Themed collection Supramolecular chemistry in OBC

Covalent nanosynthesis of fluorene-based macrocycles and organic nanogrids
This paper presents an overview of synthetic approaches for fluorene-based cyclic compounds by examining four different connection models of fluorenes, involving 2,7-, 3,6-, 9,9-, and 2,9-linked patterns.
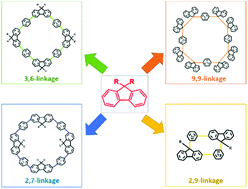
Org. Biomol. Chem., 2022,20, 73-97
https://doi.org/10.1039/D1OB01558C
Recent advances on triptycene derivatives in supramolecular and materials chemistry
In this review, recent researches on triptycene-based macrocyclic arenes, organic cages, porous materials and TADF materials are summarized.
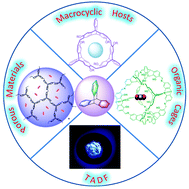
Org. Biomol. Chem., 2021,19, 10047-10067
https://doi.org/10.1039/D1OB01818C
Molecular cages for biological applications
This review compiles selected relevant examples of synthetic receptors defining a closed three-dimensional cavity (cages) with applications in chemical biology and biomedicine.
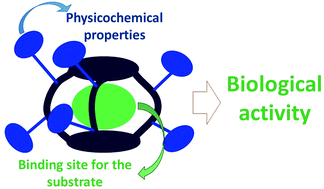
Org. Biomol. Chem., 2021,19, 9527-9540
https://doi.org/10.1039/D1OB01737C
Indicator displacement assays: from concept to recent developments
This review lays emphasis on background concept and summarizes the recent developments of various competitive IDAs. Moreover, current challenges relating to the development of new IDAs and potential future perspectives revealing the fate of IDAs have also been given.
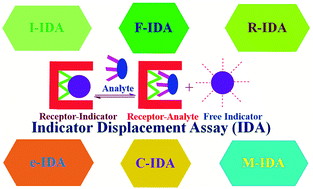
Org. Biomol. Chem., 2021,19, 5926-5981
https://doi.org/10.1039/D1OB00518A
Phosphorus containing porous organic polymers: synthetic techniques and applications in organic synthesis and catalysis
This review features the recent advancement made in phosphorus-containing POPs, highlighting different synthetic techniques for their preparation and application in organic synthesis.

Org. Biomol. Chem., 2021,19, 4174-4192
https://doi.org/10.1039/D1OB00137J
Crown ether–pillararene hybrid macrocyclic systems
In this review, we will fully discuss the synthesis/preparation and applications of crown ether–pillararene hybrid macrocyclic systems/compounds.
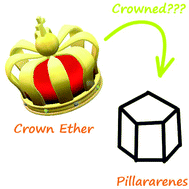
Org. Biomol. Chem., 2021,19, 3287-3302
https://doi.org/10.1039/D1OB00222H
Molecular recognition and sensing of dicarboxylates and dicarboxylic acids
This review outlines challenges faced in recognition and detection of dicarboxylic acids and dicarboxylates and strategies used to obtain effective and observable interactions in the period from 2014 to 2020.
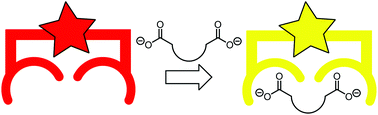
Org. Biomol. Chem., 2020,18, 8236-8254
https://doi.org/10.1039/D0OB01761B
Synthetic strategies towards mechanically interlocked oligomers and polymers
Synthetic methodologies towards the preparation of oligomeric and polymeric mechanically interlocked molecules are described, including both covalent strategies and self-assembly approaches.
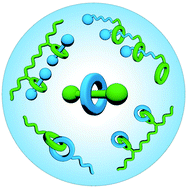
Org. Biomol. Chem., 2020,18, 6757-6780
https://doi.org/10.1039/D0OB01583K
The synthesis and applications of porphyrin-containing pillararenes
Recent progress regarding the combination of porphyrins and pillararenes into hybrid compounds and supramolecular systems is summarized in this review.
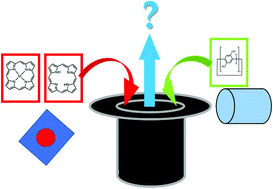
Org. Biomol. Chem., 2020,18, 4894-4905
https://doi.org/10.1039/D0OB00763C
Enantioselective synthesis enabled by visible light photocatalysis
This review summarizes recent developments in visible-light enantioselective photocatalysis reactions, which provide convenient and effective tools for asymmetric synthesis.
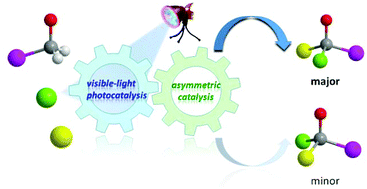
Org. Biomol. Chem., 2020,18, 4298-4353
https://doi.org/10.1039/D0OB00759E
Macrocyclization via C–H functionalization: a new paradigm in macrocycle synthesis
The emergence and applications of the C–H activation logic as a new paradigm in macrocyclization reactions are captured in this review.
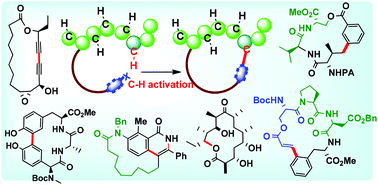
Org. Biomol. Chem., 2020,18, 1851-1876
https://doi.org/10.1039/C9OB02765C
Supramolecular assembly of functional peptide–polymer conjugates
The following review gives an overview about synthetic peptide–polymer conjugates as macromolecular building blocks and their self-assembly into a variety of supramolecular architectures, from supramolecular polymer chains, to anisotropic 1D arrays, 2D layers, and more complex 3D networks.

Org. Biomol. Chem., 2019,17, 6719-6734
https://doi.org/10.1039/C9OB01191A
Tetrathiafulvalene-calix[4]pyrrole: a versatile synthetic receptor for electron-deficient planar and spherical guests
The chemistry of tetrathiafulvalene-calix[4]pyrrole is reviewed with focus on conformational behavior, receptor properties and ionically controlled electron transfer processes.
![Graphical abstract: Tetrathiafulvalene-calix[4]pyrrole: a versatile synthetic receptor for electron-deficient planar and spherical guests](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8OB02514B&imageInfo.ImageIdentifier.Year=2019)
Org. Biomol. Chem., 2019,17, 2594-2613
https://doi.org/10.1039/C8OB02514B
Supramolecular vesicles based on pillar[n]arenes: design, construction, and applications
Recent progress in supramolecular vesicles based on pillar[n]arenes is reviewed.
![Graphical abstract: Supramolecular vesicles based on pillar[n]arenes: design, construction, and applications](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8OB03095B&imageInfo.ImageIdentifier.Year=2019)
Org. Biomol. Chem., 2019,17, 1336-1350
https://doi.org/10.1039/C8OB03095B
Reversible redox switching between local and global aromaticity for core-modified expanded carbaisophlorinoids
28π antiaromatic macrocycles with benzene and azulene units and their effects on local and global (anti)aromaticity have been described. Experimental and computaionl studies confirmed strong diatropic ring current effects in their dicationic states, respectively.
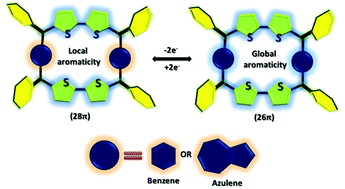
Org. Biomol. Chem., 2022,20, 2818-2821
https://doi.org/10.1039/D1OB02230J
Bis(cholyl)-based chloride channels with oxalamide and hydrazide selectivity filters
Supramolecular bis(cholyl) ion channels with oxalamide and hydrazide as selectivity filters are reported. The hydrazide system showed superior chloride transport activity to oxalamide due to better anion recognition.
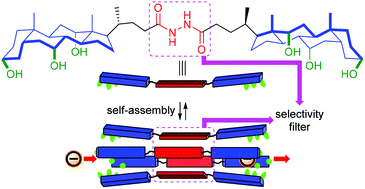
Org. Biomol. Chem., 2022,20, 2054-2058
https://doi.org/10.1039/D1OB02028E
Hydrogenation of cage-opened C60 derivatives mediated by frustrated Lewis pairs
Multiply-carbonylated fullerene derivatives were found to work as one component in frustrated Lewis pairs which caused an Si–H bond activation in the presence of B(C6F5)3, leading to the carbonyl hydrogenation in up to 99% yield.
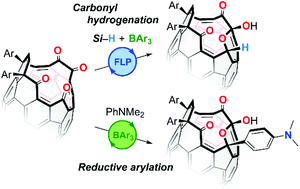
Org. Biomol. Chem., 2022,20, 1000-1003
https://doi.org/10.1039/D1OB02316K
Peptide cyclisation promoted by supramolecular complex formation
Lactamisation of phenol-ester activated peptide esters is promoted by supramolecular Zn-TPP complex formation.

Org. Biomol. Chem., 2022,20, 575-578
https://doi.org/10.1039/D1OB02309H
Glutamate carboxypeptidase II as a model system for designing host–guest units: a theoretical approach
Through a combined crystallographic and computational analysis, we designed and investigated two novel host units for the recognition of neutral and charged guests inspired by a glutamate carboxypeptidase II system and its inhibitors.
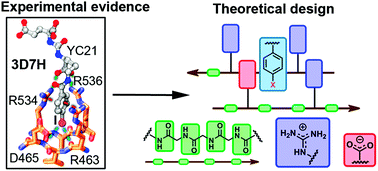
Org. Biomol. Chem., 2021,19, 7816-7821
https://doi.org/10.1039/D1OB01209F
Halogen bonding effect on electrochemical anion oxidation in ionic liquids
The properties of three imidazolium based ionic liquids have been compared and used as solvents for the electrochemical oxidation of various anions.
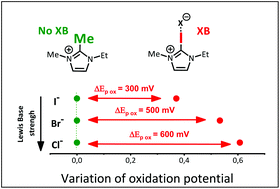
Org. Biomol. Chem., 2021,19, 7587-7593
https://doi.org/10.1039/D1OB01031J
New antimicrobial self-assembling short lipopeptides
Short lipopeptides, that self-assemble into supramolecular structures, show antimicrobial activity.
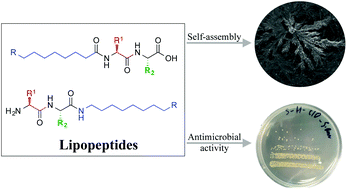
Org. Biomol. Chem., 2021,19, 6797-6803
https://doi.org/10.1039/D1OB01227D
Calixarene-decorated liposomes for intracellular cargo delivery
Liposomes equipped at the outer membrane with positively charged calixarenes show improved efficiency in cargo delivery. This is facilitated by the interaction between the macrocycle units and heparan sulfate proteoglycans surrounding the cell.

Org. Biomol. Chem., 2021,19, 6598-6602
https://doi.org/10.1039/D1OB01055G
Comparison of [Pd2L4][BF4]4 cages for binding of n-octyl glycosides and nitrate (L = isophthalamide or dipicolinamide linked dipyridyl ligand)
Isophthalamide (X = CH) and a dipicolinamide (X = N) derived dipyridyl ligands both form a [Pd2L4][BF4]4 cage in solution, but only the isophthalamide derived cage easily binds to glycosides and nitrate.
![Graphical abstract: Comparison of [Pd2L4][BF4]4 cages for binding of n-octyl glycosides and nitrate (L = isophthalamide or dipicolinamide linked dipyridyl ligand)](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D1OB01185E&imageInfo.ImageIdentifier.Year=2021)
Org. Biomol. Chem., 2021,19, 6633-6637
https://doi.org/10.1039/D1OB01185E
Chalcogen bonding mediates the formation of supramolecular helices of azapeptides in crystals
Supramolecular M- and P-helices are formed via intermolecular S⋯S and S⋯O chalcogen bonding of alanine-based azapeptides containing a β-turn structure and equipped with a thiophene group at the N- or C-terminus, respectively.
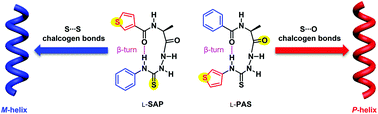
Org. Biomol. Chem., 2021,19, 6397-6401
https://doi.org/10.1039/D1OB01053K
Water binding stabilizes stacked conformations of ferrocene containing sheet-like aromatic oligoamides
The encapsulation of discrete water molecules in flexible sheet-like aromatic oligoamide foldamers, as evidenced by structural and spectroscopic studies, stabilizes their structure.
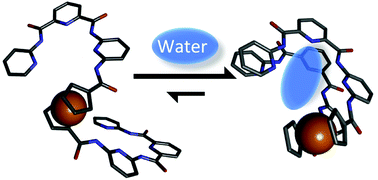
Org. Biomol. Chem., 2021,19, 5521-5524
https://doi.org/10.1039/D1OB00580D
An easily accessible, lower rim substituted calix[4]arene selectively binds N,N-dimethyllysine
A single substitution on p-sulfonatocalix[4]arene, away from its binding face, modifies its selectivity towards a smaller and less hydrophobic guest (dimethyllysine) without making direct contact with the guest.
![Graphical abstract: An easily accessible, lower rim substituted calix[4]arene selectively binds N,N-dimethyllysine](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D1OB00524C&imageInfo.ImageIdentifier.Year=2021)
Org. Biomol. Chem., 2021,19, 4691-4696
https://doi.org/10.1039/D1OB00524C
Facile access to foldable redox-active flavin-peptide conjugates
A convenient approach for the synthesis of foldable redox-active flavin peptide conjugates was established.
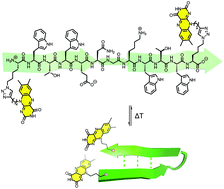
Org. Biomol. Chem., 2021,19, 4483-4486
https://doi.org/10.1039/D1OB00414J
Supramolecular brush polymers prepared from 1,3,4-oxadiazole and cyanobutoxy functionalised pillar[5]arene for detecting Cu2+
The self-assembly of an A1/A2 disubstituted pillar[5]arene was used to construct a supramolecular brush polymer.
![Graphical abstract: Supramolecular brush polymers prepared from 1,3,4-oxadiazole and cyanobutoxy functionalised pillar[5]arene for detecting Cu2+](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D0OB02587A&imageInfo.ImageIdentifier.Year=2021)
Org. Biomol. Chem., 2021,19, 1287-1291
https://doi.org/10.1039/D0OB02587A
Mukaiyama aldol reaction catalyzed by (benz)imidazolium-based halogen bond donors
A Mukaiyama aldol reaction can be catalyzed by bidentate halogen bond donors with very high efficiency. The halogenated catalysts were stable over multiple consecutive runs, which supports the halogen-bond-based mode of catalysis.
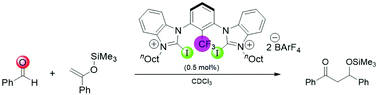
Org. Biomol. Chem., 2021,19, 770-774
https://doi.org/10.1039/D0OB02503H
A medium-firm drug-candidate library of cryptand-like structures on T7 phage: design and selection of a strong binder for Hsp90
We designed and synthesized a library of cryptand-like structures on the T7 phage; a strong binder for a cancer-related protein was selected from the library.
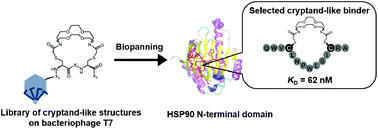
Org. Biomol. Chem., 2021,19, 146-150
https://doi.org/10.1039/D0OB01855D
Aptamer protective groups tolerate different reagents and reactions for regioselective modification of neomycin B
The aptameric protective group strategy is compatible with diverse reagents and reaction conditions for the synthesis of new neomycin B derivatives.
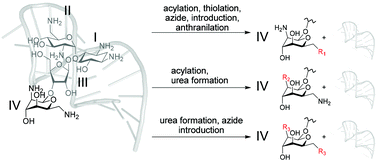
Org. Biomol. Chem., 2020,18, 9606-9610
https://doi.org/10.1039/D0OB02104K
Targeted synthesis of meso-aryl substituted aromatic trans-doubly N-confused dithia/diselena [18] porphyrins (1.1.1.1) with NIR absorption: spectroscopic and theoretical characterization
N-TIPS pyrrole and thiophene/selenophene dicarbinol: the two most promising and significant building blocks made for each other for constructing highly aromatic and NIR absorptive trans-doubly N-confused porphyrins.
![Graphical abstract: Targeted synthesis of meso-aryl substituted aromatic trans-doubly N-confused dithia/diselena [18] porphyrins (1.1.1.1) with NIR absorption: spectroscopic and theoretical characterization](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D0OB01243B&imageInfo.ImageIdentifier.Year=2020)
Org. Biomol. Chem., 2020,18, 6058-6062
https://doi.org/10.1039/D0OB01243B
A fully synthetic 6-aza-artemisinin bearing an amphiphilic chain generates aggregates and exhibits anti-cancer activities
Appendage of an amphiphilic chain by exploiting the nitrogen installed on the 6-aza-artemisinin imparted self-assembling properties. The fully synthetic 6-aza-artemisinin formed aggregates and exhibited increased in vitro anti-cancer activities.
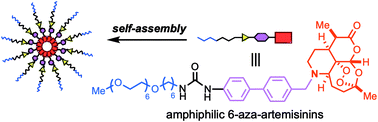
Org. Biomol. Chem., 2020,18, 5339-5343
https://doi.org/10.1039/D0OB00919A
Deprotection of a benzyl unit induces a 22π aromatic macrocycle of 3-oxypyripentaphyrin(0.1.1.1.0) with strong NIR absorption
We report a deprotection-induced aromaticity switching from nonaromatic to a 22π aromatic macrocycle of 3-oxypyripentaphyrin(0.1.1.1.0) with strong NIR absorption.
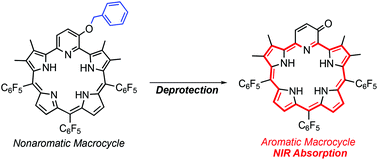
Org. Biomol. Chem., 2020,18, 5334-5338
https://doi.org/10.1039/D0OB01213K
Characterization in aqueous medium of an FMN semiquinone radical stabilized by the enzyme-like microenvironment of a modified polyethyleneimine
The elusive flavin semiquinone intermediate found in flavoproteins such as cryptochromes has been obtained in aqueous solution by single electron reduction of the natural FMN cofactor using sodium ascorbate.
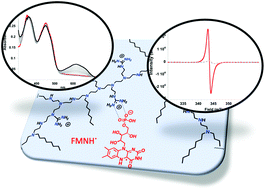
Org. Biomol. Chem., 2020,18, 4386-4389
https://doi.org/10.1039/D0OB00864H
Hierarchical self-assembly of an azobenzene dyad with inverted amide connection into toroidal and tubular nanostructures
Inversion of the amide connectivity of an azobenzene dyad, which self-assembles into chiral toroids and nanotubes, improves the thermal stability of the assemblies, however it negatively affects supramolecular chirality.
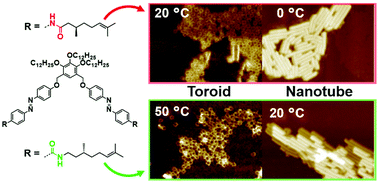
Org. Biomol. Chem., 2020,18, 3996-3999
https://doi.org/10.1039/D0OB00833H
Fluorescent supramolecular hierarchical self-assemblies from glycosylated 4-amino- and 4-bromo-1,8-naphthalimides
The investigation into the self-assembly formation of the glycan based 4-amino- and 4-bromo-1,8-naphthalimide (Nap) structures 1–3 is presented.
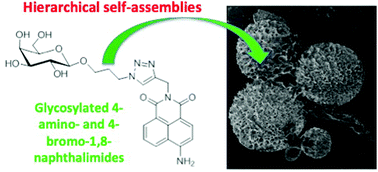
Org. Biomol. Chem., 2020,18, 3475-3480
https://doi.org/10.1039/D0OB00033G
Self-assembling purine and pteridine quartets: how do π-conjugation patterns affect resonance-assisted hydrogen bonding?
π-Conjugation patterns determine the association strengths of purine and pteridine quartets.
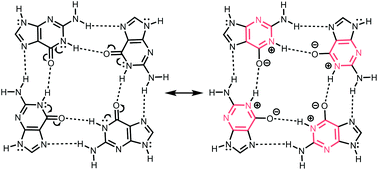
Org. Biomol. Chem., 2020,18, 1078-1081
https://doi.org/10.1039/C9OB02412C
Probing the determinants of porosity in protein frameworks: co-crystals of cytochrome c and an octa-anionic calix[4]arene
In contrast to sulfonato-calix[4]arene (sclx4), which mediates close-packed assemblies, the higher charge carboxylate-containing sclx4mc induced a crystalline framework of cytochrome c.
![Graphical abstract: Probing the determinants of porosity in protein frameworks: co-crystals of cytochrome c and an octa-anionic calix[4]arene](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C9OB02275A&imageInfo.ImageIdentifier.Year=2020)
Org. Biomol. Chem., 2020,18, 211-214
https://doi.org/10.1039/C9OB02275A
Chiral bisphosphine ligands based on quinoline oligoamide foldamers: application in asymmetric hydrogenation
Single-handed quinoline oligoamide foldamer-based bisphosphine ligands that can coordinate with Rh(cod)2BF4 were used as a platform in asymmetric hydrogenation.
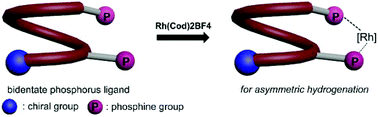
Org. Biomol. Chem., 2019,17, 9573-9577
https://doi.org/10.1039/C9OB01649J
Synthesis of helical π-conjugated polymers bearing pyridine N-oxide pendants and asymmetric allylation of aldehydes in the helical cavity
Asymmetric allylation of aldehydes was conducted inside the helical cavity of a helically folded π-conjugated polymer bearing pyridine N-oxide pendants.
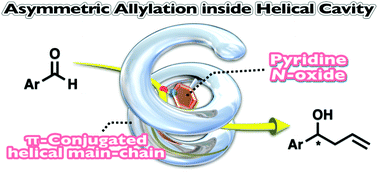
Org. Biomol. Chem., 2019,17, 8537-8540
https://doi.org/10.1039/C9OB01828J
Thermodynamically driven self-assembly of pyridinearene to hexameric capsules
For the first time the formation of hexameric pyridine[4]arene capsule has been observed in gas phase, in solution and in solid state. A thermodynamic balance between capsule formation was observed, showing favour to hexamer.
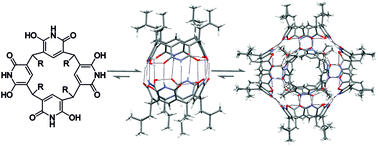
Org. Biomol. Chem., 2019,17, 6980-6984
https://doi.org/10.1039/C9OB01383K
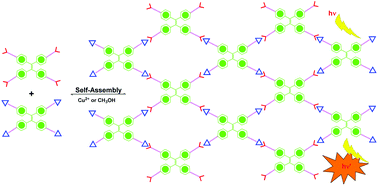
A convenient one-pot nanosynthesis of a C(sp2)–C(sp3)-linked 3D grid via an ‘A2 + B3’ approach
The synthesis of fluorene-based 3D-grid-FTPA, which shows excellent chemical, thermal, and photo-stabilities, has been developed using a one-pot Friedel–Crafts reaction.
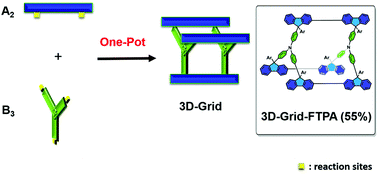
Org. Biomol. Chem., 2019,17, 6574-6579
https://doi.org/10.1039/C9OB00754G
meso-Aryl substituted stable unorthodox 5,10-porphodimethenes with α,β and β,β-N-methyl pyrrole connectivities: synthesis and spectroscopic, solid state and theoretical characterization
meso-Aryl substituted highly stable single conformers of doubly mutant porphodimethenes.
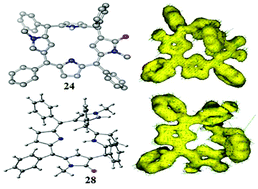
Org. Biomol. Chem., 2019,17, 6131-6135
https://doi.org/10.1039/C9OB01062A
Unidirectional complexation of pillar[4]arene[1]benzoquinoneoxime with alkyl alcohols
Unidirectional binding between a pillar[4]arene[1]benzoquinoneoxime host and n-alkyl alcoholic guests was realized with the hydroxy heads of the guests in direct contact with the oxime group of the macrocyclic host.
![Graphical abstract: Unidirectional complexation of pillar[4]arene[1]benzoquinoneoxime with alkyl alcohols](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C9OB00665F&imageInfo.ImageIdentifier.Year=2019)
Org. Biomol. Chem., 2019,17, 4975-4978
https://doi.org/10.1039/C9OB00665F
Acid/base- and base/acid-switchable complexation between anionic-/cationic-pillar[6]arenes and a viologen ditosylate salt
Acid/base and base/acid-controllable molecular switches were constructed based on water-soluble anionic pillar[6]arene or cationic pillar[6]arene and a viologen ditosylate salt.
![Graphical abstract: Acid/base- and base/acid-switchable complexation between anionic-/cationic-pillar[6]arenes and a viologen ditosylate salt](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C9OB00398C&imageInfo.ImageIdentifier.Year=2019)
Org. Biomol. Chem., 2019,17, 4430-4434
https://doi.org/10.1039/C9OB00398C
Acid–base controlled multiple conformation and aromaticity switches in tren-capped hexaphyrins
Upon protonation, a tren-capped hexaphyrin undergoes simple conformational exchanges between rectangular, Möbius and triangular shapes affording different aromaticities and cage environments, and ultimately leading to a host–guest complex.
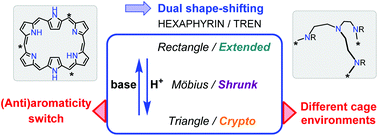
Org. Biomol. Chem., 2019,17, 3718-3722
https://doi.org/10.1039/C9OB00489K
Using reversible non-covalent and covalent bonds to create assemblies and equilibrating molecular networks that survive 5 molar urea
Molecules that assemble through reversible covalent and noncovalent interactions achieve self-assembly at extreme levels of urea and NaCl.
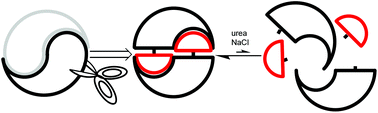
Org. Biomol. Chem., 2019,17, 2081-2086
https://doi.org/10.1039/C8OB02909A
Photodeamination to quinone methides in cucurbit[n]urils: potential application in drug delivery
A prodrug is encapsulated in CB[7] and is photochemically transformed into an active drug inside this supramolecular complex.
![Graphical abstract: Photodeamination to quinone methides in cucurbit[n]urils: potential application in drug delivery](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8OB02605J&imageInfo.ImageIdentifier.Year=2018)
Org. Biomol. Chem., 2018,16, 8908-8912
https://doi.org/10.1039/C8OB02605J
Stereo- and regioselective photocycloaddition of extended alkenes using γ-cyclodextrin
Photoexcitation of dibenzalacetones in homogeneous media and solid state yields a mixture of products with poor conversions. Reactivity of the substrate encapsulated within γ-cyclodextrin results in efficient 2 + 2 photocycloaddition between two reactants on both alkenes groups, yielding a single product with remarkable regio- and stereoselectivity at high conversions.
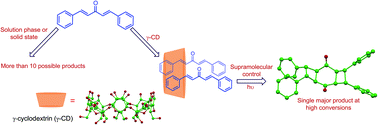
Org. Biomol. Chem., 2018,16, 6870-6875
https://doi.org/10.1039/C8OB01966E
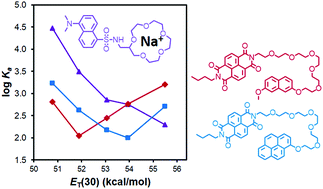
Tuning the fluorescence of tetraphenylethylene in dilute solutions via modulating multiple-hydrogen-bonding interactions between a Hamilton receptor and cyanuric acid
A hydrogen bonded luminescent supramolecular network from HTPE and CTPE can be further tuned.
Org. Biomol. Chem., 2018,16, 4429-4432
https://doi.org/10.1039/C8OB00814K
Intramolecularly enhanced molecular tweezers with unusually strong binding for aromatic guests in unfavorable solvents
Molecular tweezers using aromatic interactions for binding normally work best in polar instead of nonpolar solvents due to the strong solvophobic effect in the binding.
Org. Biomol. Chem., 2018,16, 3885-3888
https://doi.org/10.1039/C8OB00786A
Binding-promoted chemical reaction in the nanospace of a binding site: effects of environmental constriction
Chemical reactions in a confined nanospace can be very different from those in solution.
Org. Biomol. Chem., 2018,16, 2855-2859
https://doi.org/10.1039/C8OB00590G
Aromatically functionalized pseudo-crown ethers with unusual solvent response and enhanced binding properties
Intramolecularly enhanced flexible receptors outperformed traditional rigid receptors when the direct binding force was weakened by solvent competition.
Org. Biomol. Chem., 2018,16, 1627-1631
https://doi.org/10.1039/C8OB00100F
Narcissistic chiral self-sorting of molecular face-rotating polyhedra
Narcissistic chiral self-sorting prevailed in the assembly of molecular face-rotating polyhedra from a C3h building block 5,5,10,10,15,15-hexabutyl-truxene-2,7,12-tricarbaldehyde and racemic mixtures of 1,2-diamines.
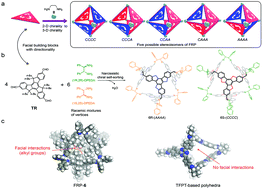
Org. Biomol. Chem., 2018,16, 34-37
https://doi.org/10.1039/C7OB02727C
Taking advantage of Co(II) induced enhanced VCD for the fast and sensitive determination of enantiomeric excess
Large Co(II)-induced VCD signals provide a method for determining the enantiomeric excess of α-amino acids. This strategy can be followed for building new VCD protocols.
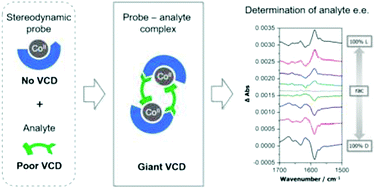
Org. Biomol. Chem., 2017,15, 9800-9803
https://doi.org/10.1039/C7OB02380D
A supramolecular host for phosphatidylglycerol (PG) lipids with antibacterial activity
Various small molecules containing boronic acid and urea functionalities are shown to bind selectively to the bacterial lipid PG (phosphatidylglycerol) and exert antibacterial activity through a membrane-related mechanism.
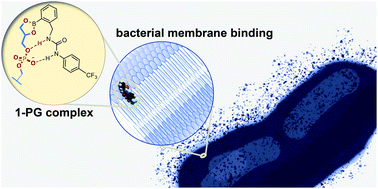
Org. Biomol. Chem., 2022,20, 5958-5966
https://doi.org/10.1039/D1OB02298A
Click synthesis of novel dendronized curcumin and analogs. Strengthening of physicochemical properties toward biological applications
Dendronization by click chemistry as a tool to improve the physicochemical properties of bioactive organic molecules toward biological applications.
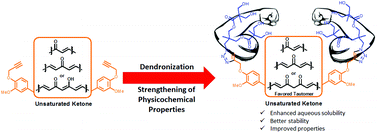
Org. Biomol. Chem., 2022,20, 2643-2650
https://doi.org/10.1039/D2OB00284A
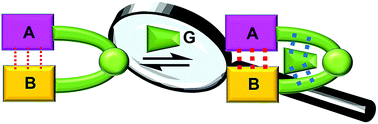
Quantifying the barrier for the movement of cyclobis(paraquat-p-phenylene) over the dication of monopyrrolotetrathiafulvalene
Two positive charges generated by oxidation of a monopyrrolotetrathiafulvalene unit (green) raise an electrostatic barrier to decrease the speed of the deslipping of a tetracationic ring (blue) that take place in a [2]pseudorotaxane.
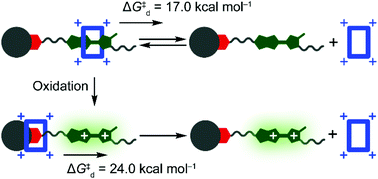
Org. Biomol. Chem., 2022,20, 2233-2248
https://doi.org/10.1039/D1OB02263F
Controlling chirality in the synthesis of 4 + 4 diastereomeric amine macrocycles derived from trans-1,2-diaminocyclopentane and 2,6-diformylpyridine
All 4 + 4 diastereomeric amine macrocycles derived from trans-1,2-diaminocyclopentane and 2,6-difromylpyridine have been synthesized. Chiral compounds exhibit chiral resolution towards 10-camphorsulfonic and tartaric acids.
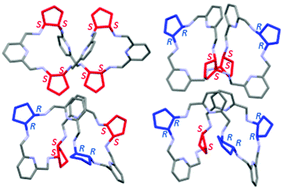
Org. Biomol. Chem., 2022,20, 1080-1094
https://doi.org/10.1039/D1OB02410H
Bio-inspired asymmetric aldehyde arylations catalyzed by rhodium-cyclodextrin self-inclusion complexes
The high enantioselective arylation was pseudo-biologically accererated by self-inclusion of the Rh complex bearing a γ-cyclodextrin.
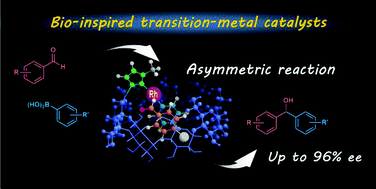
Org. Biomol. Chem., 2022,20, 801-807
https://doi.org/10.1039/D1OB02014E
Anion binding to a cationic europium(III) probe enables the first real-time assay of heparan sulfotransferase activity
We present a new luminescent europium(III)-based anion receptor that binds to PAP (adenosine-3′,5′-diphosphate). The increased emission intensity and lifetime of the receptor-PAP complex was used to develop the first real-time assay of heparan sulfotransferase activity.
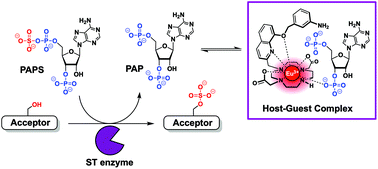
Org. Biomol. Chem., 2022,20, 596-605
https://doi.org/10.1039/D1OB02071D
Four- and two-armed hetero porphyrin dimers: their specific recognition and self-sorting behaviours
Self-assembled/self-sorted hetero dimer capsules consisting of pairs of two- and four-armed porphyrins were constructed. Small and large aromatic guests were selectively recognized by two- and four-armed dimers, respectively.
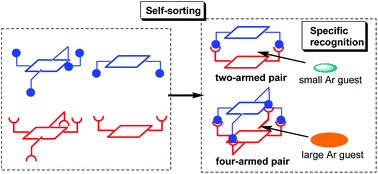
Org. Biomol. Chem., 2022,20, 387-395
https://doi.org/10.1039/D1OB01694F
Molecular tetrominoes: selective masking of the donor π-face to control the configuration of donor–acceptor complexes
π-face masking opens up a new pathway to control the location of acceptor along the donor backbone and consequently donor–acceptor binding interaction strength and percentage of acceptor ionization.
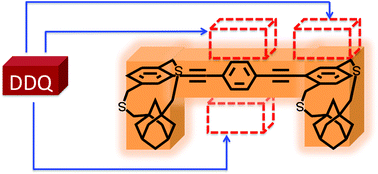
Org. Biomol. Chem., 2022,20, 375-386
https://doi.org/10.1039/D1OB02293H
Dissipative control of the fluorescence of a 1,3-dipyrenyl calix[4]arene in the cone conformation
A fuel driven control of the shape and geometry of the calix[4]arene scaffold allows ON/OFF/ON dissipative fluorescence cycles.
![Graphical abstract: Dissipative control of the fluorescence of a 1,3-dipyrenyl calix[4]arene in the cone conformation](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D1OB02096J&imageInfo.ImageIdentifier.Year=2022)
Org. Biomol. Chem., 2022,20, 132-138
https://doi.org/10.1039/D1OB02096J
Spiro-based diamond-type nanogrids (DGs) via two ways: ‘A1B1’/‘A2 + B2’ type gridization of vertical spiro-based fluorenol synthons
Diamond-type nanogrids (DGs), can helically expand to form spiro-linked polymers. Herein, we designed and synthesized two types of DGs, DGs-1 (A1B1 mode) in 44–50% yields, and DGs-2 (A2 + B2 mode) in 64% yield.
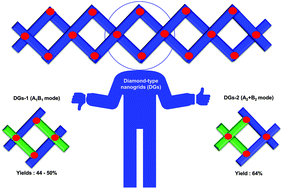
Org. Biomol. Chem., 2021,19, 10408-10416
https://doi.org/10.1039/D1OB01907D
Macrocyclic vs. [2]catenane btp structures: influence of (aryl) substitution on the self templation of btp ligands in macrocyclic synthesis
The synthesis of four 2,6-bis(1,2,3-triazol-4-yl)pyridine(btp) olefin based ligands 3, 4, 11 and 12 is described and their formation of macrocyclic products using ring closing metatheses (RCM) reactions rather than mechanically interlocked molecules.
![Graphical abstract: Macrocyclic vs. [2]catenane btp structures: influence of (aryl) substitution on the self templation of btp ligands in macrocyclic synthesis](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D1OB02032C&imageInfo.ImageIdentifier.Year=2021)
Org. Biomol. Chem., 2021,19, 10189-10200
https://doi.org/10.1039/D1OB02032C
Gold-catalyzed oxidative cyclization of amide-alkynes: access to functionalized γ-lactams
An efficient gold-catalyzed oxidative cyclization of amide-alkynes for the synthesis of functionalized γ-lactams is described.
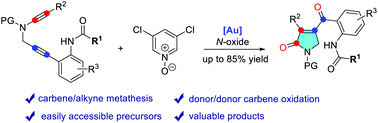
Org. Biomol. Chem., 2021,19, 9688-9691
https://doi.org/10.1039/D1OB01846A
Acridinone-based anion transporters
The acridinone 1,9-bis(thio)urea scaffold was repurposed for application in anion transport by appending a variety of electron-withdrawing groups to the peripheral phenyl moieties. High levels of activity were achieved which facilitated strictly electroneutral transport.
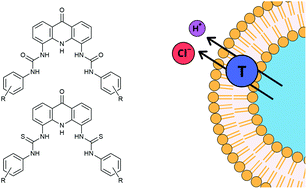
Org. Biomol. Chem., 2021,19, 9659-9674
https://doi.org/10.1039/D1OB01545A
Duplex vs. folding: tuning the self-assembly of synthetic recognition-encoded aniline oligomers
We present a strategy for characterising and preventing the undesired folding between adjacent units in duplex-forming synthetic hetero-oligomers.
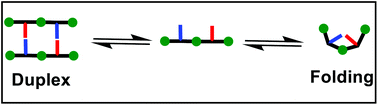
Org. Biomol. Chem., 2021,19, 8947-8954
https://doi.org/10.1039/D1OB01882E
About this collection
The latest articles published in Organic and Biomolecular Chemistry related to supramolecular chemistry research.