Themed collection Selective Chemistry with Peptides and Proteins

Bioconjugation – using selective chemistry to enhance the properties of proteins and peptides as therapeutics and carriers
Both peptide and protein therapeutics are becoming increasingly important for treating a wide range of diseases. Functionalisation of these via site-selective chemical modification leads to enhancement of their therapeutic properties.
Org. Biomol. Chem., 2016,14, 8002-8013
https://doi.org/10.1039/C6OB00808A
Site-selective incorporation and ligation of protein aldehydes
The incorporation of aldehyde handles into proteins, and subsequent chemical reactions thereof, is rapidly proving to be an effective way of generating homogeneous, covalently linked protein constructs that can display a vast array of functionality.

Org. Biomol. Chem., 2016,14, 7622-7638
https://doi.org/10.1039/C6OB00778C
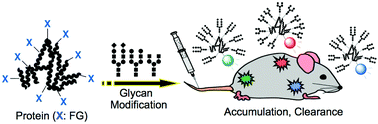
Chemically synthesized glycoconjugates on proteins: effects of multivalency and glycoform in vivo
The biodistributions and in vivo kinetics of chemically prepared glycoconjugates on proteins are reviewed.
Org. Biomol. Chem., 2016,14, 7610-7621
https://doi.org/10.1039/C6OB00788K
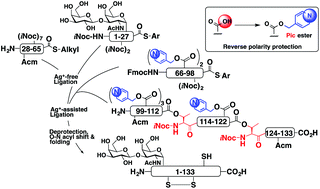
A strategy for the synthesis of hydrophobic proteins and glycoproteins
The hydrophobic glycoprotein was successfully synthesized by the reverse polarity protection strategy combined with the O-acylisopeptide method, which will be useful for the synthesis of various hydrophobic (glyco)proteins.
Org. Biomol. Chem., 2016,14, 6368-6374
https://doi.org/10.1039/C6OB00827E
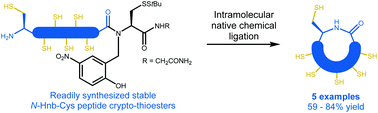
Efficient synthesis of cysteine-rich cyclic peptides through intramolecular native chemical ligation of N-Hnb-Cys peptide crypto-thioesters
We herein introduce a straightforward synthetic route to cysteine-containing cyclic peptides. It is based on the intramolecular native chemical ligation of thioesters generated in situ from N-Hnb-Cys crypto-thioesters. The strategy is applied to a representative range of natural cyclic disulfide-rich peptide sequences.
Org. Biomol. Chem., 2017,15, 316-319
https://doi.org/10.1039/C6OB02546C
Arginine side-chain modification that occurs during copper-catalysed azide–alkyne click reactions resembles an advanced glycation end product
An adduct of dehydroascorbate with arginine forms during copper-catalysed azide–alkyne click reactions and resembles an advanced glycation end product.
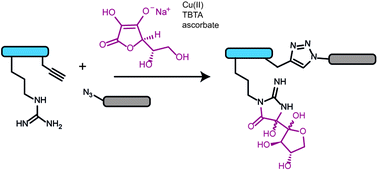
Org. Biomol. Chem., 2016,14, 6205-6211
https://doi.org/10.1039/C6OB00932H
Efficient synthesis of longer Aβ peptides via removable backbone modification
This paper describes a new method for the efficient chemical synthesis of longer Aβ peptides with the combination of the RBM strategy and native chemical ligation.
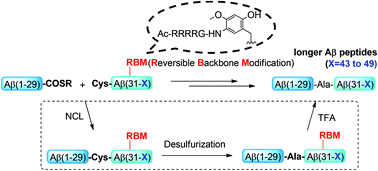
Org. Biomol. Chem., 2016,14, 5012-5018
https://doi.org/10.1039/C6OB00712K
The total synthesis and functional evaluation of fourteen stereoisomers of yaku'amide B. The importance of stereochemistry for hydrophobicity and cytotoxicity
Yaku'amide B is a highly unsaturated linear tridecapeptide and an extremely potent cytotoxin.
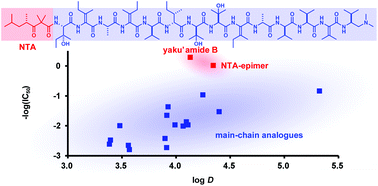
Org. Biomol. Chem., 2016,14, 4199-4204
https://doi.org/10.1039/C6OB00640J
Hmboff/on as a switchable thiol protecting group for native chemical ligation
A new thiol protecting group Hmboff/on is described, which has a switchable activity that may be useful in the chemical synthesis of complex proteins or peptides.

Org. Biomol. Chem., 2016,14, 4194-4198
https://doi.org/10.1039/C6OB00450D
A perfluoroaromatic abiotic analog of H2 relaxin enabled by rapid flow-based peptide synthesis
We report on the rapid-flow based synthesis and functional characterization of a H2 relaxin analog that takes advantage of perfluoroarylation-cysteine SNAr chemistry for a disulfide replacement strategy.

Org. Biomol. Chem., 2016,14, 3345-3349
https://doi.org/10.1039/C6OB00208K
Chemical synthesis and enzymatic properties of RNase A analogues designed to enhance second-step catalytic activity
Adenine covalently attached to the RNase A enzyme molecule decreased the rate of transphosphorylation and increased the rate of hydrolysis.
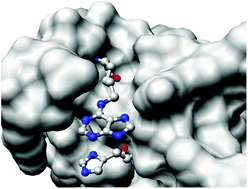
Org. Biomol. Chem., 2016,14, 8804-8814
https://doi.org/10.1039/C6OB01163B
6-Bromo-7-hydroxy-3-methylcoumarin (mBhc) is an efficient multi-photon labile protecting group for thiol caging and three-dimensional chemical patterning
Photochemical release of thiol groups allows spatio-temporal control of biological processes.
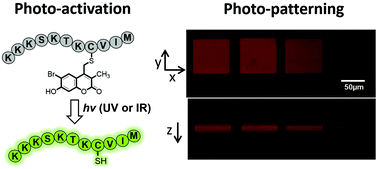
Org. Biomol. Chem., 2016,14, 8289-8300
https://doi.org/10.1039/C6OB01045H
Bis(arylmethyl)-substituted unsymmetrical phosphites for the synthesis of lipidated peptides via Staudinger-phosphite reactions
With this study we introduce new unsymmetrical phosphites to obtain lipidated peptide-conjugates starting from easily accessible azide-modified amino acid or peptide precursors.

Org. Biomol. Chem., 2016,14, 7500-7508
https://doi.org/10.1039/C6OB00843G
Insight into the SEA amide thioester equilibrium. Application to the synthesis of thioesters at neutral pH
Peptide alkylthioesters can be prepared at neutral pH by bis(2-sulfanylethyl)amide-thiol exchange.
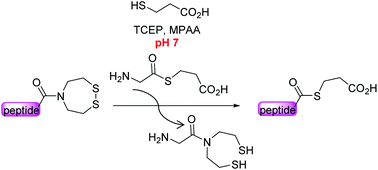
Org. Biomol. Chem., 2016,14, 7211-7216
https://doi.org/10.1039/C6OB01079B
Selenocysteine containing analogues of Atx1-based peptides protect cells from copper ion toxicity
Seleno-substituted model peptides of copper metallochaperone proteins display particularly high Cu(I) affinity and in vitro anti-oxidative reactivity.

Org. Biomol. Chem., 2016,14, 6979-6984
https://doi.org/10.1039/C6OB00849F
Replacing a single atom accelerates the folding of a protein and increases its thermostability
The conformational attributes of proline can have a substantial effect on the folding of polypeptide chains into a native structure and on the stability of that structure.

Org. Biomol. Chem., 2016,14, 6780-6785
https://doi.org/10.1039/C6OB00980H
Chemoselective modifications for the traceless ligation of thioamide-containing peptides and proteins
Optimized reaction conditions permit selective desulfurization of thiols or deselenization of selenols in the presence of thioamides to enable traceless thioamide incorporation by peptide ligation.

Org. Biomol. Chem., 2016,14, 6262-6269
https://doi.org/10.1039/C6OB01020B
Synthesis and in vitro bone cell activity of analogues of the cyclohexapeptide dianthin G
Dianthin G and its dicarba analogue were both shown to increase the number of human osteoblasts without affecting bone resorption.
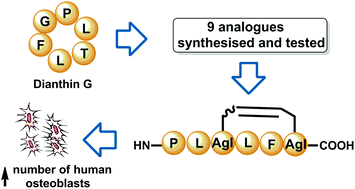
Org. Biomol. Chem., 2016,14, 6231-6243
https://doi.org/10.1039/C6OB00983B
Synthesis of misfolded glycoprotein dimers through native chemical ligation of a dimeric peptide thioester
Misfolded glycoprotein dimers were synthesized through double native chemical ligation between a dimeric peptide-α-thioester, glycopeptide, and non-glycosylated peptide.
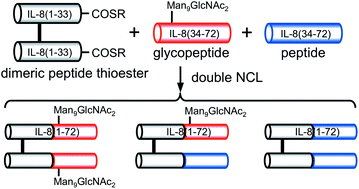
Org. Biomol. Chem., 2016,14, 6088-6094
https://doi.org/10.1039/C6OB00928J
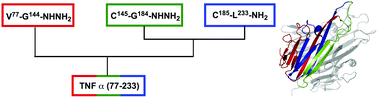
Synthesis of tumor necrosis factor α for use as a mirror-image phage display target
Chemical synthesis of TNFα, a central regulator of inflammation, for use as a mirror-image phage display target.
Org. Biomol. Chem., 2016,14, 5298-5303
https://doi.org/10.1039/C6OB00824K
Genetic incorporation of 1,2-aminothiol functionality for site-specific protein modification via thiazolidine formation
Thiazolidine ligation was used to modify site-specifically proteins harbouring a 1,2-aminothiol moiety introduced by amber codon suppression technology.

Org. Biomol. Chem., 2016,14, 5282-5285
https://doi.org/10.1039/C6OB00854B
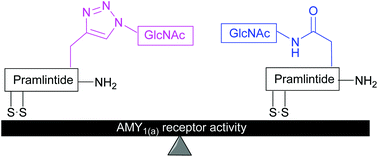
Synthesis and amylin receptor activity of glycomimetics of pramlintide using click chemistry
Synthetic clicked pramlintide glycomimetics maintained AMY1(a) activity and are expected to possess superior synthetic and pharmacokinetic properties than N-glycosylated analogues.
Org. Biomol. Chem., 2016,14, 5238-5245
https://doi.org/10.1039/C6OB00850J
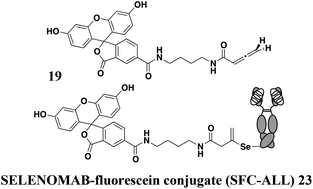
Assessment of reagents for selenocysteine conjugation and the stability of selenocysteine adducts
The allenamide functional group reacts with selenocysteine with notably high efficiency, leads to antibody conjugates with remarkable stability, and shows exquisite selectivity for selenocysteine conjugation.
Org. Biomol. Chem., 2016,14, 5141-5147
https://doi.org/10.1039/C6OB00775A
On-resin Diels–Alder reaction with inverse electron demand: an efficient ligation method for complex peptides with a varying spacer to optimize cell adhesion
The DARinv on resin is a new orthogonal reaction in peptide synthesis and the benefits for cell adhesion are discussed.

Org. Biomol. Chem., 2016,14, 4809-4816
https://doi.org/10.1039/C6OB00314A
Assessing histidine tags for recruiting deoxyribozymes to catalyze peptide and protein modification reactions
We evaluate the ability of hexahistidine tags to recruit deoxyribozymes for covalently modifying peptides and proteins.

Org. Biomol. Chem., 2016,14, 4697-4703
https://doi.org/10.1039/C6OB00716C
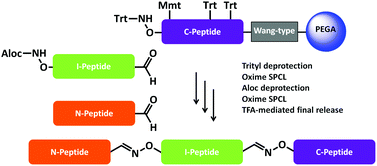
Solid phase oxime ligations for the iterative synthesis of polypeptide conjugates
All on-resin! An efficient C-to-N iterative strategy for solid phase chemical ligations (SPCL).
Org. Biomol. Chem., 2014,12, 5536-5543
https://doi.org/10.1039/C4OB00760C
About this collection
This themed issue highlights some of the most recent exciting research in the field of peptides and protein chemistry, including native chemical ligation and other chemical reactions to prepare peptides and proteins, and selective peptide and protein modification strategies.
The issue was guest-edited by Professor Philip Dawson (The Scripps Research Institute, USA). New articles will be added to this collection as they are published.