In-depth structure–selectivity investigations on asymmetric, copper-catalyzed oxidative biaryl coupling in the presence of 5-cis-substituted prolinamines†
Abstract
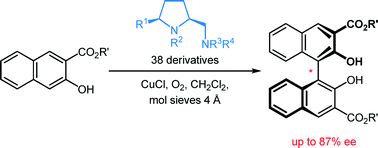
Thirteen new and 25 known prolinamines carrying an additional 5-cis substituent were evaluated as the chiral ligands in asymmetric copper-catalyzed, oxidative biaryl coupling of 3-hydroxy-2-naphthoates. Comprehensive structure–selectivity investigations revealed that a phenyl group in the 5-cis position and a small substituent at the pyrrolidine nitrogen (e.g., Me) are essential for high levels of chirality transfer. The sense of the asymmetric induction depends on the steric demand of the exocyclic amino function. In the coupling of methyl 2-hydroxy-3-naphthoate, a primary amino group permitted up to 36% ee in favor of the P-enantiomer, while up to 64% ee in favor of the M-enantiomer was reached with secondary and tertiary amino functions (e.g. NMe2, (S)-NHCH(Me)Ph). A fully linear relationship between the enantiomeric excess of the prolinamine and the binaphthol was observed. A mechanism consistent with all stereochemical findings is proposed, indicating that 3-hydroxy-2-naphthoates with bulkier ester groups should permit better stereocontrol. Indeed, the enantiomeric excess was raised to good 87% when tert-butyl 3-hydroxy-2-naphthoate was used as the substrate.

 Please wait while we load your content...
Please wait while we load your content...