Electrochemical activation of an oblique angle deposited Cu catalyst film for H2 production
Abstract
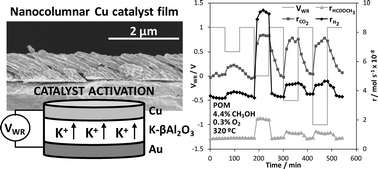
A novel Cu catalyst film was prepared by oblique angle physical vapour deposition (OAD) on a K-βAl2O3 solid electrolyte (alkaline ionic conductor) for catalytic/electrocatalytic purposes. This technique allowed us to obtain a highly porous and electrically conductive Cu catalyst electrode which was tested in the partial oxidation of methanol (POM) reaction for H2 production and its catalytic activity was in situ enhanced via electrochemical promotion of catalysis (EPOC). The electropromotional effect was reversible and reproducible, and allowed us to increase both hydrogen and methyl formate production rates by almost three times under optimal promotion conditions (320 °C, 2.2 × 10−7 mol of K+ transferred). The observed promotional effect was attributed to a decrease in the Cu catalyst work function as a consequence of the controlled migration of electropositive K+ ions which favoured the chemisorption of electron acceptor molecules (O2) at the expense of the electron donor ones (CH3OH). Under the reaction conditions these ions formed some kinds of potassium surface compounds as demonstrated by SEM, EDX and XPS post-reaction characterization analyses. The obtained results demonstrate the interest of the used catalyst-electrode preparation technique for the electrochemical activation of non-noble metal catalyst films.

 Please wait while we load your content...
Please wait while we load your content...