The total synthesis and functional evaluation of fourteen stereoisomers of yaku'amide B. The importance of stereochemistry for hydrophobicity and cytotoxicity†
Abstract
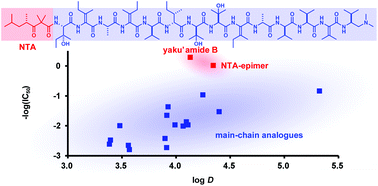
Yaku'amide B is a highly unsaturated linear tridecapeptide and an extremely potent cytotoxin. Herein, we describe the synthesis of fourteen new stereoisomers of yaku'amide B using a unified assembly strategy. The hydrophobicities and cytotoxicities of these analogues were analyzed, along with those of four previously prepared isomers. Although all of the analogues share a common planar structure, their log D values varied significantly (3.39–5.32), presumably reflecting their distinct three-dimensional shapes. Subnanomolar-level cytotoxicity was observed for the natural yaku'amide B and its epimer of the N-terminal acyl group, whereas the other sixteen isomers exhibited 13- to 1200-fold weaker activities than that of the natural isomer. These data indicated the importance of the overall stereostructure of the 13-mer sequence of yaku'amide B for exerting its potent toxicity.
- This article is part of the themed collections: Selective Chemistry with Peptides and Proteins and Contemporary Synthetic Chemistry in Drug Discovery

 Please wait while we load your content...
Please wait while we load your content...