Extended-nano fluidic systems for analytical and chemical technologies
Kazuma
Mawatari
a,
Takehiko
Tsukahara
b,
Yasuhiko
Sugii
a and
Takehiko
Kitamori
*a
aDepartment of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo, Tokyo 113-8656, Japan. E-mail: kitamori@icl.t.u-tokyo.ac.jp; Fax: +81-3-5841-6039; Tel: +81-3-5841-7231
bResearch Laboratory for Nuclear Reactors, Tokyo Institute of Technology, 2-12-1-N1-32 O-Okayama, Meguro, Tokyo 152-8550, Japan
First published on 7th July 2010
Abstract
Recently, integrated chemical systems have been further downscaled to the 101–103 nm scale, which we call extended-nano space. The extended-nano space is a transient space from single molecules to bulk condensed phase, and fluidics and chemistry have not been explored. One of the reasons is the lack of research tools for the extended-nano space, because the space locates the gap between the conventional nanotechnology (100–101 nm) and microtechnology (>1μm). For these purposes, basic methodologies were developed such as nanofabrication, fluidic control, detection methods, and surface modification methods. Especially, fluidic control is one of the important methods. By utilizing the methodologies, new specific phenomena in fluidics and chemistry were reported, and the new phenomena are increasingly applied to unique applications. Microfluidic technologies are now entering new research phase combined with the nanofluidic technologies. In this review, we mainly focus on pressure-driven or shear-driven extended-nano fluidic systems and illustrate the basic nanofluidics and the representative applications.
 Kazuma Mawatari | Kazuma Mawatari is a lecturer at the School of Engineering at the University of Tokyo. He received his PhD (2006) degree at the University of Tokyo in the area of the thermal lens microscope. He has recently taken interest in thermal lens microscopes for ultrasensitive detection, integration of chemical systems on nano/microchips, and fluidics and chemistry in extended-nano space. |
 Takehiko Tsukahara | Takehiko Tsukahara is an assistant professor at the Research Laboratory for Nuclear Reactors at the Tokyo Institute of Technology. He received his PhD (2003) degree at the Tokyo Institute of Technology in the area of solution chemistry. He has recently taken interest in nanofabrication, nanofluidics, physical chemistry in nanospaces, supercritical fluids, and NMR spectroscopy. |
 Yasuhiko Sugii | Yasuhiko Sugii is an associate professor at the School of Engineering at the University of Tokyo. He received his PhD (2000) degree from Osaka Prefecture University in the area of the fluid dynamics and measurement technique. He has recently taken interest in the measurement technique and fluid dynamics of micro and nano flow, bio-fluid mechanics in blood flow. |
 Takehiko Kitamori | Takehiko Kitamori is a professor at the School of Engineering at the University of Tokyo. He has pioneered microfluidic research in Japan and is the inventor of thermal lens microscopy (TLM). He is co-author of more than 210 international papers and is the vice president of the Chemical and Biological Microsystems Society (CBMS). He has recently taken great interest in extended nanospace, defined as 10–1000 nm, and is currently exploring new phenomena in this regime. |
1. Introduction
In the past decade, integrated micro chemical systems on a chip have been intensively developed. Superior performances were verified compared with macro-scale chemical systems, such as high speed, small sample and reagent volume, and can be used for functional and compact instrumentations for analysis, synthesis, bio- and related sciences and technologies. Among the many fundamental technologies required for the microtechnologies, fluidic control methods and basic study of microfluidic properties are essential. There were mainly two fluidic formats utilized: pressure-driven flow and electro-osmotic flow. Fluidic control utilizing electro-osmotic flow was mainly utilized for initial microfluidic systems, because most of the applications were in DNA separation and detection on a microfluidic chip. However, the targets and applications were limited due to the principle utilizing electrostatic force. In contrast, pressure-driven flow can target many kinds of molecules and even living cells and can provide general chemical systems. So far, several fluidic formats utilizing the multiphase flow were realized based on parallel flow,1 slug flow2 and droplets.3 With these formats by pressure-driven flow, gas, liquid and solid phases can be flexibly incorporated into the microchips which enable general microchemical processes.Recently, the integrated microfluidic systems are being applied to the 101–103 nm scale fluidic systems which we call “extended-nano” to distinguish from 100–101 nm conventional nanotechnology (carbon nanotube, nanopore etc.). The extended-nano space bridges the gap between single molecule and condensed phase, and the fluidics and chemistry in extended-nano space have not been well explored (Fig. 1). This is because the extended-nano space is larger than bottom-up technologies and smaller than the top-down technologies and cannot be fully covered with conventional technologies. In order to solve these problems, the basic research tools (fabrication, fluidic control, detection, surface modification and so on) were rapidly developed in recent years. Then, various new specific phenomena in fluidics and chemistry were increasingly reported.4 By fully utilizing the unique liquid properties, new extended-nano fluidic systems were reported for chemical and analytical technologies which were quite difficult just by microfluidic technologies. The features of the extended-nano space are extremely small volumes (smaller than a single cell) and extremely high surface-to-volume ratio, which can be advantageous for single cell and single molecule analysis. The unique liquid properties in the extended-nano space will lead to novel applications which are not achieved by microfluidics.
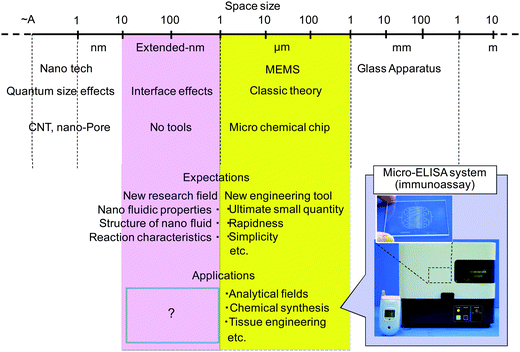 | ||
| Fig. 1 Size hierarchy and extended-nano space. | ||
Currently, fluidic control by electro-osmotic flow or electrophoresis is mainly utilized for these applications due to easy operation.5 This is partially because fluidic control by pressure-driven flow becomes difficult in extended-nano space due to the high fluidic resistance. However, as was verified in microtechnologies, pressure-driven flow can provide general chemical systems and expected to be a key technology to fully exploit nanofluidic properties. Shear-driven flow or simple capillary force can be other solutions to overcome the problems of high fluidic resistance.
In this review, we mainly target the pressure-driven flow and shear-driven flow for integrated nanofluidic systems. We illustrated the basic theory, fluidic control methods and the measurement methods. Then, several unique applications are introduced utilizing pressure-driven flow and shear driven flow. Finally, future perspectives are briefly illustrated.
2. Nanofluidics
2.1 Basic theory
The flows in nanofluidic systems are characterized by the molecular interactions at fluid–solid interface due to the large surface-to-volume ratio so that the flows in nanofluidic systems are quite different from that in macro and micro fluidic systems. Although the no-slip boundary condition was used in the continuum regime, the standard assumptions of the classical continuum theory with no-slip boundary condition often break down in nanofluidic systems.To characterize the rarefaction in gas flow, the Knudsen number (Kn) is used as:
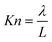 | (1) |
Kn < 0.001: continuum flow regime
0.001 < Kn < 0.1: slip flow regime
0.1 < Kn < 10: transition flow regime
10 < Kn: free molecular flow regime
These classifications are due to the fact that the mean path length of a molecule of air at 100 Pa and 25 °C is approximately 70 nm, and the Knudsen number of gas flow in extended-nano space ranges from 0.07 to 7, which corresponded to the slip flow regime or transition flow regime.
In the continuum flow regime, the channel size is large enough so that the flow obeys the Navier–Stokes equation. In the slip flow regime, slip phenomena such as the velocity slip and temperature jump appear in the thin gas layer adjacent to the solid wall named the Knudsen layer. The slip produces friction drag reduction and may affect mass, momentum and energy transport at the fluid-solid interface. Slippage over a hydrophobic surface even at microscales, NOT in the slip flow regime, has been discovered.7,8 The superhydrophobic surface generated by micro- or nanostructures on a channel wall surface can enhance the slippage of liquid because of the entrapped gas in these microstructures.9 Several kinds of slip models, such as the Burnett equation, Grad moment method and so on, were proposed.10
In the free molecular flow regime, not only do the no-slip conditions break down, but also the surface to volume effects overcome the inertial effects and electro-kinetics is accounted for. A molecular dynamics (MD) technique has been developed to investigate the free molecular flow, where the continuum assumption is replaced by discrete representation of the fluid as interacting particles.11,12 Lattice Boltzmann (LB)13 and direct simulation Monte Carlo (DSMC)14 methods were mainly used for analysis of flow in nanofluidics.
In the extended-nano channel the friction drag becomes significantly large in the continuum flow regime. The friction drag is caused by the pressure drop ΔP, whose value of laminar flow without the slip flow in the circular pipe is theoretically expressed by Hagen-Poiseuille law as follows:
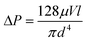 | (2) |
In the slip flow regime, the friction drag reduction occurs. In the free molecular flow regime, the friction drag deviates from the values calculated from eqn (2). It was reported that the viscosity of liquid and friction drag in the extended-nano space became larger than that in the bulk.
In addition, the surface force becomes dominant because of the large surface-to-volume ratio in the extended-nano channel. One of the important parameters is the Laplace pressure ΔPLaplace caused by the interfacial tension between gas-liquid or liquid-liquid phases. Normally, the Laplace pressure can be estimated using the Young–Laplace equation as:
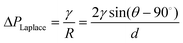 | (3) |
2.2 Nanofluidic control methods
Several types of nanofluidic control methods, such as capillary action, pressure-driven flow, electro-osmotic flow, and shear-driven flow, were established in extended-nano spaces. Generally, liquids can be introduced into the nanospaces due to capillary action, because the nano-confinement induces negative pressure of liquids. Tas et al. analyzed the meniscus curvatures of water plugs in hydrophilic silicon oxide one-dimensional (1D) extended-nano channels with nanometre-depth and micrometre-width using optical microscope. They found that negative pressure of water could be induced by the nano-confinement. A similar shape of meniscus at the liquid/air interface was observed from experimental and theoretical results of ethanol in 1D extended-nano spaces consisting of a SiO2/Si3N4 membrane. For hydrogen-bonded solvents, the very characteristic shape of liquid meniscus with both convex and concave curvatures was found to be formed in the 1D extended-nano spaces.15,16 The capillary filling speeds of hydrogen-bonded solvents such as water and alcohols into 1D and two-dimensional (2D) extended-nano spaces with nanometre-width and -depth on various substrates were systematically measured using optical microscope or a high speed camera. The authors concluded that the filling speed of these solvents was reduced with a decrease in size, because the liquid viscosity in extended-nano space became about 3–4 times larger than bulk liquid.17–19The pressure-driven flow is appropriate for general chemical operations including solvent extraction and reaction. For controlling fluid flows in extended-nano spaces, the nanofluidic control system, which provides both a high pressure of MPa and a low flow rate of pL min−1, are required because of the large pressure drop in extended-nano spaces. Kitamori's groups developed a nanofluidic control system in which the flow rates could be simply controlled by applying air pressure in a sample solution.20 Liquid introduction and mixing of different solutions could be precisely achieved by the developed nanofluidic control systems which were composed by a Y-shaped extended-nano space channel and U-shaped microchannels on a glass chip. As shown in Fig. 2, the continuous mixing of a fluorescein solution and a buffer solution was demonstrated in a Y-shaped extended-nano space channel using an air-pressure-based method, and the performances were evaluated from changes of fluorescence intensities before and after mixing of fluorescein aqueous solution and non-fluorescent buffer solution. These results showed that the nanofluid flow in the extended-nano spaces could be stably controlled in the pressure range of 0.003–0.4 MPa, flow rate range of 0.16–21.2 pL min−1 and residence time range of 24 ms–32.4 s.
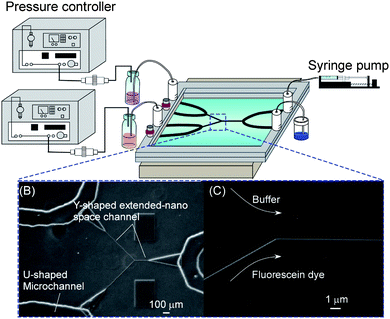 | ||
| Fig. 2 Pressure-driven nanofluidic control system. | ||
Shear-driven flow is expected to be an alternative fluidic control method to pressure-driven flow and electro-osmotic flow. After the solution is introduced into the nanometre-sized gap between upper and lower substrates using capillary action, the upper substrate is moved in the direction parallel with a bottom plate. The materials, such as flat plate, rotatin disk, or flexible belt, can be utilized as the moving upper substrates. Since the solution is automatically dragged in the gap according to the viscous effect of solution, a hydrodynamic flow called a shear-driven flow can be induced even in the extended-nano spaces. It makes it possible to control the nanofluid velocity by a given moving speed, and distance of upper substrate moved and high speed liquid flow can be realized. Actually, Desmet et al. demonstrated that the fluorescent dye, which was dropped in front of a 400 nm-sized gap (1D extended-nano channel), could be driven rapidly in the extended-nano channel by dragging the upper surface.21
2.3 Measurement methods
In order to develop novel functional and practical nanofluidic devices, it is necessary to investigate the velocity profile, which plays an important role for the transport phenomenon and separation in nanofluidics. Micro particle image velocimetry technique (μPIV)22 and molecular tagging velocimetry (MTV)23,24 have been developed to measure a velocity distribution in microfluidics. Although these techniques are useful to investigate the flow field on the microscale, it is difficult to improve the spatial resolution of velocity better than 200 nm due to an optical limitation known as Abbe's diffraction limit.25 Therefore, these methods cannot be applied to investigate flow distribution in nanofluidic systems.In order to overcome the limitation, a nano-PIV technique based on evanescent wave illumination by total internal reflection, which can measure the velocity distribution within a distance of 100 nm from the solid wall in the microchannel, has been developed.26,27Fig. 3 shows an arrangement of optics for total internal reflection, which generates evanescent wave illumination closer to the wall region. Since the intensity of the evanescent wave decays exponentially with the distance normal to the wall, it is possible to observe the illuminated tracers only closer to the wall. However, the technique improves the spatial resolution only in the normal direction to the wall, not that in direction parallel to the wall, due to optical limitation.
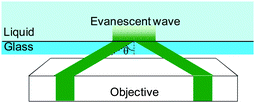 | ||
| Fig. 3 Arrangement of optics for total internal reflection for nanoPIV. | ||
As another approach for improving the spatial resolution, an optical point measurement method with a 20 nm spot size utilizing far-field optical microscope, called stimulated emission depletion (STED),28 has been proposed.29Fig. 4 shows the basic principles of STED. The STED consists of the excitation laser beam through an objective lens to be focused to a spot in the flow field, and a second laser beam with a doughnut-shape by a phase plate modulation is introduced coaxially with the excitation laser beam. The second beam is used to quench the fluorescent dye so that the dye at the outer edge of the excitation spot is quenched preferentially. The center of the laser spot becomes a smaller effective fluorescence spot. The velocity is calculated by measuring a molecular tracer of fluorescence dye and measuring the photobleaching effect by STED which depends on the velocity. A spatial resolution of 70 nm for determination of flow velocity spatial profile was achieved.
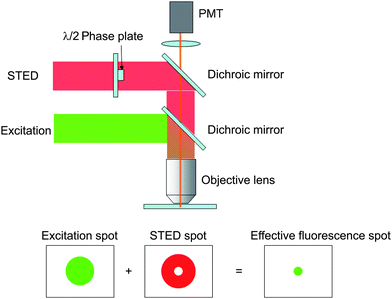 | ||
| Fig. 4 Principles of STED for high resolution imating. | ||
The flow meter is an important method in the extended-nano space. A novel technique for measuring the streaming potential/current in 2D extended-nano space fabricated on fused-silica glass substrates has been proposed to evaluate surface states directly.30 A streaming potential and current of liquid flow were generated by pressure-driven flow in extended-nano channels and were detected using an electrometer. Since the obtained voltage value showed a good linear correlation with the applied pressure, it is possible to measure flow rate in nanochannels. This method will be an important method as a nano-flowmeter to control the nanofluid without adding particles as in PIV.
In order to investigate the surface force, such as the Laplace pressure, it is important to measure the shape of the interface and contact angle on the nanoscale. A liquid bridge inclusion constrained between two clearly-defined menisci in a closed-end carbon nanotube with 10 nm diameter was approximately visualized using a transmission electron microscope (TEM).31 The dynamic behavior of a water plug close to the open end of the carbon tube using environmental scanning electron microscope (ESEM) has also been observed.32 The technique might be applied to interfaces in the extended-nano space.
3. Applications
3.1 Simple mixing and reaction system
Simple reaction systems involving only mixing and reaction are important as tools for investigating basic chemical processes, while the fluidic control is difficult in the extended-nano spaces. There is an example by electro-osmotic flow. Patel et al. demonstrated the electrokinetic DNA hybridization in a T-shaped 1D extended-nano space channel with 10 μm width and 200 nm depth.33 By using an electric field of 220 V cm−1, two solutions without gel could be diffusion-mixed at the T-intersection in the extended-nano channel, and almost all the single-stranded DNA (ssDNA) fragments could be converted into double-stranded DNA (dsDNA) fragments at a hybridization time of about 600 s. This nano-hybridization is a highly efficient technique, because there is no need to provide not only long hybridization time but also sample labeling and sieving structure.The extended-nano regions are expected to produce unique confinement-induced nanospatial effects for the important chemical reactions, because of its large interfacial area ratio and dominant electrical double layer. Kitamori et al. utilized a pressure-driven nanofluidic control system and realized an enzyme reaction in which the nonfluorescent substrate TokyoGreen-β-galactoside (TG-β-gal) was hydrolyzed to the fluorescein derivative TokyoGreen (TG) and β-galactose by the β-galactosidase enzyme acting as a calalyst, as shown in Fig. 5.20 They evaluated size-confinement effects on the kinetic parameters, such as the Michaelis constant (Km), the maximum reaction rate (Vmax) and the first-order rate constant of the enzyme reaction (kcat) for the enzyme reaction, and found that the enzymatic reaction in a 2D extended-nano space was more than two times faster than those in the bulk and microspace. Moreover, from the results of pH dependence, they suggested that the acceleration of the rate of reaction was attributable to an enhancement of proton mobility of water in the extended-nano space.
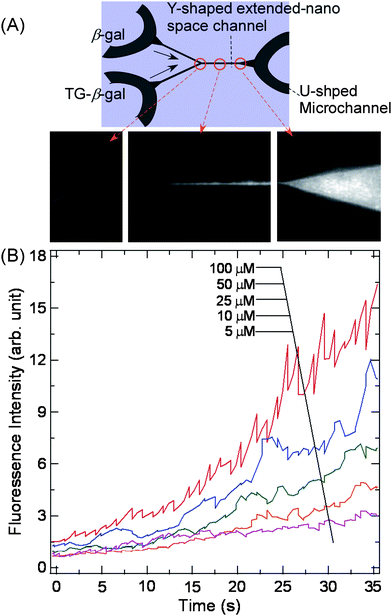 | ||
| Fig. 5 Enzymatic reaction to produce fluorophore by mixing and reacting TokyoGreen-β-galactoside (TG-β-gal) and β-galactosidase (β-gal). Adapted from ref. 20. | ||
Poncet et al. demonstrated the syntheses of colloidal particles or droplets with controlled sizes and morphologies using a nanofluidic device invoking the 1D extended-nano space channels with depths of 420 to 1000 nm and U-shaped microchannels.34 The water and oil solutions that filled the different microchannels were driven into a cross-shaped 1D extended-nano space channel using pressure-driven flow, and the immiscible solutions formed the sheath flow at the cross junction. When the sheath flow was released downstream of the nano-space channel into a microspace chamber, the nanometre-sized droplets were formed, because the interfacial tensions between the immiscible solutions were strongly influenced by sizes and structures of the nanochannel. In this nanofluidic device, it is possible to produce simple and multiple droplets with diameters as small as 900 nm.
3.2 Electrochemical system
Electrochemical systems will provide unique reaction systems due to the small space. A nanofluidic device, in which two platinum electrodes and a 1D extended-nano channel with ∼50 nm height are embedded in a SiO2 substrate, was fabricated using nanofabrication techniques, and the chemically reversible redox reaction of a ferrocenedimethanol (Fc(MeOH)2) solution confined in the extended-nano space were demonstrated using cyclic voltammetry. The results showed that the heterogeneous electron-transfer rate constant of Fc(MeOH)2 was quite fast, and changed by the addition of the supporting electrolytes. The rate constant was expected to depend on the structure of the electrical double layer formed in the extended-nano space, because the double layer can become comparable to the size of the diffusion layer.353.3 Separation system
Separation is one of the important methods in analytical technologies. So far, several separation methods have been reported in the extended-nano space. The most popular method is electrophoresis separation due to the easy fluidic control just by applying voltage. In addition to the advantages obtained just by downscaling the separation, such as small sample volumes (< pL), several unique separation modes were demonstrated which were difficult in the microspace. For example, nanochannel arrays for biomolecule separations based on Ogston sieving, reptation and entropic recoil have been reported in 1D extended-nano space.36 The deep and shallow channels were periodically arranged to induce the separation. For smaller analytes, the radius of gyration can be smaller than the channel dimension. In this case, Ogston sieving becomes the dominant separation mechanism in which smaller molecules elute faster than the larger molecules. If the channel dimension is larger than the analytes, entropy trapping will be dominant. The possibility of entering the 1D extended-nano channel dominates the separation process, and the larger molecules can elute faster than the smaller molecules. The performance of this device was shown from the variety of separations demonstrated, including entropic trapping separation of macromolecules (λ-DNA-Hind III digest) and Ogston sieving of smaller PCR markers.Although there are many possibilities for the electrophoretic separation, separation utilizing pressure-driven flow will also provide a wide range of applications. Liquid chromatography is one of the applications which is widely used in chemistry. Previously, the separation techniques were attempted on microchips by utilizing packed column microchannels, while the resolution was not improved.37 Chromatography systems operating on the microscale face considerable technical difficulties of being able to pack the stationary phase in the separation channel with high reproducibility and accuracy. This problem can be overcome by the reduction of the channel size to a few hundred nanometres, resulting in an interfacial area to volume ratio of the extended-nano channel similar to that of a conventional HPLC column. Therefore, an extended-nano channel surface could be used as a stationary phase. This would remove limitations of current LC systems, namely, the generation of heterogeneous pathways for the solute (eddy diffusion) and the resultant peak broadening by the controlled channel size. Furthermore, unique properties of liquids in the extended-nano space would provide unique separation modes.
Desmet et al. reported an open column chromatography utilizing 1D extended-nano channels.38 Fluidic control by shear-driven flow provided a very fast flow velocity (∼mm s−1) in the extended-nano channels compared with that by pressure-driven flow. The surface of the extended-nano channel was coated with C18 monolayer, and separation of peptide in reverse-phase separation mode was demonstrated in a very short time (0.2 s). They also evaluated the theoretical plate number for the separation of three coumarin dyes, and a good theoretical plate number (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900–24
900–24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) within a short separation time (<1 s) was demonstrated, while conventional HPLC would require several minutes.39
100) within a short separation time (<1 s) was demonstrated, while conventional HPLC would require several minutes.39
The unique separation mode was also reported. The size of the extended-nano space becomes comparable with the length of electrical double layer, and the ions distribute unevenly inside the channel depending on the change of the analyte and wall. Although the flow velocity profile in the extended-nano space is still not clear, the flow velocity in the center of the cross-section will be larger than that near the wall. Therefore, the counter-ions (different charge with the wall) will move faster than the co-ions. Liu et al. reported pressure-driven open column capillary chromatography.40 A fused-silica capillary column with a radius of several-hundred nm was used. Separations of fluorescent molecules depending on the charge were successfully demonstrated without applying an external voltage. The excellent separation efficiency was demonstrated to be approximately 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 plates per metre. However, a relatively large injection volume (pL or sub-pL scale samples) was required. Kitamori et al. reported integrated chromatography systems by utilizing 2D extended-nano space.41 Injection of aL samples and separation functions were integrated on a glass substrate (Fig. 6). Extended-nano channels were connected to four microchannels to which pressure was applied by a compressor and air-pressure controller. Similar to the separation mode reported by Liu et al. (bare glass and aqueous solution), separation of fluorescent molecules was demonstrated depending on the charge which was consistent with the surface negative charge. The efficient separation was shown in approximately 170
000 plates per metre. However, a relatively large injection volume (pL or sub-pL scale samples) was required. Kitamori et al. reported integrated chromatography systems by utilizing 2D extended-nano space.41 Injection of aL samples and separation functions were integrated on a glass substrate (Fig. 6). Extended-nano channels were connected to four microchannels to which pressure was applied by a compressor and air-pressure controller. Similar to the separation mode reported by Liu et al. (bare glass and aqueous solution), separation of fluorescent molecules was demonstrated depending on the charge which was consistent with the surface negative charge. The efficient separation was shown in approximately 170![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 plates per metre.
000 plates per metre.
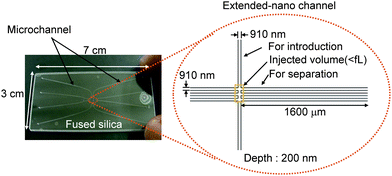 | ||
| Fig. 6 Illustration of extended-nano chromatography system. Adapted from ref. 41. | ||
Particle separation is also important in many fields. Separation of microparticles was demonstrated by several microfluidic technologies such as pinched-flow fractionation.42 However, the separation of nanoparticles was usually difficult due to the large difference of the channel and particle dimension. For this purpose, there were several reports on nanoparticle separation utilizing the extended-nano space. van den Berg et al. reported a hydrodynamic chromatography device utilizing 1D extended-nano space.43 The principle is based on the size-dependent exclusion from the wall in a channel in which a pressure-driven flow is applied. The separation of polystyrene nanoparticles (26–180 nm) was successfully demonstrated. By further miniaturizing the separation space, macromolecule separation utilizing this principle will become possible.
3.4 Bioanalytical system
The volume of the extended-nano space is typically smaller than fL (= 1 μm3). It is very suitable for single cell analysis, because cell volumes are typically in the range of 10 pL. Since the analytical scale is much smaller than cell volumes, future medical and bioanalysis advances may rely on the application of extended-nano space to implement single cell proteomics or metabolomics which is required in the near future. In addition to the extremely small volume, the surface-to-volume ratio of the extended-nano space is several orders larger than that in the microspace. Therefore, precise trap and detection at the single molecule level can be expected. Therefore, many analytical devices will be expected in the extended-nano space for single molecule and single cell analysis when combined with microfluidic technologies.Integration of immunoassay into the extended-nano space will become one of the key future technologies. However, basic methods for the integration were not developed. So far, the basic methods were reported for the integration of nano-in-microchannel fused silica chips, with pressure driven flow by a pressure controller. It was thus demonstrated, for the first time, that immunoassay could be successfully performed for detection of alpha-fetoprotein (AFP) in 1D extended-nano channel.44 The immunoassay included many liquid operations such as immunoreaction (two times for sandwich immunoassay), enzymatic reaction and washing (many times). The well-defined structure of the artificial extended-nano space will be essential to realize the complex chemical processes by pressure-driven flow. Currently, sensitivity enhancement utilizing the ELISA (enzyme linked immunosorbent assay) format and thermal lens microscope (TLM) detection are in development, and approximately 20 molecules per detection area (1 μm2) has been successfully demonstrated.45
The surface properties play significant roles for fluidic and reaction controls. For example, local hydrophobic and hydrophilic patterning can be used to control the two phase multiphase flow. The local immobilization of the proteins and DNA can be utilized for single biomolecule capture and analysis. So far, there have been few reports on the local surface modification in the extended-nano space. This is due to the difficulties of the partial surface modification to closed extended-nano space. Kitamori et al. developed a local DNA immobilization method in the extended-nano space using a photo-linker (Fig. 7) and photochemical reaction.46 A photo-linker of PEG-benzophenone was synthesized. Benzophenone reacts with the CH group in the target DNA by photochemical reaction, and the target DNA was immobilized by this reaction. PEG worked as a protector for nonspecific adsorption. The hydrophilic property of PEG was also important for the introduction of liquid with moderate pressure because the Laplace pressure becomes so high (∼MPa) in the extended-nano space. The local DNA patterning was confirmed by fluorescence microscope after hybridizing with fluorophore-labeled complementary DNA and also investigation of cross reaction with non-complementary DNA labeled with other fluorophores.
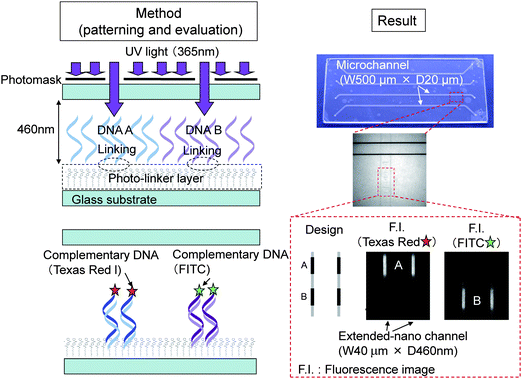 | ||
| Fig. 7 Principle of DNA patterning method and results. Adapted from ref. 46. | ||
Currently, there are only a few reports on the bioanalytical applications. By combining the microfluidic technologies, these devices become innovative tools for single cell bio and diagnosis (e.g. cancer diagnosis at single cell level).
3.5 Energy devices
Extended-nano space is comparable with electric double layer. Co-ions and counter-ions unevenly distribute depending on the charge of the wall. When the counter-ions in the electrical double layer are transported in the extended-nano spaces by the pressure-driven hydrodynamic flow, the streaming potential/current can be induced. Therefore, fluidic energy by pressure-driven flow can be easily converted to electrical power. The energy devices using this principle will become novel hydraulic power generation devices with very simple structures compared with the conventional macro-scale plants. Actually, Dekker et al. demonstrated power generation utilizing 1D silica extended-nano channel.47 By adjusting the ion strength and load resister, a power of 240 pW was obtained for a single channel (width: 50 μm, depth: 490 nm and length: 4.5 mm) by applying a pressure of 3 bar. The highest efficiency of 3% was obtained for a 70 nm deep channel, when the electric double layer was overlapping.There is another unique liquid property in the extended-nano space for fuel cell applications. Matzke et al. reported that the apparent proton conductivity inside a nanochannel could be enhanced by several orders of magnitude due to the electrical double layer overlap utilizing 1D extended-nano channels.48 This unique property was used for fuel cell applications with the extended-nano channel working as conventional proton exchange membrane. In total, 55 parallel extended-nano channels were arrayed to bridge the two U-type microchannels. The depth of the extended nano channels was changed from 50 nm to 50 μm with a constant width of 100 μm. The apparent conductivity of proton increased when the depth of the channels was below 1 μm. The size for transition was larger than the length of the electrical double layer (approximately 100 nm) calculated by conventional Debye–Hückel theory. The reason for this increase is not clear. Kitamori et al. suggested that size confinement of water enhanced proton exchange rate, which was 20 times that of the value in the bulk water. The increase was also observed from 800 nm,49 and they introduced a proton exchange layer and proposed three-layer model to explain the size effect. These phenomena might be closely related, and a more detail study is required. These nanofluidic devices could be applied to fuel cell applications by densely packing the extended-nano channels, which work as proton exchange membranes.
4. Conclusion and future perspectives
In this review, we targeted extended-nano fluidic systems utilizing fluidic control by pressure-driven flow and shear-driven flow as a widely-applicable method. The number of the applications is still small, but the reported systems are quite unique and range from chemistry to energy devices due to the generality of the fluidic control method. However, the basic nanofluidics of pressure-driven flow has not been well explored at present and we cannot answer the basic questions on the fluidic properties. For example, specific liquid properties, such as viscosity increase, enhanced proton exchange, slipping and electrokinetic effect, may influence the flow velocity profile inside the extended-nano space which might be different from the Hagen-Poiseuille profile for pressure-driven flow. For this basic study, new detection methods of the flow profile are required which has a nanometre scale spatial resolution. For technical problems, sensing technologies for flow velocity or pressure will be required for fluidic control. Single molecule detection method with wide applicability is also one of the key technologies for analytical applications.References
- T. Kitamori, M. Tokeshi, K. Sato and A. Hibara, Anal. Chem., 2005, 76, 52A–60A.
- J. R. Burns and C. Ramshaw, Lab Chip, 2001, 1, 10–15 RSC.
- B. Zheng, J. D. Tice, L. S. Roach and R. F. Ismagilov, Angew. Chem., Int. Ed., 2004, 43, 2508–2511 CrossRef CAS.
- T. Tsukahara, K. Mawatari and T. Kitamori, Chem. Soc. Rev., 2010, 39, 1000–1013 RSC.
- M. L. Kovarik and S. C. Jacobson, Anal. Chem., 2007, 79, 1655–1660 CrossRef CAS.
- S. A. Schaaf and P. L. Chambre, Flow of Rarefied Gases, Princeton University Press, USA, 1961 Search PubMed.
- S. Luk, R. Mutharasan and D. Apelianff, Ind. Eng. Chem. Res., 1987, 26, 1609–1616 CrossRef CAS.
- J. Baudry, E. Charlaix, A. Tonck and D. Mazuyer, Langmuir, 2001, 17, 5232–5236 CrossRef CAS.
- R. S. Voronov, D. V. Papavassiliou and L. L. Lee, Ind. Eng. Chem. Res., 2008, 47, 2455–2477 CrossRef CAS.
- B. Y. Cao, J. Sun, M. Chen and Z. Y. Guo, Int. J. Mol. Sci., 2009, 10, 4638–4706 Search PubMed.
- F. H. Stillinger and A. Rahman, J. Chem. Phys., 1972, 57, 1281–1292 CrossRef CAS.
- M. P. Allen and D. J. Tildesley, Computer Simulation of Liquid, Oxford University Press, UK, 1987 Search PubMed.
- G. Chen, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 57, 14958–14973 CrossRef CAS.
- G. A. Bird, Molecular Gas Dynamics and Simulation of Gas Flows, Oxford University Press, UK, 1994 Search PubMed.
- N. R. Tas, P. Mela, T. Kramer, J. W. Berenschot and A. van den verg, Nano Lett., 2003, 3, 1537–1540 CrossRef CAS.
- J. W. van Honschoten, M. Escalante, N. R. Tas and M. Elwenspoek, J. Colloid Interface Sci., 2009, 329, 133–139 CrossRef CAS.
- N. R. Tas, J. Haneveld, H. V. Jansen, M. Elwenspoek and A. van den verg, Appl. Phys., 2004, 85, 3274–3276 CAS.
- A. Hibara, T. Saito, H.-B. Kim, M. Tokeshi, T. Ooi, M. Nakao and T. Kitamori, Anal. Chem., 2003, 36, 605–612.
- A. Han, G. Mondin, N. G. Hegelbach, N. F. de Rooij and U. Saufer, J. Colloid Interface Sci., 2006, 293, 151–157 CrossRef CAS.
- A. Hibara, T. Tsukahara and T. Kitamori, J. Chromatogr., A, 2009, 1216, 673–683 CrossRef CAS.
- D. Clicq, K. Pappaert, S. Vankrunkelsven, N. Vervoort, G. V. Baron and G. Desmet, Anal. Chem., 2004, 76, 430A–438A CAS.
- J. G. Santiago, S. T. Wereley, C. D. Meinhart, D. J. Beebe and R. J. Adrian, Exp. Fluids, 1998, 25, 316–319 CrossRef CAS.
- P. H. Paul, M. G. Garguilo and D. J. Rakestraw, Anal. Chem., 1998, 70, 2459–2467 CrossRef CAS.
- B. Mosier, J. Molho and J. Santiago, Exp. Fluids, 2002, 33, 545–554 CAS.
- E. Abbe, Arch. Mikrosk. Anat., 1873, 9, 413–420 Search PubMed.
- C. Zettner and M. Yoda, Exp. Fluids, 2003, 34, 115–121 CAS.
- S. Jin, P. Huang, J. Park, J. Y. Yoo and K. S. Breuer, Exp. Fluids, 2004, 37, 825–833 CrossRef.
- S. W. Hell and J. Wichmann, Opt. Lett., 1994, 19, 780–782 Search PubMed.
- C. Kuang and G. Wang, Lab Chip, 2010, 10, 240–245 RSC.
- K. Morikawa, K. Mawatari, M. Kato, T. Tsukahara and T. Kitamori, Lab Chip, 2010, 10, 871–875 RSC.
- C. M. Megaridis, A. G. Yazicioglu, J. A. Libera and Y. Gogotsi, Phys. Fluids, 2002, 14, L5–L8 CrossRef CAS.
- M. P. Rossi, H. Ye, Y. Gogotsi, S. Babu, P. Ndungu and J. C. Bradley, Nano Lett., 2004, 4, 989–993 CrossRef CAS.
- D. E. Huber, M. L. Markel, S. Pennathur and K. D. Patel, Lab Chip, 2009, 9, 2933–2940 RSC.
- F. Malloggi, N. Pannacci, R. Attia, F. Monti, P. Mary, H. Willaime, P. Tabeling, B. Cabane and P. Poncet, Langmuir, 2010, 26, 2369–2373 CrossRef CAS.
- M. A. G. Zevenbergen and B. L. Wolfrum, J. Am. Chem. Soc., 2009, 131, 11471–11477 CrossRef CAS.
- J. Fu, R. B. Schoch, A. L. Stevens, S. Tannenbaum and J. Han, Nat. Nanotechnol., 2007, 2, 121–128 CrossRef CAS.
- C. Ericson, J. Holm, T. Ericson and S. Hjerten, Anal. Chem., 2000, 72, 81–87 CrossRef CAS.
- S. Vankrunkelsven, D. Clicq, D. Cabooter, W. D. Malsche, J. G. E. Gardeniers and G. Desmet, J. Chromatogr., A, 2006, 1102, 96–103 CrossRef CAS.
- V. Fekete, D. Clicq, W. D. Malsche, H. Gardeniers and G. Desmet, J. Chromatogr., A, 2008, 1189, 2–9 CrossRef CAS.
- X. Wang, J. Kang, S. Wang, J. J. Lu and S. Liu, J. Chromatogr., A, 2008, 1200, 108–113 CrossRef CAS.
- M. Kato, M. Inaba, T. Tsukahara, K. Mawatari and T. Kitamori, Anal. Chem., 2010, 82, 543–547 CrossRef.
- N. Pamme, Lab Chip, 2007, 7, 1644–1659 RSC.
- M. T. Blom, E. Chmela, R. E. Oosterbroek, R. Tijssen and A. van den Berg, Anal. Chem., 2003, 75, 6761–6768 CrossRef CAS.
- R. Kojima, K. Mawatari, T. Tsukahara, R. Bjorn and T. Kitamori, Microchim. Acta, 2009, 164, 307–310 CrossRef CAS.
- F. Hiruma, K. Mawatari, T. Tsukahara and T. Kitamori, Proc. microTAS., 2008, 221–223 Search PubMed.
- B. Renberg, K. Sato, K. Mawatari, N. Idota, T. Tsukahara and T. Kitamori, Lab Chip, 2009, 9, 1517–1523 RSC.
- F. H. J. van der Heyden, D. J. Bonthuis, D. Stein, C. Meyer and C. Dekker, Nano Lett., 2007, 7, 1022–1025 CrossRef CAS.
- S. Liu, Q. Pu, L. Gao, C. Korzeniewski and C. Matzke, Nano Lett., 2005, 5, 1389–1393 CrossRef CAS.
- T. Tsukahara, W. Mizutani, K. Mawatari and T. Kitamori, J. Phys. Chem. B, 2009, 113, 10808–10816 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
