CrossRef.
- J. Dewulf, G. Van der Vorst, K. Denturck, H. Van Langenhove, W. Ghyoot, J. Tytgat and K. Vandeputte, Resour., Conserv. Recycl., 2010, 54, 229–234 CrossRef.
- P. Poizot and F. Dolhem, Energy Environ. Sci., 2011, 4, 2003–2019 CAS.
- H. Nishide, K. Koshika and K. Oyaizu, Pure Appl. Chem., 2009, 81, 1961–1970 CrossRef CAS.
- P. Novak, K. Muller, K. S. V. Santhanam and O. Haas, Chem. Rev., 1997, 97, 207–281 CrossRef CAS.
- L. Nyholm, G. Nyström, A. Mihranyan and M. Strømme, Adv. Mater., 2011, 23, 3751–3769 CAS.
- K. Nakahara, K. Oyaizu and H. Nishide, Chem. Lett., 2011, 40, 222–227 CrossRef CAS.
- M. Yao, H. Senoh, S. Yamazaki, Z. Siroma, T. Sakai and K. Yasuda, J. Power Sources, 2010, 195, 8336–8340 CrossRef CAS.
- H. Y. Chen, M. Armand, M. Courty, M. Jiang, C. P. Grey, F. Dolhem, J.-M. Tarascon and P. Poizot, J. Am. Chem. Soc., 2009, 131, 8984–8988 CrossRef CAS.
- X. Han, C. Chang, L. Yuan, T. Sun and J. Sun, Adv. Mater., 2007, 19, 1616–1621 CrossRef CAS.
- X. Han, G. Qing, J. Sun and T. Sun, Angew. Chem., Int. Ed., 2012, 51, 5147–5151 CrossRef CAS.
- Z. P. Song, H. Zhan and Y. H. Zhou, Angew. Chem., Int. Ed., 2010, 49, 8444–8448 CrossRef CAS.
- Z. Song, T. Xu, M. L. Gordin, Y.-B. Jiang, I.-T. Bae, Q. Xiao, H. Zhan, J. Liu and D. Wang, Nano Lett., 2012, 12, 2205–2211 CrossRef CAS.
- J. Q. Geng, J.-P. Bonnet, S. Renault, F. Dolhem and P. Poizot, Energy Environ. Sci., 2010, 3, 1929–1933 CAS.
- S. Renault, J. Q. Geng, F. Dolhem and P. Poizot, Chem. Commun., 2011, 47, 2414–2416 RSC.
- C. Sotiriou-Leventis and Z. Mao, J. Heterocycl. Chem., 2000, 37, 1665–1667 CrossRef CAS.
- P. R. Ashton, S. E. Boyd, A. Brindle, S. J. Langford, S. Menzer, L. Perez-García, J. A. Preece, F. M. Raymo, N. Spencer, J. F. Stoddart, A. J. P. White and D. J. Williams, New. J. Chem., 1999, 23, 587–602 RSC.
- H. Sachdev, EU. Pat., EP 2 390 253 A1, 2011 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/c2ra21239k/ |
| This journal is © The Royal Society of Chemistry 2012 |
