Synthesis of Ag/Ag2CO3 heterostructures with high length–diameter ratios for excellent photoactivity and anti-photocorrosion†
Nan Yu,
Ruohao Dong,
Jinjin Liu,
Kuangfu Huang and
Baoyou Geng*
College of Chemistry and Materials Science, Key Laboratory of Functional Molecular Solids, Ministry of Education, Anhui Laboratory of Molecular-Based Materials, Center for Nanoscience and Technology, Anhui Normal University, Wuhu 241000, P. R. China. E-mail: bygeng@mail.ahnu.edu.cn
First published on 18th October 2016
Abstract
Promising photocatalysts, Ag/Ag2CO3 one-dimensional heterostructures with high length–diameter ratios, are synthesized on a large scale by a simple one-pot method. The photocatalysis results prove that they have excellent photoactivity and stability under visible light. We propose a possible mechanism to explain the photocatalytic performance of the as-prepared heterostructures.
Introduction
Metal–semiconductor hybrid nanostructures have been attracting considerable research attention because of their unique properties which are extremely different from those of the single components.1 They have been widely applied in diverse fields, including optics, electronics, catalytic reactions, etc.2 Metal–semiconductor hybrid nanostructures in particular, exhibit interesting advantages as photocatalysts for organic pollutant degradation and water splitting because of plasmonic effects and rapid photoexcited electron–hole separation.3–5 In the photocatalytic process, metal nanoparticles not only promote visible light absorption effectively, but also act as good electron acceptors to prevent photoexcited electrons recombining with holes.6,7 So far, significant effort has been made toward a variety of hybrid photocatalysts. Among those, silver compounds loaded with noble metals (mainly Ag) were obtained by extensive synthesis routes since the breakthrough discovery of the Ag3PO4 semiconductor that exhibited high photocatalytic capabilities for water-splitting and organic dye decomposition under visible light.8 For example, Ye and co-workers successfully utilized Ag nanoparticles and nanowires to promote the Ag3PO4 photocatalytic performance.9–11Additionally, as a silver compound with a narrow band gap (2.3 eV), the Ag2CO3 photocatalyst has also been found to exhibit an excellent visible light response to degrade organic pollutants and bactericidal activity with high efficiency.12–15 However, it is well-know that Ag2CO3 encounters the problem of severe photo-corrosion, which manifests as Ag+ being reduced to metallic Ag by photoexcited electrons in the photocatalytic process. At present, two approaches have been exploited for photocorrosion inhibition. One way is to introduce a sacrificial reagent to be an electron acceptor in the photocatalytic system, such as AgNO3;12 the other method is synthesis of various Ag2CO3 nanostructures to improve the photochemical activity and anti-photocorrosion ability of Ag2CO3, such as Ag2CO3 nanorods,16 Ag2CO3/AgX (X = Cl, Br, I) complexes,17 nanofibers containing Ag2CO3 nanoparticles,18 Ag2CO3/semiconductor composites19–22 and graphene–Ag2CO3.23 However, compared with other silver compounds such as Ag3PO4 and Ag3VO4, the investigation of Ag2CO3 photocatalysts is still insufficient. In particular, there are no reports about Ag nanoparticle loaded Ag2CO3 photocatalysts formed by a simple one-pot method. Although, Hu17 et al. utilized visible-light to irradiate Ag2CO3 nanorods in order to obtain Ag2CO3/Ag. A “two-step” method was used in their research, which is a frequently-used strategy for obtaining metal–semiconductor hybrid nanostructures. More importantly, Ag2CO3/Ag hybrid nanostructures with super high length–diameter ratios (nanowires) have not been successfully prepared previously. However, many physical properties, chemical behaviors and chemical reactivities can be improved significantly on the nanowire surface, which benefits their photocatalytic performance.24
Herein, we successfully developed a facile one-pot strategy to fabricate Ag/Ag2CO3 hybrid nanostructures assisted by PVP and urea. The as-prepared Ag/Ag2CO3 heterostructured photocatalysts exhibit extremely high activity and stability. The morphology can be controlled via changing of the reaction conditions, which influences the photocatalytic properties of Ag2CO3 in the degradation of MO and RhB. The experimental results show that a higher length–diameter ratio in the hybrid nanostructure improves the photocatalytic performance; moreover, its photocurrent response time is very fast under visible light in photoelectrochemical reactions. We also discuss in detail the relationship between morphology and photoactivity. The study shows promise for the application of Ag/Ag2CO3 hybrid nanostructures in photodegradation and water splitting under visible light.
Experimental section
The typical synthesis of Ag/Ag2CO3 hybrid structures
Ag/Ag2CO3 hybrid structures were prepared using a simple one-pot hydrothermal synthesis method. Typically, 2 mmol of AgNO3 and 0.4 g of PVP was dissolved into 20 mL of deionized water at room temperature with vigorous stirring. Then 2 mmol of urea was added gradually into the above mixed solution, and continuously stirred until a homogeneous solution was obtained. The resulting mixture was transferred into a Teflon-lined autoclave and heated at 120 °C for 8 h. After cooling naturally to room temperature, the products were separated by centrifugation and washed a few times with deionized water and ethanol alternatingly to remove ionic remnants. The final products were dried in a vacuum drying oven for 12 h.Photocatalyst characterization
An XRD-6000 X-ray diffractometer with Cu Kα radiation (λ = 1.54060 Å) was used to obtain X-ray powder diffraction (XRD) spectra with a scanning rate of 0.1° s−1. The morphology of the products was analyzed by scanning electron microscopy (SEM, HITACHI S-4800) with an attached energy-dispersive X-ray (EDX) system and transmission electron microscopy (TEM) images were also acquired (FEI TECNAI G20). X-ray photoelectron spectroscopy (XPS) was carried out on a Physical Electronics 5400 ESCA spectrometer with a base pressure of 109 Torr and Mg Kα X-ray radiation at a power of 200 W. UV-vis diffuse reflectance spectra were obtained on a UV-vis spectrophotometer (UV-24500, SHIMADZU).Photocatalytic performance measurements
The organic dye photodecomposition measurements were taken on RhB and MO solutions. Firstly, RhB or MO powder was dissolved into distilled water with a concentration of 10 mg L−1. 0.1 g of the as-prepared Ag/Ag2CO3 hybrid structure was dispersed into the above organic dye solutions. Then to complete the adsorption–desorption equilibrium between the dye and the photocatalyst, they were magnetically stirred for 30 min in the dark. Visible-light was obtained from a 300 W Xe arc lamp (PLS-SXE 300, Beijing), and UV light was optically filtered out. The above suspensions were irradiated under visible-light with magnetic stirring. Monitoring of the absorbance of the dye solutions in the degradation process was carried out on a UV-vis spectrophotometer (UV-4100, HITACHI) every 2 min after removal of the photocatalyst from the photodecomposition systems by centrifugal separation. Radical-trapping experiments were executed by the above process, but 3 mL of DMSO was added into the photocatalytic system.Photoelectrochemical measurements
The photocurrents were measured with an electrochemical workstation (Zahner-Zennium Germany) in a standard three-electrode system with 0.5 M Na2SO4 electrolyte. A platinum wire was used as a counter electrode with Ag/AgCl (saturated KCl) as a reference electrode and ITO as a working electrode. 5 mg of sample powder was dispersed ultrasonically in a mixed solution of 850 μL of distilled water, 50 μL of Nafion solution and 100 μL of ethylene glycol. 20 μL of the dispersed colloid was spin-coated onto a piece of ITO with a fixed area of 1.5 × 1.5 cm2, and then dried completely in air at room temperature. To obtain the photocurrent analysis, the ITO slice, as a working electrode, was irradiated and shielded at intervals of 60 s under a 500 W Xe arc lamp (San-ei Electric Japan).Results and discussion
The following are the main reactions, which are interlaced with each other in the closed higher temperature and pressure system:| CO(NH2)2 + H2O → NH3 + CO2 |
| NH3 + Ag+ → Ag(NH3)2+ |
Ag(NH3)2+ + CO32− ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) Ag2CO3 + NH3 Ag2CO3 + NH3 |
The crystalline structure and phase purity of the as-prepared samples were examined by X-ray powder diffraction (XRD), as shown in Fig. 1a. The result is in agreement with the standard pattern of Ag2CO3 (JCPDS, card no. 70-2184). All of the diffraction peaks can be indexed to Ag2CO3 and metallic Ag without other peaks being found in the pattern, suggesting that the as-prepared products are high purity and crystallinity, with metallic Ag existing in them. The Ag/C molar ratio (2.72) was determined by energy dispersive X-ray spectrometry (Fig. 1b), which further indicates the existence of metallic Ag. To further prove the presence of Ag0 in the products, X-ray photoelectron spectroscopy (XPS) was introduced to reveal the valence state and elementary components. It was easily found that the products were composed of Ag, C and O elements, from the spectrum shown in (Fig. 1c). The higher resolution XPS data shows only one O 1s peak located at 531.1 eV, which is assigned to Ag2CO3, and not to Ag2O, according to previous studies.25–27 Fig. 1d shows the XPS spectrum of Ag 3d in the products. It clearly shows two individual peaks located at 368.2 eV and 374.2 eV, which were assigned to the Ag 3d5/2 and Ag 3d3/2 binding energies, respectively. Four peaks can be obtained by further dividing Ag 3d5/2 and Ag 3d3/2. The strong peaks at 368.0 eV and 374.0 eV are assigned to Ag+ in Ag2CO3 and the bands at 368.9 eV and 374.9 eV are attributed to Ag0 species.28 The XPS results confirm that the as-prepared products are a Ag and Ag2CO3 hybrid structure without other impurities, which is consistent with the XRD and EDX results.
 | ||
| Fig. 1 Analysis of the crystalline structure and elemental composition of the as-prepared product. (a) XRD, (b) EDX and (c) and (d) XPS. | ||
The morphology of the typical product was revealed by scanning electron microscopy (Fig. 2a and b). It was clearly found that many nanoparticles were homogeneously loaded onto the one-dimensional nanowires with a diameter of about 100 nm and a length of 5–10 μm. There are no impurity particles or aggregates in the products. The structure details were further investigated by TEM. The TEM image in the dark field (Fig. 2c) shows that the nanowires are composed of nanoparticles and that they are actually porous structures, which is somewhat different to the SEM results. However, we also clearly observe that many small nanoparticles with sizes in the range of 5–10 nm are present on the surface of the nanowires. Moreover, photocorrosion phenomena did not occur when Ag2CO3 was illuminated in the electron beam of the TEM, which shows that the hybrid nanostructures are more stable than Ag2CO3 particles deposited at room temperature (Fig. S1, ESI†). The HR-TEM image (inset in Fig. 2d) reveals that the lattices of Ag and Ag2CO3 are in close contact, indicating that strong interactions were established between Ag and Ag2CO3 during the synthesis process.
 | ||
| Fig. 2 Electron microscopy images of a typical product. (a and b) SEM images and (c and d) TEM images (the inset image is a HR-TEM image of Ag nanoparticles). | ||
To investigate the growth process of hybrid nanowires, we executed many parallel experiments. Fig. S2† shows the morphologies of products synthesized with different amounts of PVP. We could not obtain nanowires without PVP in the reaction, as shown in Fig. S2a.† A great many nanorods appeared and scattered particles were not found in the products with an increasing amount of PVP. Fig. S2b† shows a SEM image of the nanorods synthesized with 0.2 g of PVP. With an increasing amount of PVP, the nanorods changed to a more uniform and longer form (Fig. S2c†). However, a large number of disharmonic nanoparticles appeared when the amount of PVP exceeded 0.4 g (Fig. S2d†). The influence of urea quantity on the morphology and composition of the products was also studied (Fig. S3†). Many non-uniform nanoparticles were observed when we did not add urea into the reaction (Fig. S3a†) and no wires were found in the products. The EDX pattern shows that the products formed are pure Ag nanoparticles (inset in Fig. S3a†). More and more nanowires appeared and the Ag nanoparticle content in the products increased with an increase in urea until the particles changed totally to wire-shaped structures. When the amount of urea was over 0.24 g, a great many nanorods with a non-uniform size appeared in the products.
The reaction time also influences the morphology of the as-synthesized catalysts. Fig. S4† shows the SEM images of the different products formed from 2 mmol of urea, 2 mmol of AgNO3 and 0.4 g of PVP, but with different reaction times. It was easily found that 1-dimensional structures do not appear in the products from shorter reaction times, while a great many spindle-like nanostructures are obtained when the reaction time is increased to 12 h. We presume that Ag2CO3 goes through several recrystallization phenomena as the reaction time is prolonged. To study the relationship between catalyst morphology and photocatalytic performance, we chose the as-synthesized samples with different morphologies and bulk Ag2CO3 prepared by precipitating Ag+ and CO32− at room temperature for comparison. Based on these results, we summarize the relationship between the reaction conditions and the morphology in Table 1.
Fig. S5† shows SEM and TEM images and XRD and EDX patterns of the as-prepared samples formed under different reaction conditions. It is easily found that they are rod and spindle shaped structures with lengths of ∼1 μm and diameters of ∼100 nm. XRD and EDX data (inset in Fig. S5†) display peaks all corresponding to an elemental composition of Ag and Ag2CO3, which indicates that both of the structures are Ag/Ag2CO3 hybrid products. It is clear that C 1s, O 1s, and Ag 3d and 3p all appear in the XPS results (Fig. S6†), which further proves that the products synthesized using the reaction conditions outlined in Table 1 are Ag/Ag2CO3 hybrid products.
The photoactivity of the as-prepared products was studied by degradation of organic pollutants under visible light irradiation (λ > 400 nm). Taking MO and RhB as model molecules, we did not introduce any sacrificial reagents into the photocatalytic experiments. Fig. 3 shows the UV-vis spectra of RhB and MO after exposure to visible light for different lengths of time in the presence of typical wire-shaped photocatalysts. These spectra show that the intensities of the absorption peaks at 550 nm and 460 nm decrease as the illumination time increases, in other words, the concentrations of the organic dyes in the aqueous solutions fall by 95% over 12 min and 16 min respectively. Fig. 3c and d directly present the photocatalytic performance of different catalysts for RhB and MO degradation respectively; Co is the concentration of dye after completion of the adsorption/desorption equilibrium in the dark for 30 min. We easily find out the following results from the graphs: firstly, it is very difficult for P25 to degrade organic dyes under visible light because its band gap is too large (∼3.2 eV) to absorb light of lower energy. Secondly, the photoactivities of the as-prepared catalysts are superior to that of bulk Ag2CO3 precipitated by AgNO3 and NaHCO3 at room temperature, because they possess Ag nanoparticles and a high ratio of length to diameter. Thirdly, the samples with larger length–diameter aspect ratios exhibit the best performance among all the catalysts because of their higher surface area and larger amount of active interfaces. Finally, the rate of RhB degradation is faster than MO, which should be attributed to the different photocatalytic processes.
It is important to test the stability of photocatalysts in light. Fig. 4 shows the photocatalytic stability of the four Ag/Ag2CO3 structures prepared under different synthesis conditions. Notably, the rod, spindle and wire shaped nanostructures exhibit better photoactivity than bulk Ag2CO3, as they still decompose nearly 90% of MO and RhB; the nanowires in particular show degradation beyond 90% even after three successive cycles. Meanwhile the photoactivity of bulk Ag2CO3 reduces greatly because of serious photocorrosion after irradiation for 30 min. The results indicate that the hybrid one-dimensional structures improve the separation and migration efficiency of hole-carriers generated by visible light excitation. In comparison to Ag2CO3 nanorods and nanospindles, the nanowires possess a shorter transfer path for photo-induced electrons and more reaction active sites because of their smaller diameter and larger specific surface area, which effectively facilitates electron transfer out of Ag2CO3.
 | ||
| Fig. 4 Comparison of the photocatalytic stability of bulk Ag2CO3, and Ag/Ag2CO3 nanowires, nanospindles and nanorods in recycling reactions. (a) RhB and (b) MO. | ||
Furthermore, photocurrent analysis was executed to clarify the improved photoactivity of wire-shaped Ag/Ag2CO3 hybrid nanostructures in a three-electrode PEC cell. Fig. S7a† displays the current density–potential curve (j–v) recorded in 0.5 M Na2SO4 aqueous solution. It is easily found that the dark current is very weak up to 1.0 V (vs. Ag/AgCl), but that the current in visible light increases obviously from 0.868 V (vs. Ag/AgCl), where electrocatalytic oxygen evolution could start. The photoelectric response and stability of the photocatalyst is also observed from the current–time (i–t) curve of on/off light cycles. The i–t investigation was carried out with a xenon lamp time interval of 60 s that turned on and turned off at a potential of 0.6 V (vs. Ag/AgCl). Fig. S7b (ESI†) shows that the anodic current increased rapidly in 0.39 s when the light was turned on, which indicates that photoinduced holes migrate to the surface for surface state filling (non-faradaic current) and to oxidize water (faradaic current). The photocurrent remains steady after a quick decline because of faradaic current decay. When the irradiation is over, a cathodic current spike appears rapidly in 0.55 s due to photoexcited electrons recombining with the holes stored in the surface states.29 Moreover, the Ag/Ag2CO3 nanowires have a steady performance after 30 min illumination under visible light.
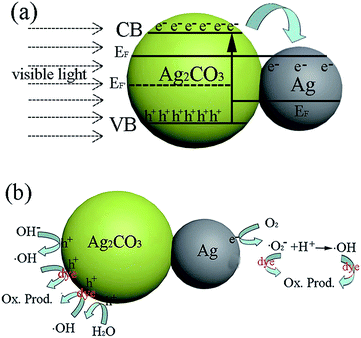
According to the above photocatalytic results, we propose a possible mechanism to explain the better photocatalytic performance of Ag/Ag2CO3 one-dimensional hybrid nanostructures than bulk Ag2CO3 under visible light, especially the wire-shaped structure with the biggest length–diameter ratio. This is schematically illustrated in Fig. 5. Firstly, Ag nanoparticles loaded in situ onto the one-dimensional Ag2CO3 semiconductor greatly improve the absorption in the visible light region. UV-visible diffuse reflectance spectra were used to investigate the optical absorbance of the different photocatalysts, which are shown in Fig. S8a.† The bulk Ag2CO3 has an absorption edge around 480 nm and its absorption range extends from the UV to the visible region. However, the as-prepared one-dimensional hybrid nanostructures have interesting strong absorption in the visible light region, especially the wire-shaped structures due to the surface plasmonic resonance (SPR) effect. Considering the fact that Ag2CO3 is an indirect bandgap semiconductor,21 the plots of the transformed Kubelka–Munk function of light energy (αhν)1/2 versus energy (hν) of bulk Ag2CO3 and the as-synthesized hybrids are shown in Fig. S7b.† The bandgap width of bulk Ag2CO3 is estimated to be 2.4 eV, while those of the Ag/Ag2CO3 nanowires, nanospindles and nanorods are 1.6 eV, 1.8 eV and 2.1 eV, respectively.
Secondly, a great many photoexcited electrons (e−) and photoinduced holes (h+) are produced in Ag2CO3 semiconductors by illumination with visible light. The close contact of the Ag nanoparticles and Ag2CO3 equilibrates their Fermi levels, which causes an energy level close to the conduction band of the Ag2CO3 semiconductor. Ag nanoparticles are good electron acceptors because their excellent conductivity greatly promotes interfacial charge-transfer kinetics among Ag and Ag2CO3, which further accelerates the effective separation of photoexcited electrons and photoinduced holes to decrease the recombination opportunity (Fig. 5a).30 This is why the as-prepared Ag/Ag2CO3 one-dimensional hybrid nanostructures are more stable than bulk Ag2CO3. The electrons in the conduction band of Ag2CO3 can migrate quickly through the Schottky barrier into metallic Ag, which effectively facilitates their participation in the multiple electron reduction reaction.13
| O2 + e− → ˙O2− |
| ˙O2− + 2H+ + 2e− → H2O2 |
| H2O2 + e− → ˙OH + OH− |
While the remaining holes on Ag2CO3 oxidize OH− from the above reaction and water. The oxidation reactions are as follows:
| OH− + h+ → ˙OH |
| H2O + h+ → ˙OH + H+ |
The highly reactive ˙O2− and ˙OH species from the electron reduction and hole oxidation are sufficient to destroy the structure of a dye molecule, which is an important reason for the decomposition of the pollutants.
Thirdly, Ag/Ag2CO3 one-dimensional hybrid nanostructures provide high surface areas and large numbers of active interfaces, especially the nanowires with their high length–diameter ratio. The larger specific surface area of the nanostructures can promote charge transfer across material interfaces, such as solid–solid and solid–liquid, which can provide a large number of active sites for the quick degradation of organic molecules.31
We can explain the difference in degradation rate between RhB and MO by combining the above photocatalytic mechanismwith Chen’s study;13 the oxyradicals mainly oxidizing RhB and MO are ˙O2− and ˙OH species respectively. It is presumed that the faster degradation rate of RhB compared to MO is because the ˙O2− concentration is higher than the ˙OH concentration, which indicates that a great many photoexcited electrons are transferred to Ag nanoparticles and can further reduce O2 to ˙O2−. We also performed radical trapping experiments, which are shown in Fig. S19.† The degradation rates of the dyes decrease obviously when DMSO is introduced into the photocatalytic system, indicating that ˙O2− and ˙OH play important roles in the photooxidation of RhB and MO. However, it is clearly found that the rate decrease of MO is faster than that of RhB, which shows that the radicals oxidizing MO and RhB are mainly ˙OH and ˙O2−, respectively.
Conclusions
In summary, we successfully prepared one-dimensional Ag/Ag2CO3 hybrid nanostructures with high length–diameter ratios via a facile and low-cost one-pot method. They exhibit superior photocatalytic activities and stabilities in RhB and MO degradation processes under visible light compared to P25 and bulk Ag2CO3. This should be attributed to many Ag nanoparticles existing in the as-synthesized nanostructures and their special one-dimensional morphology, which improves the light absorption and quick separation of the photoexcited electrons and photoinduced holes.Acknowledgements
This study was supported by the National Natural Science Foundation of China (21271009, 21471006), the Doctoral Fund of the Ministry of Education of China (20123424110002), the Programs for Science and Technology Development of Anhui Province (1501021019), the Program for Postgraduate Innovative Research of Anhui Normal University (2014yks072), the Program for Innovative Research Team of Anhui Education Committee, the Program for Talents Team of University in Anhui and the Graduate Student Research Innovation Project of Anhui Normal University (2015cxsj131zd).Notes and references
- C. Gong, L. Colombo, R. M. Wallace and K. Cho, Nano Lett., 2014, 14, 1714–1720 CrossRef CAS PubMed; R. B. Jiang, B. X. Li, C. H. Fang and J. F. Wang, Adv. Mater., 2014, 26, 5274–5309 CrossRef PubMed; Y. P. Du, B. Chen, Z. Y. Yin, Z. Q. Liu and H. Zhang, Small, 2014, 10, 4727–4734 CrossRef PubMed; D. W. Ding, K. Liu, S. N. He, C. B. Gao and Y. D. Yin, Nano Lett., 2014, 14, 6731–6736 CrossRef PubMed; Y. Z. Chen, D. Q. Zeng, M. B. Cortie, A. Dowd, H. Z. Guo, J. B. Wang and D. L. Peng, Small, 2015, 11, 1460–1469 CrossRef PubMed; L. Nahar, R. J. A. Esteves, S. Hafiz, U. Ozgür and I. U. Arachchige, ACS Nano, 2015, 9, 9810–9821 CrossRef PubMed.
- U. Banin, Y. B. Shahar and K. Vinokurov, Chem. Mater., 2014, 26, 97–110 CrossRef CAS.
- S. K. Ghosh and T. Pal, Chem. Rev., 2007, 107, 4797–4862 CrossRef CAS PubMed.
- D. B. Ingram and S. Linic, J. Am. Chem. Soc., 2011, 133, 5202–5205 CrossRef CAS PubMed.
- H. Tada, T. Kiyonaga and S. Naya, Chem. Soc. Rev., 2009, 38, 1849–1858 RSC.
- J. Chen, J. Wu, P. Wu and D. Tsai, J. Phys. Chem. C, 2011, 115, 210–216 CAS.
- P. Wang, B. Huang, Z. Lou, X. Zhang, X. Qin, Y. Dai, Z. Zheng and X. Wang, Chem.–Eur. J., 2010, 16, 538–544 CrossRef CAS PubMed.
- Z. Yi, J. Ye, N. Kikugawa, T. Kako, S. Ouyang, H. Stuart-Williams, H. Yang, J. Cao, W. Luo, Z. Li, Y. Liu and R. Withers, Nat. Mater., 2010, 9, 559–564 CrossRef CAS PubMed.
- Y. P. Bi, H. Y. Hu, S. X. Ouyang, Z. B. Jiao, G. X. Lu and J. H. Ye, J. Mater. Chem., 2012, 22, 14847–14850 RSC.
- Y. P. Bi, H. Y. Hu, S. X. Ouyang, Z. B. Jiao, G. X. Lu and J. H. Ye, Chem.–Eur. J., 2012, 18, 14272–14275 CrossRef CAS PubMed.
- H. Y. Hu, Z. B. Jiao, T. Wang, J. H. Ye, G. X. Lu and Y. P. Bi, J. Mater. Chem. A, 2013, 1, 10612–10616 CAS.
- G. Dai, J. Yu and G. Liu, J. Phys. Chem. C, 2012, 116, 15519–15524 CAS.
- H. J. Dong, G. Chen, J. X. Sun, C. M. Li, Y. G. Yu and D. H. Chen, Appl. Catal., B, 2013, 134, 46–54 CrossRef.
- J. J. Buckley, P. L. Gai, A. F. Lee, L. Olivi and K. Wilson, Chem. Commun., 2008, 34, 4013–4015 RSC.
- C. Xu, Y. Liu, B. Huang, H. Li, X. Qin, X. Zhang and Y. Dai, Appl. Surf. Sci., 2011, 257, 8732–8736 CrossRef CAS.
- J. J. Li, W. L. Yang, J. Q. Ning, Y. J. Zhong and Y. Hu, Nanoscale, 2014, 6, 5612–5615 RSC.
- H. Xu, J. X. Zhu, Y. X. Song, T. T. Zhu, W. K. Zhao, Y. H. Song, Z. L. Da, C. B. Liu and H. M. Li, J. Alloys Compd., 2015, 622, 347–357 CrossRef CAS; J. J. Li, Y. L. Xie, Y. J. Zhong and Y. Hu, J. Mater. Chem. A, 2015, 3, 5474–5481 Search PubMed.
- G. Panthi, S. J. Park, T. W. Kim, H. J. Chung, S. T. Hong, M. Park and H. Y. Kim, J. Mater. Sci., 2015, 50, 4477–4485 CrossRef CAS; P. S. Saud, B. Pant, Z. K. Ghouri, G. Panthi, S. J. Park, W. D. Han, M. Park and H. Y. Kim, Fibers Polym., 2015, 16, 1336–1342 CrossRef.
- Y. F. Li, L. Fang, R. X. Jin, Y. Yang, X. Fang, Y. Xing and S. Y. Song, Nanoscale, 2015, 7, 758–764 RSC; H. T. Ren, S. Y. Jia, S. H. Wu, T. H. Zhang and X. Han, Mater. Lett., 2015, 142, 15–18 CrossRef CAS.
- J. Wang, C. Dong, B. B. Jiang, K. L. Wu, J. Sun, X. Z. Li, W. J. Zhang, B. Zhang and X. W. Wei, Mater. Lett., 2014, 131, 108–111 CrossRef CAS.
- C. L. Yu, G. Li, S. Kumar, K. Yang and R. C. Jin, Adv. Mater., 2014, 26, 892–898 CrossRef CAS PubMed.
- C. L. Wu, Mater. Lett., 2014, 136, 62–264 Search PubMed; C. L. Wu, Mater. Lett., 2015, 152, 76–78 CrossRef CAS.
- C. Dong, B. B. Jiang, K. L. Wu, Y. Hu, S. H. Xia and X. W. Wei, Mater. Lett., 2015, 146, 37–39 CrossRef CAS; C. Dong, K. L. Wu, X. W. Wei, X. Z. Li, L. Liu, T. H. Ding, J. Wang and Y. Ye, CrystEngComm, 2014, 16, 730–736 RSC.
- A. I. Hochbaum and P. D. Yang, Chem. Rev., 2010, 110, 527–546 CrossRef CAS PubMed.
- J. F. Weaver and G. B. Hoflund, Chem. Mater., 1994, 6, 1693–1699 CrossRef CAS.
- C. T. Campbell and M. T. Paffett, Surf. Sci., 1984, 143, 517–535 CrossRef CAS.
- R. W. Bigelow, Appl. Surf. Sci., 1988, 32, 122–140 CrossRef CAS.
- C. H. An, J. Z. Wang, W. Jiang, M. Y. Zhang, X. J. Ming, S. T. Wang and Q. H. Zhang, Nanoscale, 2012, 4, 5646–5650 RSC; Y. F Li, K. Li, Y. Yang, L. J. Li, Y. Xing, S. Y. Song, R. C. Jin and M. Li, Chem.–Eur. J., 2015, 21, 17739–17747 CrossRef CAS PubMed.
- D. W. Kim, S. C. Riha, E. J. DeMarco, A. B. F. Martinson, O. K. Farha and J. T. Hupp, ACS Nano, 2014, 8, 12199–12207 CrossRef CAS PubMed.
- H. Yamada, A. Bhattacharyya and J. Maier, Adv. Funct. Mater., 2006, 16, 525 CrossRef CAS; P. Kamat, J. Phys. Chem. C, 2007, 111, 2834 Search PubMed; T. Tatsuma, K. Takada and T. Miyazaki, Adv. Mater., 2007, 19, 1249 CrossRef; M. Antoniadoua and P. Lianos, Appl. Catal., B, 2010, 99, 307 CrossRef; Y. Bi and J. Ye, Chem. Commun., 2010, 46, 1532 RSC.
- F. E. Osterloh, Chem. Soc. Rev., 2013, 42, 2294–2320 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, SEM, TEM, XRD, XPS, UV-vis diffuse reflectance spectra, j–v and i–t curves. See DOI: 10.1039/c6ra21325a |
| This journal is © The Royal Society of Chemistry 2016 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: