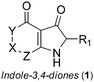
Synthesis and anti HSV-1 evaluation of novel indole-3,4-diones†
Angela
Scala
a,
Massimiliano
Cordaro
a,
Antonino
Mazzaglia
b,
Francesco
Risitano
a,
Assunta
Venuti
c,
Maria Teresa
Sciortino
c and
Giovanni
Grassi
*a
aDipartimento di Chimica Organica e Biologica, Università, Vill.S.Agata, I-98166, Messina, Italy. E-mail: ggrassi@unime.it; Fax: +39 090 393897; Tel: +39 090 6765513
bISMN-CNR c/o Dipartimento di Chimica Inorganica, Chimica Fisica e Chimica Analitica, Università, Vill.S.Agata, I-98166, Messina, Italy
cDipartimento di Scienze della Vita “M. Malpighi”, Sezione Scienze Microbiologiche, Genetiche e Molecolari; Università, Vill.S.Agata, I-98166, Messina, Italy
First published on 20th December 2010
Abstract
A novel class of water soluble indole-3,4-diones has been synthesized and evaluated in vitro for antiviral activity against HSV-1. The results showed lack of cytotoxicity and significant antiviral activity. The cellular internalization efficiency and the antiherpetic effect were successfully increased by incorporation into nanoaggregates of an amphiphilic β-cyclodextrin.
Viral infections are one of the most serious threats to health worldwide. Herpes simplex virus (HSV) is a double-stranded DNA virus of the Herpes viridae family that frequently infects human beings, causing diseases ranging from mild uncomplicated mucocutaneous infection to some that are life-threatening.1 The treatment of choice is Acyclovir (ACV), a guanosine analogue that has to be phosphorylated three times to interfere with viral DNA replication. ACV triphosphate acts by competitive inhibition of viral DNA polymerase and is a DNA chain terminator.2 Widespread use of ACV has led to coming out of HSV strains resistant to ACV. Therefore, research efforts are continuing to develop cheaper, more potent, and less toxic alternative agents against HSV.
In this paper we report a new class of compounds interfering with HSV-1 replication in vitro and sharing attractive structural features with some inhibitors of cyclin-dependent kinases (CDKs).3,4 These compounds, namely indole-3,4-diones 1, were obtained by tandem N-deprotection/cyclization of 1,3,3′-tricarbonyl precursors 2.5
In our prior studies, an efficient chemo- and regioselective method for the generation of the powerful open-chain building blocks 2 was reported, and transformation of the latter into highly functionalized oxazoles was described.6 Apart from their synthetic utility, the appeal of these intermediates is enhanced by the presence of an enolizable 1,3,3′-tricarbonyl moiety, which also occurs in a variety of biopharmacologically significant compounds.7 In fact, triketones8 and their derivatives have long been known for their uses as biologically active substances in their own right. Moreover, their “tricarbonyl Y-topology” bestows upon those molecules significant characteristics,9 including enhanced stability and synthetic versatility as multidentate ligands. For instance, parent α,γ-diketoacids and their analogues have been recently proposed to inhibit HIV integrase and HCV-NS5b polymerase by a mechanism involving chelation of active site magnesium ions, thereby blocking the elongation step in the RNA synthesis.10
Altogether, these findings encouraged us to expand our ongoing studies on the synthetic usefulness of 1,3,3′-tricarbonyls. Thus, the new indole-3,4-dione system was designed and successfully synthesized. Finally, its antiviral activity against HSV-1 was investigated in vitro, and enhanced antiherpetic activity was achieved by incorporation into nanoaggregates of non-ionic heptakis[6-deoxy-6-hexylthio-2-oligo(ethylene glycol)]-β-cyclodextrin (SC6PEG) that can form very stable aqueous colloidal dispersion.11,12
The indolone scaffold is a key structural feature of many naturally-occurring alkaloids with potent biological properties.13 The diversity of the structures encountered, as well as their biological and pharmaceutical relevance, has motivated research aimed at the development of new, economical, efficient, and selective synthetic strategies, particularly for the decoration of indole-type ring systems.14
Thus, we developed an original synthetic methodology for the construction of the selected indole-3,4-diones 6, bearing H-atoms or Me groups on the sp3-C-atoms. The reaction was based on a one-pot acid-promoted N-deprotection-cyclization of the appropriate open-chain derivatives 5 prepared6 from cyclic 1,3-dicarbonyls 3 and 4-methyl-2-phenyl-1,3-oxazol-5(4H)-one 4 (Scheme 1). The products 6 can exist in various tautomeric forms; however, under our experimental conditions, spectroscopic data are consistent only with the depicted 3,4-dione structure. Indeed, the NMR results show exclusively this form (see ESI†). In particular, C2–H and C3a–H are not exchangeable with deuterium (D2O) and 1H-13C correlation experiments (HSQC and HMBC) confirm that C3a is a C–H and unequivocally linked to two C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups.
O groups.
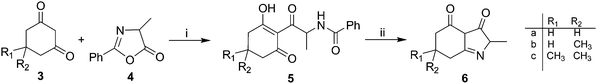 | ||
| Scheme 1 Synthetic route to compounds 6a–c. Reagents and conditions: (i) CH3CN, DBU, MW; (ii) 6N HCl, glacial CH3COOH, reflux. | ||
Compounds 6, containing an Y-iminodicarbonyl moiety likely capable of undergoing prototropic changes, showed semipolarity and consequent high solubility in organic solvents as well as water. Therefore the new structure displays drug-like properties including physicochemical characteristics that typically afford favorable pharmacokinetic and pharmacodynamic properties in vivo. Accordingly, these molecules could potentially overcome the limited solubility in water and limited oral bioavailability of Acyclovir15 in the treatment of herpetic infections.
The cytotoxic effect of 6a–c against Vero cells was assessed using the trypan blue exclusion test. Experiments were performed at various concentrations either in DMSO (in the range 1–100 μM) and water (40–100 μM). In both cases, no cytotoxic effect was detected even at the highest concentration.
The anti-HSV-1 activity of compounds 6a–c was studied in Vero cell cultures using a standard plaque reduction assay. The initial screening was performed in DMSO as a solvent, at three different concentrations of compounds (10, 50, 100 μM) and the results are given in Fig. 1. All three compounds inhibited plaque formation, displaying potent-to-weak dose-dependent activity.
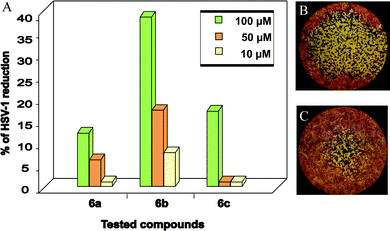 | ||
| Fig. 1 Plaque reduction assay in DMSO. A) Percentage of HSV-1 reduction in the presence of 6a–c (100, 50 and 10 μM). B) Typical size-morphology of HSV-1 plaque C) HSV-1 plaque after treatment with 6b (50 μM). Pictures were taken by using an inverted microscope (Leica). Plaques were visualized at 20x magnification. | ||
Compound 6b revealed the highest anti-HSV-1 activity (Fig. 1A). Indeed, with respect to the controls, plaque number and size-morphology were significantly reduced not only at the higher concentration (100 μM) but also, albeit to a lesser degree, at both dilutions 50 and 10 μM. The characteristic small-plaque pattern observed in treated cells (Fig. 1C) compared to the control (Fig. 1B) suggested that the indoledione derivatives inhibited viral dissemination to neighboring cells.
These findings prompted further investigation of the most promising compound, 6b, in aqueous solution and in the presence of a cyclodextrin-based carrier system under physiological conditions.
Amphiphilic β-cyclodextrins (ACyDs) have been proposed as a useful drug delivery system because they can increase the solubility and bioavailability of the entrapped pharmaceutically active guest. ACyD form nanoparticles of vesicular and micellar morphology in aqueous solutions according to the hydrophilic-hydrophobic balance.11,16 Moreover the macrocycle can be conveniently tailored both by appending groups, that confer stability to nanocarriers, and targeting groups to increase selectivity in the interaction with receptor proteins.17,18 These nanoaggregates are potentially non immunogenic due to the oligoethylene glycol grafted chains and were designed with the aim to have a greater longevity in vivo minimizing non selective scavenging and rapid clearance by phagocytosis. Furthermore ACyD nanoaggregates can deliver drug affording fascinating intracellular effects.19,20
With this in mind, we proposed SC6PEG as a carrier system for compound 6b, with the aim of increasing its cellular internalization efficacy, and improving its antiherpetic activity. To verify the ability of SC6PEG to entrap 6b, the spectroscopic investigation of the sequestration process was carried out, followed by a standard plaque reduction assay in aqueous solution using 6b/SC6PEG complex and comparing with free 6b.
The process of sequestration of 6b was studied by monitoring the changes in the UV/Vis absorption spectra of the complex compared to free compound. In Fig. 2 the spectrum of free 6b in aqueous buffered solution shows an absorption band centred at 294 nm. In the presence of increasing amount of SC6PEG up to [6b]/[SC6PEG] molar ratio of about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the extinction of the band at 294 nm rises: at lower concentration of ACyD (in the range up to 5 μM) the scattering intensity remains essentially unchanged whereas the absorption slightly increases. On the other hand, at higher SC6PEG concentrations in the 10–40 μM range, the scattering intensity rises. As shown in the inset of Fig. 2 by subtracting the scattering effect, SC6PEG/6b system exhibits an higher absorbance than free 6b (with conseguent increase of extinction molar coefficient), indicating association of 6b in SC6PEG. On the other hand the UV spectrum of the complex undergoes a slight wavelength blueshift (about 1 nm) with respect to free 6b. These effects are well-known in literature where the change of extinction coefficient indicates complexation of molecules with cyclodextrins.21,22
1, the extinction of the band at 294 nm rises: at lower concentration of ACyD (in the range up to 5 μM) the scattering intensity remains essentially unchanged whereas the absorption slightly increases. On the other hand, at higher SC6PEG concentrations in the 10–40 μM range, the scattering intensity rises. As shown in the inset of Fig. 2 by subtracting the scattering effect, SC6PEG/6b system exhibits an higher absorbance than free 6b (with conseguent increase of extinction molar coefficient), indicating association of 6b in SC6PEG. On the other hand the UV spectrum of the complex undergoes a slight wavelength blueshift (about 1 nm) with respect to free 6b. These effects are well-known in literature where the change of extinction coefficient indicates complexation of molecules with cyclodextrins.21,22
![A) Cartoon representation of SC6PEG assisted entrapment of 6b. Micellar nanoaggregate is sketched according to the structural characterization (see ref.11). B) Extinction spectra of [6b] = 50 μM in absence (a) and in presence of increasing concentration of SC6PEG (2.5, 5, 10, 20 and 40 μM from b up to e respectively) in PBS 10 mM (pH = 7.4). In the inset the spectra (obtained by subtracting scattering) of free 6b (trace a) and SC6PEG/6b at ∼1 : 1 molar ratio (trace e) are reported.](/image/article/2011/MD/c0md00190b/c0md00190b-f2.gif) | ||
Fig. 2 A) Cartoon representation of SC6PEG assisted entrapment of 6b. Micellar nanoaggregate is sketched according to the structural characterization (see ref.11). B) Extinction spectra of [6b] = 50 μM in absence (a) and in presence of increasing concentration of SC6PEG (2.5, 5, 10, 20 and 40 μM from b up to e respectively) in PBS 10 mM (pH = 7.4). In the inset the spectra (obtained by subtracting scattering) of free 6b (trace a) and SC6PEG/6b at ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio (trace e) are reported. 1 molar ratio (trace e) are reported. | ||
In line with these reports, our findings are consistent with entrapment of 6b into the AcyD nanoaggregates.23,24
Moreover, the in vitro investigation was carried out by using Vero cell lines infected with HSV-1 and treated with free 6b and 6b/SC6PEG complex (ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at concentrations ranging from 12.5 μM to 50 μM in aqueous solution. Compared to the control, plaque number was slightly changed at all tested concentrations of free 6b, but interestingly plaque size-morphology was reduced at 25 and 50 μM (Fig. 3, A and B). It is noteworthy that this potent activity in aqueous solution was achieved at concentrations that were 50% lower than those used in DMSO.
1) at concentrations ranging from 12.5 μM to 50 μM in aqueous solution. Compared to the control, plaque number was slightly changed at all tested concentrations of free 6b, but interestingly plaque size-morphology was reduced at 25 and 50 μM (Fig. 3, A and B). It is noteworthy that this potent activity in aqueous solution was achieved at concentrations that were 50% lower than those used in DMSO.
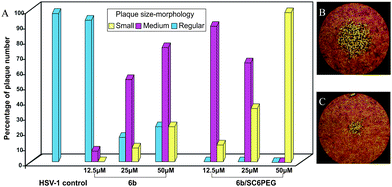 | ||
| Fig. 3 Plaque reduction assay in aqueous solution. A) Effect of 6b and 6b/SC6PEG on the plaque size-morphology. HSV-1 plaques after treatment with 6b 50μM (B) and 6b/SC6PEG50μM (C). Pictures were taken by using an inverted microscope (Leica). Plaques were visualized at 20x magnification. | ||
Furthermore, the antiviral activity of 6b entrapped into SC6PEG against HSV-1 was found to be markedly superior to that of the free compound. The plaque reduction assay revealed that 6b/SC6PEG complex reduced plaque number (∼50%) and significantly size-morphology as depicted in Fig. 3A and 3C.
Thus, the use of the amphiphilic β-CyD considerably enhanced the in vitro antiviral activity of tested compound, by increasing the ability of 6b to penetrate into target cells. Therefore, in analogy with other cyclodextrin–based delivery systems,19,20 sequestration of the drug in the ACyD nanoparticles enhances the cell internalization and, as a result, an increased amount of guest can be delivered with obvious therapeutic effect improvement.
In conclusion, in this paper a new class of indole-3,4-dione derivatives containing an Y-iminodicarbonyl moiety was synthesized and characterized. The selected investigated compounds showed semipolarity and consequent high solubility in organic solvents as well as water.
In particular the compound 6b, bearing a Me group either on C2 and C6, exhibits promising biological properties such as, i) excellent solubility under physiological conditions, ii) lack of cytotoxicity, iii) antiviral activity against HSV-1.
The entrapment of 6b in amphiphilic β-cyclodextrin–based nanocarrier increases the efficacy of internalization improving the therapeutic benefit. Indeed, 6b/SC6PEG complex decreases sensitively both the number of plaques and size morphology in comparison with free 6b (reduction of about 50%) under the same experimental conditions.
In addition, the indole-3,4-dione molecular architecture shows structural features common to some pharmacological inhibitors of cyclin-dependent kinases (CDKs), recently reported to prevent viral replication in vitro.4 All CDK inhibitors target the ATP binding pocket of the catalytic site of the kinase, which can accommodate a large diversity of structures, including molecules incorporating an indolone core.4
Thus, our results open new perspectives in the search for novel antiviral drugs, and further studies are currently in progress to explore their antiviral mechanism.
Notes and references
- R. J. Whitley and B. Roizman, Lancet, 2001, 357(9267), 1513–1518 CrossRef CAS.
- F. Morfin and D. Thouvenot, J. Clin. Virol., 2003, 26, 29–37 CrossRef CAS.
- M. Cordaro, G. Grassi, A. Mazzaglia, F. Risitano, A. Scala, M. T. Sciortino, A. Venuti, Abstracts of Papers, 3rd Hellenic Symposium on Organic Synthesis, From Chemistry to Biology, Medicine and Medical Sciences, Athens, Greece, 15–17 october 2009, 98 Search PubMed.
- (a) M. Knockaert, P. Greengard and L. Meijer, Trends Pharmacol. Sci., 2002, 23(9), 417–425 CrossRef CAS; (b) L. M. Schang, Antivir. Chem. Chemother., 2001, 12, 157–178 Search PubMed.
- (a) E. Altieri, M. Cordaro, G. Grassi, F. Risitano, A. Scala, Abstracts of Papers, 23th Congresso Nazionale SCI, Sorrento, Italy, 5–10 july 2009, 285 Search PubMed; (b) M. Cordaro, G. Grassi, A. Mazzaglia, F. Risitano, A. Scala, Abstracts of Papers, 33th Convegno Nazionale SCI, Divisione Chimica Organica, S. Benedetto del Tronto (AP), Italy, 12–16 september 2010, p. O39 Search PubMed.
- M. Cordaro, G. Grassi, F. Risitano and A. Scala, Synlett, 2009, 1, 103–105.
- (a) S. Lim, Y. Min, B. Choi, D. Kim, I. Yoon, S. S. Lee and I. M. Lee, Tetrahedron Lett., 2001, 42, 7645 CrossRef CAS; (b) Q. Shen, W. Huang, J. Wang and X. Zhou, Org. Lett., 2007, 9, 4491–4494 CrossRef CAS and references cited therein.
- Y. S. Chen, P. Y. Kuo, T. L. Shie and D. Y. Yang, Tetrahedron, 2006, 62, 9410–9416 CrossRef CAS and references cited therein.
- T. P. Radhakrishnan, J. Org. Chem., 2001, 66, 3215–3219 CrossRef CAS and references cited therein.
- (a) U. Koch, B. Attenni, S. Malancona, S. Colarusso, I. Conte, M. Di Filippo, S. Harper, B. Pacini, C. Giomini, S. Thomas, I. Incitti, L. Tomei, R. De Francesco, S. Altamura, V. G. Matassa and F. Narjes, J. Med. Chem., 2006, 49(5), 1693–1705 CrossRef CAS; (b) P. Pace, E. Nizi, B. Pacini, S. Pesci, V. Matassa, R. De Francesco, S. Altamura and V. Summa, Bioorg. Med. Chem. Lett., 2004, 14, 3257–3261 CrossRef CAS.
- D. Lombardo, A. Longo, R. Darcy and A. Mazzaglia, Langmuir, 2004, 20, 1057–1064 CrossRef CAS.
- R. Stancanelli, M. Guardo, C. Cannavà, G. Guglielmo, P. Ficarra, V. Villari, N. Micali and A. Mazzaglia, Journal of Pharmaceutical Sciences, 2010, 99, 3141–3149 CAS.
- (a) C. K. Sha, S. J. Huang, C. M. Huang, A. W. Hong and T. H. Jeng, Pure Appl. Chem., 2000, 72(9), 1773–1776 CrossRef CAS; (b) D. J. Watson and A. I. Meyers, Tetrahedron Lett., 2000, 41, 1519–1522 CrossRef CAS.
- M. M. Heravi, B. Baghernejad, H. A. Oskooie and R. Hekmatshoar, Tetrahedron Lett., 2008, 49, 6101–6103 CrossRef CAS and references cited therein.
- G. Brandi, L. Rossi, G. F. Schiavano, E. Millo and M. Magnani, Int. J. Antimicrob. Agents, 2009, 34, 177–180 CrossRef CAS.
- R. Donohue, A. Mazzaglia, B. J. Ravoo and R. Darcy, Chem. Commun., 2002, 2864–2865 RSC.
- A. Mazzaglia, A. Valerio, V. Villari, A. Rencurosi, L. Lay, S. Spadaro, L. Monsù Scolaro and N. Micali, New J. Chem., 2006, 30, 1662–1668 RSC.
- S. McNicholas, A. Rencurosi, L. Lay, A. Mazzaglia, L. Sturiale, M. Perez and R. Darcy, Biomacromolecules, 2007, 8, 1851–1857 CrossRef CAS.
- F. Quaglia, L. Ostacolo, A. Mazzaglia, V. Villari, D. Zaccaria and M. T. Sciortino, Biomaterials, 2009, 30, 374–382 CrossRef CAS.
- A. Mazzaglia, N. Angelini, R. Darcy, R. Donohue, D. Lombardo, N. Micali, M. T. Sciortino, V. Villari and L. Monsù Scolaro, Chem.–Eur. J., 2003, 9, 5762–5769 CrossRef CAS.
- P. Bortolus, G. Marconi, S. Monti, B. Mayer, G. Köhler and G. Grabner, Chem.–Eur. J., 2000, 6, 1578–1591 CrossRef CAS.
- S. Monti, F. Manoli, I. Manet, G. Marconi, B. Mayer, R. E. Tormos and M. A. Miranda, J. Photochem. Photobiol., A, 2005, 173, 349–357 CrossRef CAS.
- S. Sortino, S. Petralia, R. Darcy, R. Donohue and A. Mazzaglia, New J. Chem., 2003, 27, 602–608 RSC.
- Dynamic light scattering measurements show that free 6b, SC6PEG and 6b/SC6PEG can form nanoaggregates with an average size of 100 nm, 50–150 nm and 150–200 nm, respectively (see ref. 11). The association with ACyD increases the absorbance in UV spectrum of complex indicating disaggregation of 6b caused by interaction with amphiphile. A deeper investigation on the morphology of 6b/SC6PEG colloidal aggregates is currently in progress. We acknowledge Dr Norberto Micali (IPCF-CNR, Messina) for Dynamic Light Scattering Measurements.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c0md00190b |
| This journal is © The Royal Society of Chemistry 2011 |