Naphthalene strapped fluorescent calix[4]pyrrole isomers: halide ion selectivity based on strap topography†
Ritwik
Samanta
,
Sanjeev P.
Mahanta
,
Susanta
Ghanta
and
Pradeepta K.
Panda
*
School of Chemistry, University of Hyderabad, Hyderabad, India 500046. E-mail: pkpsc@uohyd.ernet.in; pradeepta.panda@gmail.com.
First published on 16th July 2012
Abstract
Two novel isomeric strapped calix[4]pyrroles were synthesized using diether straps derived from naphthalene as the fluorescent reporting unit for the first time. The receptor derived from 2,3-dihydroxynaphthalene displays highest sensitivity towards Cl− ion, whereas that derived from 2,7-analogue shows peak sensitivity for F− ion.
The unexpected discovery of anion binding by meso-octamethylcalix[4]pyrrole by Sessler and coworkers, resulted in drawing wide attention from researchers to this macrocycle, as a new class of neutral anionic host.1 Over the last two decades, several transformations on this macrocycle have been carried out, in order to modulate its anion binding ability. This includes substitution at one or more of its meso or β-pyrrolic positions and also via core modification (including core inversion).2 While these new systems help in achieving the desired selectivity to some extent, the single-side strapping approach first reported by Lee and co-workers displayed a tremendous increase in selectivity towards halides.3a Later, Lee's group independently and in association with Sessler's group carried out further detailed studies. These calix[4]pyrroles were strapped using flexible straps via 1,3-linkage to benzene derivatives and 2,5-heteroarenes with diester, diether and diamide based alkyl chains.3 Furthermore, several other reporter units or additional flexible binding domains were used as part of the strapping unit to provide new dimension to anion binding and transport studies along with their ion-pair receptor ability.4
In recent years, fluorometric sensing has emerged as a highly sensitive technique and in conjunction with appropriate receptors can enhance detection of suitable guests in trace quantity.5 In this direction, fluorescent anthracene–calix[4]pyrrole conjugates were designed, which could detect anions by quenching of their fluorescence.6 However, irrespective of whether the fluorescent reporter (anthracene in this case) is conjugated or not, fluoride always remained the most effective quencher. Further, anion sensing of calix[4]pyrrole could be controlled via photoinduced electron transfer using coumarin as the fluorescent reporter in the strap as observed in 1 (Fig. 1).7 Here, the fluorescence emission could be manipulated using appropriate cations and anions. Similarly, acridine was also used as a fluorescent anchor (additionally also acts as a proton acceptor) to the strap 2 and it displays a large selectivity of chloride over bromide ion.3e,3f In the benzo-strapped calix[4]pyrrole 3 Lee's group reported a large downfield shift of the inner aromatic CH proton upon anion binding.3b It is envisaged that this CH proton helps additionally in holding the anions of appropriate size. However, recently, in a related receptor 4, we observed similar binding affinity towards halide ions and hence could conclude that in spite of large deshielding of CH resonance, its contribution towards enhancement of binding affinity of anion is minimal and may be more regulatory in nature.8
![Some of the recently developed strapped calix[4]pyrroles.](/image/article/2012/RA/c2ra21437g/c2ra21437g-f1.gif) | ||
| Fig. 1 Some of the recently developed strapped calix[4]pyrroles. | ||
So far, apart from varying the reporting units in the strap, only the effect of strap length on the anion binding ability was investigated. Herein, we report the synthesis and anion binding study of two novel isomeric fluorescent strapped calix[4]pyrroles. Our approach is unique, in the sense that while both receptors possess naphthalene as the fluorescent reporting units and are linked via same ether-derived straps (2,3- and 2,7-positions of naphthalene), their disposition towards the calix[4]pyrrole moiety is different. Owing to this topographical difference, both the receptors display varied selectivity towards anions. Both the isomeric receptors 5 and 6 were synthesized following the slightly modified Lee's method we have earlier used to synthesize 4 (Scheme 1).8 Briefly, 2,3-dihydroxynaphthalene and 2,7- dihydroxynaphthalene were converted to their corresponding diketones 7 (78%) and 8 (84%) using potassium carbonate and 7-bromo-2-heptanone in acetone. The diketones were converted to respective bisdipyrromethanes 9 (63%) and 10 (55%) using excess pyrrole and TFA. Finally, condensation of the bisdipyrromethanes in acetone using catalytic BF3·etherate led to the formation of only the cis-strapped calix[4]pyrroles 5 and 6 as off white powders in 11 and 6% yield, respectively.
![Synthetic route for the strapped calix[4]pyrroles 5 and 6.](/image/article/2012/RA/c2ra21437g/c2ra21437g-s1.gif) | ||
| Scheme 1 Synthetic route for the strapped calix[4]pyrroles 5 and 6. | ||
All the compounds are rigorously characterized by 1H and 13C NMR, mass spectroscopy, IR spectroscopy and elemental analysis.
Preliminary anion binding studies of these receptors, using 1H NMR titration, display irreversible binding towards anions and hence quantitative estimation of binding affinities is not possible. However, Job plot analysis confirmed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry for all bound anions (ESI†). Subsequently, we carried out anion binding studies using fluorescence emission spectroscopy and isothermal titration calorimetry (ITC). Fluorescence quenching experiments were carried out by addition of solutions of anions as their tetrabutylammonium (TBA) salts to a ∼30 μM solution of compounds 5 and 6 in acetonitrile; the degree of quenching with gradual addition of different anions is displayed in Fig. 2. In the case of receptor 5, significant fluorescence quenching was observed upon addition of F−, Cl−, Br−, CN−, CH3COO− and H2PO4−, whereas no significant quenching was observed in the case of NO3−, NO2−, HSO4−, ClO4−. Further addition of I− displays very weak binding (Ka = 0.9) and hence the observed quenching may be attributed to its heavy atom effect.6 On the other hand, receptor 6 shows significant quenching with fluoride ion along with very weak quenching by chloride ion only.
1 binding stoichiometry for all bound anions (ESI†). Subsequently, we carried out anion binding studies using fluorescence emission spectroscopy and isothermal titration calorimetry (ITC). Fluorescence quenching experiments were carried out by addition of solutions of anions as their tetrabutylammonium (TBA) salts to a ∼30 μM solution of compounds 5 and 6 in acetonitrile; the degree of quenching with gradual addition of different anions is displayed in Fig. 2. In the case of receptor 5, significant fluorescence quenching was observed upon addition of F−, Cl−, Br−, CN−, CH3COO− and H2PO4−, whereas no significant quenching was observed in the case of NO3−, NO2−, HSO4−, ClO4−. Further addition of I− displays very weak binding (Ka = 0.9) and hence the observed quenching may be attributed to its heavy atom effect.6 On the other hand, receptor 6 shows significant quenching with fluoride ion along with very weak quenching by chloride ion only.
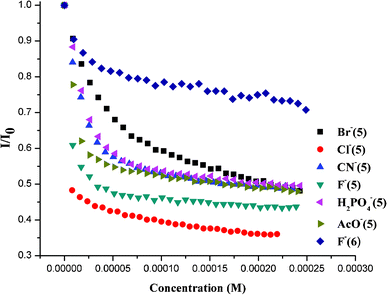 | ||
| Fig. 2 The quenching effect of various anions on 5 and 6 in CH3CN. | ||
A detailed inspection of the association constants (Table 1) reveals strongest preference of 5 (quenching response) towards chloride ion compared to other studied anions including fluoride ion.9 The association constant (Ka) for compound 5 towards chloride is about one order higher compared to fluoride ion and Ka decreases in the order: CH3COO− > CN− > H2PO4− > Br−. This type of chloride selectivity over fluoride is hitherto not reported in calixpyrrole chemistry and is also a rare phenomenon in host–guest chemistry.10 Interestingly, in the case of receptor 5, Ka for fluoride ion appears one order less than that observed for the very closely related 4 under identical conditions (Table 1). Moreover, the association constants measured from ITC (Table 1) also agreed quite well with that obtained from the emission study, except for the chloride complex of 6 and its complexes with larger anions where no quenching is detected. In order to understand the binding mechanism, DFT study was performed on the fluoride and chloride complexes of receptors 4–6. In the free hosts, the aromatic moiety resides in a tilted position (of various degrees) compared to the mean plane of the calix[4]pyrroles. Interestingly, in case of the 5·F− complex, the naphthalene moiety is highly tilted and resides almost in a plane parallel to that of the mean plane of calix[4]pyrrole (Fig. 3).
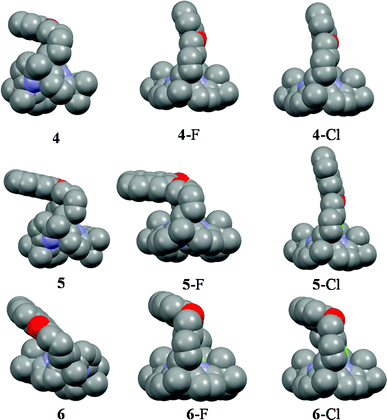 | ||
| Fig. 3 DFT minimized structures of 4, 5 and 6, along with their fluoride and chloride complexes. Colour code: red: O, blue: N, grey: C and dark green: Cl, yellowish green: F. | ||
| Anion | 4 a b | 5 c | 5 a | 6 c | 6 a |
|---|---|---|---|---|---|
| a Determined by ITC. b Ref. 8. c Determined by fluorescence study. d Measured in 0.5% water-acetonitrile. e Binding constants could not be evaluated. f Not reported. | |||||
| F− | — | 2.42 × 105 | 2.42 × 105 | 4.20 × 104 | 4.60 × 104 |
1.17 × 106![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
— | 2.26 × 105![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
— | — | |
| Cl− | 2.12 × 106 | 2.28 × 106 | 2.96 × 106 | 7.20 × 102 | 1.01 × 105 |
| Br− | 1.95 × 106 | 2.92 × 104 | 2.40 × 104 | —e | 3.38 × 104 |
| CN− | —f | 6.31 × 104 | 6.84 × 104 | —e | 3.35 × 104 |
| H2PO4− | 1.07 × 105 | 5.30 × 104 | 4.91 × 104 | —e | 1.36 × 104 |
| CH3COO− | 1.28 × 105 | 1.21 × 105 | 1.13 × 105 | —e | 5.60 × 104 |
On the other hand, this type of spatial disposition is neither observed for the chloride complexes of both 4 and 5, nor for the fluoride complex of 4 (Fig. 3). As the overall association constants result from the combination of four N–H⋯X− hydrogen bonding interactions and the anion–fluorophore interaction, we believe the almost parallel disposition of naphthalene moiety in the case of the 5·F− complex not only hinders the latter's interaction with the fluoride ion but also adversely affects its binding to calix[4]pyrrole in 5, probably due to some unfavourable anion–π interaction. It also indicates that the bigger chloride ion, owing to better fitting inside the binding domain of the strap, could force the bulky naphthalene moiety into an orthogonal orientation, where effective interaction between anion and fluorophore seems favourable. On the basis of the experimental and computational data, the proposed binding model is depicted in Fig. 4a. However, the fluorescence quenching study of receptor 6 displays maximum quenching by fluoride with Ka about two orders higher than that observed for the chloride ion, albeit weakly (Table 1). Here, the DFT optimized structures exhibit a tilted disposition of naphthalene moiety for both the fluoride and the chloride complex, thereby resulting in weaker anion–fluorophore interactions. However, ITC data reveals that while the binding constant measured for interaction of 6 with fluoride is similar to that obtained from the fluorescence quenching experiment, that evaluated for the chloride interaction is more than two orders higher (Table 1). Further acetate, bromide, cyanide and dihydrogenphosphate ions also bind to 6 in similar order but interestingly do not display any quenching in the fluorescence study (Table 1). This discrepancy in anion binding behaviour of 6, can be explained on the basis of the proposed model (Fig. 4b), assuming a relatively constrained binding domain, where smaller anions like fluoride and chloride bind between the strap and the calix[4]pyrrole (hence able to interact with both of them), whereas other larger anions bind to only the calix[4]pyrrole with the naphthalene moiety residing away from it and hence no fluorescence quenching could be observed upon anion binding.3c The above proposed model was well supported by DFT optimized structures, particularly in the case of larger anions like acetate and dihydrogenphosphate (ESI†). This supposition received further credence from the 1H NMR study (ESI†), where the inner naphthalene CH singlet undergoes a downfield shift (0.4–0.6 ppm) depending on their proximity to the anions. For example, fluoride being the smallest in size exerts a very minimal downfield shift compared to the larger dihydrogenphosphate ion, where the inner CH protons (Hb) comes closer to the anion, thereby enhancing the H-bonding type interaction (Fig. 5). However, the tilting away of the fluorophore probably negates anion–fluorophore interaction and hence the observation of resultant lack of quenching in emission. Chloride seems to be an intermediate case, which probably interacts weakly with the fluorophore and relatively strongly with the calix[4]pyrrole with an overall higher Ka (from ITC). The greater overall affinity for chloride is again supported by enthalpy driven complexation for 6·Cl− leading to maximum stabilization in ΔG (ESI†).
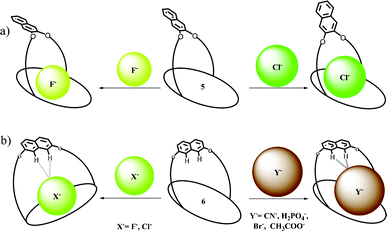 | ||
| Fig. 4 Plausible binding modes of different anions for 5 and 6. | ||
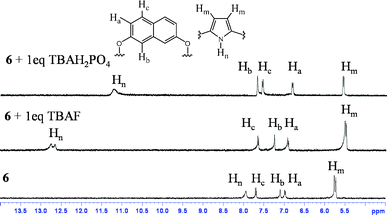 | ||
| Fig. 5 Change in 1H NMR spectrum (partial) of 6 after addition of 1 equivalent of TBAF and TBAH2PO4 in CD3CN. | ||
In conclusion, we have designed and synthesized two new isomeric strapped calix[4]pyrroles using naphthalene as the fluorescent reporting unit. Depending on the disposition of the reporter, sensitivity of the receptors toward anions could be modulated and hence may provide further impetus to host design to enhance sensitivity and selectivity towards anions.
This work is supported by CSIR, India (Project no. 01(2449)/10/EMR–II). RS and SPM thank CSIR for financial support.
References
- (a) P. A. Gale, J. L. Sessler, V. Kral and V. Lynch, J. Am. Chem. Soc., 1996, 118, 5140 CrossRef CAS; (b) A. Baeyer, Ber. Dtsch. Chem. Ges., 1886, 19, 2184 CrossRef.
- (a) P. A. Gale, P. Anzenbacher and J. L. Sessler, Coord. Chem. Rev., 2001, 222, 57 CrossRef CAS; (b) P. A. Gale, J. L. Sessler and V. Kral, Chem. Commun., 1998, 1 RSC; (c) S. P. Mahanta, B. S. Kumar and P. K. Panda, Chem. Commun., 2011, 47, 4496 RSC; (d) J. L. Sessler, W. Roznyatovskiy and V. Lynch, J. Porphyrins Phthalocyanines, 2009, 13, 322 CrossRef CAS; (e) A. Aydogan, J. L. Sessler, A. Akar and V. Lynch, Supramol. Chem., 2008, 20, 11 CrossRef CAS; (f) K. X. Ji, D. S. C. Black, S. B. Colbran, D. C. Craig, K. M. Edbey, J. B. Harper and G. D. Willett, Tetrahedron, 2005, 61, 10705 CrossRef; (g) G. Bruno, G. Cafeo, F. H. Kohnke and F. Nicolo, Tetrahedron, 2007, 63, 10003 CrossRef CAS; (h) J. C. Woods, S. Camiolo, M. E. Light, S. J. Coles, M. B. Hursthouse, M. A. King, P. A. Gale and J. W. Essex, J. Am. Chem. Soc., 2002, 124, 8644 CrossRef; (i) R. Nishiyabu and P. Anzenbacher, J. Am. Chem. Soc., 2005, 127, 8270 CrossRef CAS; (j) J. Yoo, Y. Kim, S. J. Kim and C. H. Lee, Chem. Commun., 2010, 46, 5449 RSC; (k) B. Garg, T. Bisht and S. M. S. Chauhan, New J. Chem., 2010, 34, 1251 RSC; (l) S. M. S. Chauhan, B. Garg and T. Bisht, Supramol. Chem., 2009, 21, 394 CrossRef CAS; (m) P. Anzenbacher, R. Nishiyabu and M. A. Palacios, Coord. Chem. Rev., 2006, 250, 2929 CrossRef CAS; (n) S. Fukuzumi, K. Ohkubo, Y. Kawashima, D. S. Kim, J. S. Park, A. Jana, V. M. Lynch, D. Kim and J. L. Sessler, J. Am. Chem. Soc., 2011, 133, 15938 CrossRef CAS; (o) S. K. Kim, D. E. Gross, D. G. Cho, V. M. Lynch and J. L. Sessler, J. Org. Chem., 2011, 76, 1005 CrossRef CAS.
- (a) D. W. Yoon, H. Hwang and C. H. Lee, Angew. Chem., Int. Ed., 2002, 41, 1757 CrossRef CAS; (b) C. H. Lee, H. K. Na, D. W. Yoon, D. H. Won, W. S. Cho, V. M. Lynch, S. V. Shevchuk and J. L. Sessler, J. Am. Chem. Soc., 2003, 125, 7301 CrossRef CAS; (c) C. H. Lee, J. S. Lee, H. K. Na, D. W. Yoon, H. Miyaji, W. S. Cho and J. L. Sessler, J. Org. Chem., 2005, 70, 2067 CrossRef CAS; (d) D. W. Yoon, D. E. Gross, V. M. Lynch, J. L. Sessler, B. P. Hay and C. H. Lee, Angew. Chem., Int. Ed., 2008, 47, 5038 CrossRef CAS; (e) C. H. Lee, H. Miyaji, D. W. Yoon and J. L. Sessler, Chem. Commun., 2008, 24 RSC; (f) S. D. Jeong, J. Yoo, H. K. Na, D. Y. Chi and C. H. Lee, Supramol. Chem., 2007, 19, 271 CrossRef CAS; (g) S. J. Hong, J. Yoo, D. W. Yoon, J. Yoon, J. S. Kim and C. H. Lee, Chem.–Asian J., 2010, 5, 768 CrossRef CAS.
- (a) P. K. Panda and C. H. Lee, J. Org. Chem., 2005, 70, 3148 CrossRef CAS; (b) H. Miyaji, S. J. Hong, S. D. Jeong, D. W. Yoon, H. K. Na, S. J. Hong, S. Ham, J. L. Sessler and C. H. Lee, Angew. Chem., Int. Ed., 2007, 47, 2508 CrossRef; (c) J. Yoo, M. S. Kim, S. J. Hong, J. L. Sessler and C. H. Lee, J. Org. Chem., 2009, 74, 1065 CrossRef CAS; (d) M. G. Fisher, P. A. Gale, J. R. Hiscock, M. B. Hursthouse, M. E. Light, F. P. Schmidtchen and C. C. Tong, Chem. Commun., 2009, 3017 RSC; (e) M. Yano, C. C. Tong, M. E. Light, F. P. Schmidtchen and P. A. Gale, Org. Biomol. Chem., 2010, 8, 4356 RSC; (f) J. L. Sessler, S. K. Kim, D. E. Gross, C.H. Lee, J. S. Kim and V. M. Lynch, J. Am. Chem. Soc., 2008, 130, 13162 CrossRef CAS; (g) I. W. Park, J. Yoo, B. Kim, S. Adhikari, S. K. Kim, Y. Yeon, C. J. E. Haynes, J. L. Sutton, C. C. Tong, V. M. Lynch, J. L. Sessler, P. A. Gale and C. H. Lee, Chem.–Eur. J., 2012, 18, 2514 CrossRef CAS.
- (a) S. W. Thomas III, G. D. Joly and T. M. Swager, Chem. Rev., 2007, 107, 1339 CrossRef; (b) S. Shanmugaraju, S. A. Joshi and P. S. Mukherjee, J. Mater. Chem., 2011, 21, 9130 RSC.
- H. Miyaji, P. Anzenbacher Jr, J. L. Sessler, E. R. Bleasdale and P. A. Gale, Chem. Commun., 1999, 1723 RSC.
- H. Miyaji, H. K. Kim, E. K. Sim, C. K. Lee, W. S. Cho, J. L. Sessler and C. H. Lee, J. Am. Chem. Soc., 2005, 127, 12510 CrossRef CAS.
- R. Samanta, S. P. Mahanta, S. Chaudhuri, P. K. Panda and A. Narahari, Inorg. Chim. Acta, 2011, 372, 281 CrossRef CAS.
- A. C. Tedescoa, D. M. Oliveiraa, Z. G. M. Lacavab, R. B. Azevedob, E. C. D. Limac and P. C. Moraisd, J. Magn. Magn. Mater., 2004, 272–276, 2404 CrossRef.
- (a) J. L. Sesler, S. Camiolo and P. A. Gale, Coord. Chem. Rev., 2003, 240, 17 CrossRef; (b) B. Schazmann, N. Alhashimy and D. Diamond, J. Am. Chem. Soc., 2006, 128, 8607 CrossRef CAS; (c) K. C. Nam, S. O. Kang and S. W. Ko, Bull. Korean Chem. Soc., 1999, 20, 953 CAS; (d) J. L. Sessler, T. D. Mody, D. A. Ford and V. Lynch, Angew. Chem., Int. Ed. Engl., 1992, 31, 452 CrossRef; (e) J. L Sessler, P. A. Gale and W. S. Cho, Anion Receptor Chemistry, Royal Society of Chemistry, 2006 Search PubMed.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental details, ITC curves and electronic structure calculation are available. See DOI: 10.1039/c2ra21437g/ |
| This journal is © The Royal Society of Chemistry 2012 |
