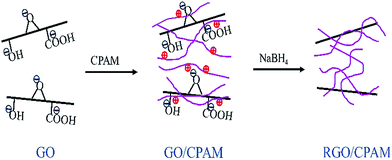
Preparation via flocculation of reduced graphene oxide/cationic polyacrylamide composites and their electrochemical properties†
Li-Li Wu,
Fei-Rong Deng and
Chao-Can Zhang*
School of Materials Science and Engineering, Wuhan University of Technology, Wuhan 430070, China. E-mail: polymers@whut.edu.cn
First published on 18th October 2016
Abstract
Composites of reduced graphene oxide (RGO) and cationic polyacrylamide (CPAM) are prepared through a facile method. The effect of CPAM content on the specific capacitance of the composite is investigated. Compared with the pure RGO, the composite shows a relatively high capacitance, due to the fact that CPAM improves the wettability and surface area of RGO.
Supercapacitors have become promising energy storage devices for portable electronic devices and electric vehicles because of their high power capabilities and power densities, ultra-long cycling life, wide thermal operating ranges, and low maintenance costs.1,2 Graphene, a new kind of carbon material with a unique two-dimensional layer atomic structure, has been proven to possess excellent mechanical, electronic and catalytic properties.3 A high conductivity and an in-plane surface area make it a potential electrode material for use in supercapacitors.4
Graphene oxide (GO) is often considered as a precursor to low-cost and scalable production of graphene based materials. It bears a large number of hydroxyl, epoxide and carbonyl groups,5,6 which can be eliminated by chemical reduction or thermal annealing. However, irreversible agglomeration while removing oxygen containing groups is the main problem. So far, many efforts have been made to prepare functionalized or dispersed graphene, either by chemical reduction of GO in the presence of stabilizers, such as amphiphilic polymers7 or polypyrrole microspheres,8 or by the reduction of functional GO with organic ionic surfactants9 or isocyanates.10
Cationic polyacrylamide (CPAM), a common polymer electrolyte, is widely used in wastewater treatment11 and paper-making.12 It can be used as a flocculant13 or a dispersing agent14 owing to the active amide groups in the polymer chain. GO sheets with negatively charged oxygen groups can intercalate with CPAM chains via electrostatic interactions. Yu et al.15 reported a simple and highly effective method for the preparation of a conductive graphene-based thin film by self-assembly of graphene oxide on a CPAM layer, followed by chemical reduction. The RGO/CPAM composite film exhibited a sheet resistance of 3.1 kΩ sq−1. Furthermore, CPAM can be used as a flocculant for GO, owing to the co-action of charge neutralization and polymer bridging. Preparation of a RGO/CPAM composite through the process of flocculation in an aqueous solution has never been reported.
In the present work, we report a simple route for the preparation of a RGO/CPAM composite (Scheme 1). A certain amount of CPAM solution was added to the completely exfoliated GO aqueous solution, and the mixture was stirred with glass rods for several minutes. It was observed that the GO sheets were flocculated due to the use of CPAM (see Fig. S1†). The resulting brown GO/CPAM flocks were subsequently reduced by sodium borohydride, which is an environmentally friendly chemical in comparison with hydrazine.16 The entire reaction finished in just 2 h and the black soft RGO/CPAM composites were easily obtained by centrifugation. This reduced graphene oxide, which was flocculated with CPAM, can be obtained in gram quantities quickly, and exhibits good dispersibility in aqueous solvents. CPAM intercalation between graphene sheets can prevent the aggregation of graphene sheets during reduction. Meanwhile, the presence of hydrophilic CPAM in graphene materials may enhance their surface wettabilities and improve their specific capacitances. Finally, RGO/CPAM composites with different weight ratios (GO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CPAM), 10
CPAM), 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 10
1 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, were fabricated for the purpose of studying the effect of CPAM addition on the electrochemical performances of the products. The obtained composites were named as RGO/CPAM1 and RGO/CPAM2, respectively.
2, were fabricated for the purpose of studying the effect of CPAM addition on the electrochemical performances of the products. The obtained composites were named as RGO/CPAM1 and RGO/CPAM2, respectively.
To investigate the morphology of the prepared RGO and the RGO/CPAM composite, SEM and TEM images were obtained. The SEM images (see Fig. S2†) show that the surface of RGO is smooth, which indicates that the RGO sheets are stacked together. Meanwhile, the CPAM-intercalated RGO nanosheets are well dispersed in pieces, which suggests that the graphene sheets were stabilized by CPAM during the reduction treatment. More details can be observed from the TEM images (Fig. 1). The RGO sheets show typical wrinkles and crumple-like structures (Fig. 1a and c). The inset of Fig. 1c shows a selected area of the electron diffraction pattern (SAED) of RGO, showing a clear diffraction spot that is indexed to a hexagonal graphite crystal structure. Compared with RGO, some dark flakes are seen in the images of the RGO/CPAM2 composite (Fig. 1b and d), due to parts of the CPAM chains being mutually entangled, aggregated and randomly dispersed between the RGO sheets. This indicates that CPAM was attached to the RGO sheets.17 The SAED pattern of CPAM shows the crystalline nature of the polymer (inset of Fig. 1d).18
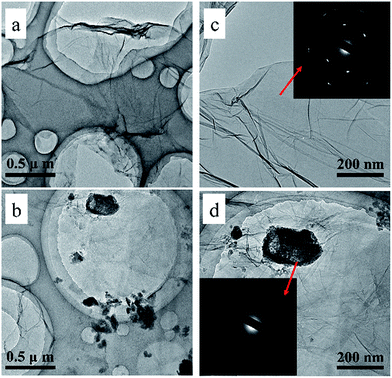 | ||
| Fig. 1 TEM images of RGO (a and c) and the RGO/CPAM2 composite (b and d) under different magnifications (0.5 μm and 200 nm scale bar). | ||
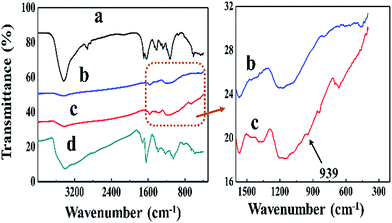
The FTIR spectra of GO, RGO, the RGO/CPAM2 composite and pure CPAM were taken and the results are shown in Fig. 2. The spectrum of GO illustrates the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (νC
O (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1719 cm−1), benzenoid C
O at 1719 cm−1), benzenoid C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (νC
C (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C at 1647 cm−1), C–O–C (νC–O–C at 1501 cm−1), and O–H (νH–O at 3404 cm−1).19,20 For pure CPAM, C
C at 1647 cm−1), C–O–C (νC–O–C at 1501 cm−1), and O–H (νH–O at 3404 cm−1).19,20 For pure CPAM, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the –CO–NH2 group, –NH2 bending vibrations and C–N stretching vibrations were observed at 1694, 1638 and 1280 cm−1 respectively. Compared with GO, the spectra of RGO and the RGO/CPAM2 composite have no peaks at 1719 and 1501 cm−1, which indicates the successful removal of the oxygen-containing groups from the sheets. The image on the right shows the spectra of RGO and the RGO/CPAM2 composite in the range of 1500 to 400 cm−1. Compared with RGO, new peaks at 1367 and 939 cm−1 are observed for the RGO/CPAM2 composite, which is attributed to the C–N stretching vibrations. This proves that CPAM had intercalated with the RGO sheets.
O stretching of the –CO–NH2 group, –NH2 bending vibrations and C–N stretching vibrations were observed at 1694, 1638 and 1280 cm−1 respectively. Compared with GO, the spectra of RGO and the RGO/CPAM2 composite have no peaks at 1719 and 1501 cm−1, which indicates the successful removal of the oxygen-containing groups from the sheets. The image on the right shows the spectra of RGO and the RGO/CPAM2 composite in the range of 1500 to 400 cm−1. Compared with RGO, new peaks at 1367 and 939 cm−1 are observed for the RGO/CPAM2 composite, which is attributed to the C–N stretching vibrations. This proves that CPAM had intercalated with the RGO sheets.
The structures of RGO, CPAM and the RGO/CPAM2 composite were further studied using their Raman spectra as shown in Fig. 3. It can be seen that both RGO and the RGO/CPAM2 composite show double peaks at about 1320 and 1590 cm−1, while CPAM does not. The peak at ∼1320 cm−1 (D band) is attributed to the vibrations of sp3 carbon atoms in defects and disorders, and the peak at ∼1590 cm−1 (G band) corresponds to the vibration of sp2 carbon atoms in the 2D hexagonal lattice. Compared with RGO, the D/G ratio of the RGO/CPAM2 composite is slightly increased from 2.6 to 2.77. This illustrates that the RGO/CPAM2 composite has a lower percentage of sp2 domains but they are larger in size.21 This can be explained by considering that CPAM is still connected to the RGO sheets via polymer bridging after the removal of oxygen groups, which leads to the larger size of the graphitic domains.
XRD patterns were also collected to study the structure of RGO and the RGO/CPAM2 composite (see Fig. S3†). The interlayer distance of the RGO/CPAM2 composite is 0.39 nm, larger than that of RGO (0.35 nm). This indicates that CPAM is intercalated between RGO sheets after the removal of oxygen-containing groups in the plane. However, the increase of the interlayer distance is minimal, which is due to the polymer bridging of CPAM between the RGO sheets. This is consistent with the results of the Raman spectrum.
The electrochemical performances of the RGO/CPAM composites with different weight ratios were tested. The as-obtained samples were fabricated into electrodes and characterized by cyclic voltammetry (CV) and galvanostatic charge–discharge measurements (Fig. 4). Typical CV responses of RGO, RGO/CPAM1 and RGO/CPAM2 at 5 mV s−1 are shown in Fig. 4a. The specific capacitance (CS) is proportionate to the average area of the CV curve.22 The average specific capacitances from the CV curves were calculated according to the following equation:
 | (1) |
 | ||
| Fig. 4 The cyclic voltammograms at a scan rate of 5 mV s−1 (a) and the galvanostatic charge–discharge curves at 0.1 A g−1 (b) of RGO, RGO/CPAM1 and RGO/CPAM2 in 6 M KOH solution. | ||
| Sample | RGO | RGO/CPAM1 | RGO/CPAM2 |
|---|---|---|---|
| CSCVs (F g−1) | 10.18 | 39.34 | 103.52 |
| CSgalvanostatic (F g−1) | 11.73 | 56.18 | 167.6 |
Fig. 4b shows galvanostatic charge–discharge measurements of RGO, RGO/CPAM1 and RGO/CPAM2 at 0.1 A g−1. The values of CS were calculated based on data obtained from the discharge curves according to the following equation:
| CS = IΔt/(m × ΔV) | (2) |
In addition, the relationships between the CS values and the current densities measured in a three-electrode system are presented in Fig. 5a. A small decrease (about 33%) in the CS value of RGO/CPAM2 is observed as the current density changes from 0.1 to 1.0 A g−1 (Fig. 5b). The highest specific capacitance of 167.6 F g−1 obtained at 0.1 A g−1 is similar to the value of 135 F g−1, which was obtained from reduced-graphene oxide using ultra-thin graphene membranes.25
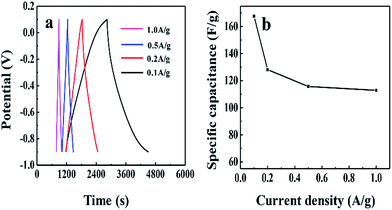 | ||
| Fig. 5 The galvanostatic charge–discharge curves of the RGO/CPAM2 composite with different current densities (a); the specific capacitance of RGO/CPAM2 at different current densities (b). | ||
In summary, we have reported a novel RGO/CPAM composite fabricated on a gram scale through a facile method, which includes flocculation of GO using CPAM, followed by reduction with sodium borohydride. The CPAM was reported to be attached to the graphene sheets after the removal of the oxygen-containing groups. In comparison with pure RGO, the specific capacitance of the RGO/CPAM composite has been improved vastly. This can be attributed to the fact that intercalated-CPAM effectively prevents aggregation of the RGO sheets and enhances the wettability of the composite electrode material. According to the present results, this original method for preparation of a RGO/CPAM composite is expected to offer a great opportunity for the application of graphene based composite materials in supercapacitor electrodes.
References
- A. S. Arico, P. Bruce and B. Scrosati, Nat. Mater., 2005, 4, 366–377 CrossRef CAS PubMed.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- G. Tsoukleri, J. Parthenios and K. Papagelis, Small, 2009, 5, 2397–2402 CrossRef CAS PubMed.
- T. Y. Kim, H. W. Lee and M. Stoller, ACS Nano, 2010, 5, 436–442 CrossRef PubMed.
- H. He, J. Klinowski and M. Forster, Chem. Phys. Lett., 1998, 287, 53–56 CrossRef CAS.
- A. Lerf, H. He and M. Forster, J. Phys. Chem. B, 1998, 102, 4477–4482 CrossRef CAS.
- S. Stankovich, R. D. Piner, X. Chen, N. Wu, S. T. Nguyen and R. S. Ruoff, J. Mater. Chem., 2006, 16, 155 RSC.
- T. Qian, C. Yu and S. Wu, J. Mater. Chem. A, 2013, 1, 6539–6542 CAS.
- K. Zhang, L. Mao and L. Zhang, J. Mater. Chem., 2011, 21, 7302–7307 RSC.
- S. Stankovich, R. D. Piner, S. T. Nguyen and R. S. Ruoff, Carbon, 2006, 44, 3342 CrossRef CAS.
- U. Ulusoy, S. Şimşek and Ö. Ceyhan, Adsorption, 2003, 9, 165–175 CrossRef CAS.
- K. A. Klimchuk, M. B. Hocking and S. Lowen, J. Polym. Sci., Part A: Polym. Chem., 2001, 39, 2525–2535 CrossRef CAS.
- M. E. Zeynali and A. Rabbii, Iran. Polym. J., 2002, 11, 269–275 CAS.
- A. Rabiee, M. E. Zeynali and H. Baharvand, Iran. Polym. J., 2005, 14, 603 CAS.
- S. Yu, N. Li and D. Higgins, ACS Appl. Mater. Interfaces, 2014, 6, 19783–19790 CAS.
- H. J. Shin, K. K. Kim and A. Benayad, Adv. Funct. Mater., 2009, 19, 1987–1992 CrossRef CAS.
- W. Song, X. Wang and Q. Wang, Phys. Chem. Chem. Phys., 2015, 17, 398–406 RSC.
- R. Pandey, K. Awasthi and R. S. Tiwari, Eprint Arxiv, 2010.
- Y. Xu, H. Bai and G. Lu, J. Am. Chem. Soc., 2008, 130, 5856–5857 CrossRef CAS PubMed.
- G. I. Titelman, V. Gelman and S. Bron, Carbon, 2005, 43, 641–649 CrossRef CAS.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558 CrossRef CAS.
- V. Srinivasan and J. W. Weidner, J. Power Sources, 2002, 108, 15–20 CrossRef CAS.
- Z. S. Wu, D. W. Wang and W. Ren, Adv. Funct. Mater., 2010, 20, 3595–3602 CrossRef CAS.
- K. Zhang, L. L. Zhang, X. S. Zhao and J. Wu, Chem. Mater., 2010, 22, 1392 CrossRef CAS.
- A. Yu, I. Roes and A. Davies, Appl. Phys. Lett., 2010, 96, 253105 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and supplementary characterisation data. See DOI: 10.1039/c6ra17371c |
| This journal is © The Royal Society of Chemistry 2016 |