Polyelectrolyte and carbon nanotube multilayers made from ionic liquid solutions†
Takuya
Nakashima
a,
Jian
Zhu
a,
Ming
Qin
a,
Szushen
Ho
a and
Nicholas A.
Kotov
*abc
aDepartment of Chemical Engineering, University of Michigan, Ann Arbor, Michigan 48109, USA. E-mail: kotov@umich.edu
bDepartment of Biomedical Engineering, University of Michigan, Ann Arbor, Michigan 48109, USA
cDepartment of Materials Science and Engineering, University of Michigan, Ann Arbor, Michigan 48109, USA
First published on 1st February 2010
Abstract
The inevitable contact of substrates with water during the traditional practice of layer-by-layer assembly (LBL) creates problems for multiple potential applications of LBL films in electronics. To resolve this issue, we demonstrate here the possibility of a LBL process using ionic liquids (ILs), which potentially eliminates corrosion and hydration processes related to aqueous media and opens additional possibilities in structural control of LBL films. ILs are also considered to be one of the best “green” processing solvents, and hence, are advantageous in respect to traditional organic solvents. Poly(ethyleneimine) (PEI) and poly(sodium styrenesulfonate) (PSS) were dispersed in a hydrophilic IL and successfully deposited in the LBL fashion. To produce electroactive thin films with significance to electronics, a similar process was realized for PSS-modified single-walled carbon nanotubes (SWNT-PSS) and poly(vinyl alcohol) (PVA). Characterization of the coating using standard spectroscopy and microscopy techniques typical of the multilayer field indicated that there are both similarities and differences in the structure and properties of LBL films build from ILs and aqueous solutions. The films exhibited electrical conductivity of 102 S m−1 with transparency as high as 98% for visible light, which is comparable to similar parameters for many carbon nanotube and graphene films prepared by both aqueous LBL and other methods.
Introduction
The layer-by-layer (LBL) adsorption technique is a simple and inexpensive process for the construction of multilayer thin films.1–5 While the LBL method was initially established for the electrostatic deposition of pairs of oppositely charged polyelectrolytes,6–10 it has also been successfully applied to thin films of inorganic nanomaterials,11–16 biopolymers,17–22 and nanoscale carbon materials.23–26 The physicochemical interactions promoting the formation of LBL films have been extensively explored. Hydrogen-bonding,27,28 metal–ligand interactions,29 charge-transfer interactions,30 covalent attachment,31 sol–gel reaction,32 molecular recognition,33 and hydrophobic interactions34,35 are considered to be significant in the multilayer formation with a different degree of influence on the final structure depending on the chemical identity of the components. Considering the large variety of these interactions, an equally large variety of organic and inorganic layers can potentially be created, but has been somewhat limited by the dominance of water-base systems in the LBL field. Without diminishing the great advantages of aqueous solutions and multiple potential applications that require water-based solvents, the use of non-aqueous systems would open up substantially novel applications of LBL films especially in the area of electronic devices36,37 for which anhydrous conditions are highly desirable.38 Elimination of water will prevent many chemical processes leading to corrosion of substrates or electro/photo active films, for instance for preparation of inexpensive solar cells or supercapacitors. The absence of water is also important to avoid the hydration of the previous coatings, which can alter the electrical properties of the films and their key functional characteristics. Hence, non-aqueous conditions realized with traditional organic solvents or a new type of solvent could be potentially very useful for LBL deposition of advanced thin films with electrical or optical functionalities.The specific interactions such as hydrogen-bonding39–41 and molecular recognition42 are common in organic solvents. The use of non-aqueous media with highly dielectric properties enables one to utilize the electrostatic interaction between oppositely charged polyelectrolytes dissolved in them,43 although the traditional components of LBL deposition have definite difficulties with being dispersed or dissolved in most organic solvents. Kamineni et al.43 reported the formation of polyelectrolyte LBL films by using formamide and also demonstrated the advantage of non-aqueous systems by incorporating a water-sensitive hydrogen storage material in an LBL film. Organic solvents with high dielectric constants usually possess the zwitter-ionic canonical structure, which contributes to the highly dielectric properties.44 There are also studies by Hao et al.40,41 and Serizawa42et al. who utilized some organic solvents, but their processes involved very specific interactions between the LBL components, such as hydrogen-bonding or the formation of stereo complexes. Without diminishing the fundamental significance of these studies, it would be important to find a more general approach to “no-water” LBL process and to demonstrate that the properties of such coatings are suitable for at least some electronic devices.
Considering the fact that the use of traditional organic solvents in the studies cited above is becoming severely restricted due to strong environmental concerns related to volatile organic compound (VOC) regulations, it would be quite interesting and technologically important to find an alternative to them in “no-water” LBL processes. Room-temperature ionic liquids (RTILs) have been receiving much interest as environmentally benign solvents for organic chemical reactions45 and separations,46 and recent developments include molecular self-assembly and supramolecular chemistry in ILs.47 The dielectric property of ILs, which consist solely of ions, could be moderately controlled depending on the ionic components to change the inter-ionic interaction.48 Consequently, the capability of dissolving polymers also depends on their ionic components,49,50 which can be tuned and tailored from a large variety of ionic combinations. Some ILs have excellent dissolution ability to poorly-soluble polymers in conventional solvent systems such as cellulose,51 silk52 and carbon nanotubes.53 They do not cause corrosion and hydration of sensitive electronic materials and substrates. ILs also eliminate environmental concerns regarding VOCs. Taking these properties into consideration, the use of ILs as media for LBL assembly seems to be fairly advantageous to the further expansion of LBL technique as well as the applications of LBL films. We describe herein the LBL multilayer assembly from IL solutions and specifically by using a spin-assisted LBL technique.54,55 PEI and PSS were used as typical examples of polyelectrolytes to produce generic polyelectrolyte multilayers. The LBL assembly from ILs was further applied to multilayer formation single-walled carbon nanotubes (SWNTs) partnered with poly(vinyl alcohol) (PVA) resulting in multilayers with competitive optoelectronic characteristics.
Experimental
Materials
1-Ethyl-3-methylimidazolium ethylsulfate ([EMIm][EtSO4]), PSS (Mw = 1![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000
000![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000), PEI (branched, Mw = 25
000), PEI (branched, Mw = 25![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000) and PVA (80% hydrolyzed, Mw = 9000 to 10
000) and PVA (80% hydrolyzed, Mw = 9000 to 10![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 000), whose chemical structures are depicted in Fig. 1, were purchased from Sigma-Aldrich and used as received without further purification. Purified P2-SWNTs (70–90% carbonaceous purity) were obtained from Carbon Solutions, Inc. PSS, PEI and PVA were individually dissolved in deionized water (DI water) at a concentration of 50 mg mL−1. The pH of the PEI solution was adjusted to 3.7 by hydrochloric acid. SWNTs were dispersed in PSS solution (1 mg mL−1) with 1 day of mild sonication. The concentration of SWNTs was adjusted to 0.5 mg mL−1. All IL solutions were prepared by the co-solvent evaporation method since direct dispersion of polymers into ILs is generally hampered by the high viscosity of ILs.49,56 Briefly, 5 mL of [EMIm][EtSO4] was individually mixed with 0.5 mL of aqueous solutions (50 mg mL−1) of PSS, PEI and PVA. The aqueous dispersion of SWNT-PSS was also added to [EMIm][EtSO4] by equivolume. All of these mixtures gave a single phase without precipitation or phase separation. The resultant solutions were lyophilized for 24 h and further dried in vacuo at 80 °C for 24 h to remove water thoroughly. The water content of the IL solutions was determined to be ca. 4.7 × 10−2 wt% for the PVA solution and below 0.02 wt% for the other three solutions using a Karl Fischer coulometer (Metrohm, 831 KF Coulometer). The final concentrations of the IL-polymer solutions were adjusted to 5 mg mL−1 for PSS, PEI and PVA and 0.5 mg mL−1 for SWNTs (with 1 mg mL−1 of PSS).
000), whose chemical structures are depicted in Fig. 1, were purchased from Sigma-Aldrich and used as received without further purification. Purified P2-SWNTs (70–90% carbonaceous purity) were obtained from Carbon Solutions, Inc. PSS, PEI and PVA were individually dissolved in deionized water (DI water) at a concentration of 50 mg mL−1. The pH of the PEI solution was adjusted to 3.7 by hydrochloric acid. SWNTs were dispersed in PSS solution (1 mg mL−1) with 1 day of mild sonication. The concentration of SWNTs was adjusted to 0.5 mg mL−1. All IL solutions were prepared by the co-solvent evaporation method since direct dispersion of polymers into ILs is generally hampered by the high viscosity of ILs.49,56 Briefly, 5 mL of [EMIm][EtSO4] was individually mixed with 0.5 mL of aqueous solutions (50 mg mL−1) of PSS, PEI and PVA. The aqueous dispersion of SWNT-PSS was also added to [EMIm][EtSO4] by equivolume. All of these mixtures gave a single phase without precipitation or phase separation. The resultant solutions were lyophilized for 24 h and further dried in vacuo at 80 °C for 24 h to remove water thoroughly. The water content of the IL solutions was determined to be ca. 4.7 × 10−2 wt% for the PVA solution and below 0.02 wt% for the other three solutions using a Karl Fischer coulometer (Metrohm, 831 KF Coulometer). The final concentrations of the IL-polymer solutions were adjusted to 5 mg mL−1 for PSS, PEI and PVA and 0.5 mg mL−1 for SWNTs (with 1 mg mL−1 of PSS).
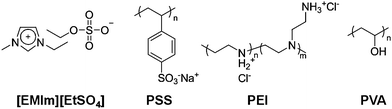 | ||
| Fig. 1 Chemical structures of the ionic liquid and polymers. | ||
LBL assembly
Silicon wafers and glass slides used as substrates for multilayer assembly were treated with piranha solution. Single crystalline CaF2 plates were used as substrates for Fourier-transform infrared (FTIR) and X-ray photoelectron spectroscopy (XPS) measurements. LBL films were obtained using LBL spin-coater (Laurell Technologies) designed in our group.57 Polymer solutions were alternately spun onto substrates, with washing steps between the layer deposition steps. 50 μL of polymer IL solution was placed on the substrate and then the substrate was rotated with a spinner according to a fixed rotation program (typically 500 rpm for 5 s, 2000 rpm for 10 s and 6000 rpm for 10 s, sequentially). After the deposition of each polyelectrolyte layer, the nonvolatile IL and non-adsorbed polymer molecules were thoroughly removed by rinsing with 0.5 mL of acetonitrile at 3000 rpm for 20 s.Instrumental analyses
Ellipsometry measurements were obtained using a BASE-160 Spectroscopic Ellipsometer produced by J. A. Woollam Co., Inc. FTIR spectra were recorded on a Jasco FT/IR 4200 spectrometer. UV–vis absorption measurements were taken using an Agilent 8453E UV–vis spectrophotometer. XPS experiments were carried out on a Kratos AXIS-165 with Monochromatic Al Kα radiation (E = 1486.6 eV). The surface morphology of obtained films was characterized by atomic force microscopy (AFM, Nanoscope III, Digital Instruments/Veeco Metrology Group) and scanning electron microscopy (SEM, FEI Nova Nanolab). The four-point probe method was employed to measure the conductivity of [PVA/(SWNT-PSS)]n LBL films. The measurements were made on an Agilent 34401A multimeter in connection with a Signatone four-point probing system. The surface resistivity for each bilayer was converted to the conductivity value by using the thickness data obtained from ellipsometry measurements. Values reported here are the averages of five measurements for different points.Results and discussion
[EMIm][EtSO4] was chosen as an IL for LBL assembly since it has a relatively high dielectric constant among the large variety of ILs investigated.48 Also, [EMIm][EtSO4] showed the ability to dissolve both cationic and anionic polymers, such as PEI and PSS, respectively. Even after the thorough removal of water, which acts initially as a co-solvent, PEI and PSS solutions in [EMIm][EtSO4] were stable for the duration of the project (more than 6 months). As a preliminary test for the formation of polyelectrolyte complexes leading eventually to LBL multilayers, we mixed aliquots of IL solutions of PEI and PSS. Once mixed together, the solution turned opaque, indicating the formation of polyelectrolyte aggregates. This observation was considered as an indication of the suitability of [EMIm][EtSO4] for LBL assembly. Although the surface charge screening from the ionic media is expected to somewhat diminish electrostatic interactions between polyelectrolytes, the multiple interactions of various nature discussed in the Introduction, can successfully lead to assembly. Moreover, given that the electrostatic interaction between polyelectrolytes would be less effective in such ionic media, the result strongly supports the consideration that the formation of polyelectrolyte complexes is fundamentally based on the ion-exchange process and dominated by the entropic contribution from the release of counterions.58 These considerations encouraged us to further investigate LBL assembly from IL solutions.Indeed, the successful step-by-step increase of film thickness was observed for consecutive deposition of PSS and PEI, hinting at the formation of (PEI/PSS)n multilayer films (Fig. 2). As an important control experiment, film growth was not observed for repetitive exposure of the substrate under analogous conditions to IL solutions of either PSS or PEI without the second component. The film thickness of the (PEI/PSS)n multilayers increases linearly with a correlation coefficient of almost a unity; the average thickness per layer pair was estimated to be about 6.7 nm. It might be interesting to compare this value with those obtained in water-based LBL. Very recently, Elzbieciak et al. have reported a 2.5 nm increment of film thickness for a PEI/PSS bilayer prepared from aqueous solutions using a standard dipping approach.59 The greater thickness obtained in our studies can be attributed to strong screening of ionic interactions in the IL. The formal salt concentration in the solvent that we used reaches as high 10 M, which can certainly induce the formation of coils of polyelectrolytes.60
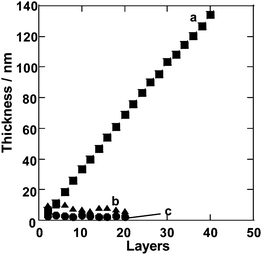 | ||
| Fig. 2 Ellipsometry thickness measurements of multilayer films made from (a) (PEI/PSS)n, (b) PSS and (c) PEI. | ||
Since ionic liquids are known to be less volatile than organic solvents, the accumulation of residual ionic liquids may adversely affect the film quality and the complete removal of ionic liquids would be crucial. The LBL film prepared from an ionic liquid solution was characterized by FTIR as shown in Fig. 3(a), which is almost identical to that of a (PEI/PSS)20 LBL film prepared from aqueous solution [Fig. 3(b)]. The absence of aromatic C–H (3150, 3110 cm−1) and ring (1570, 1450 cm−1) stretching signals as depicted in Fig. 3 by dashed lines, which are characteristic to the IL imidazolium cation, suggests that the IL molecules were effectively washed out by rinsing with acetonitrile. Although both the (PEI/PSS)20 films prepared from aqueous solutions and IL solutions exhibited almost identical FTIR spectra, a significant difference was found in water contact angles. The static contact angle of water droplet on the 10 bilayer (PEI/PSS)n film made from IL was measured to be 32 ± 3°, which was clearly larger than that of a LBL film prepared from aqueous solution (12 ± 3°) (see ESI†. This is an interesting fact that indicates that the internal organization of LBL films and specifically the packing of polymer chains is different for multilayers made in ILs than in water. The surface of the LBL film from IL solutions should have polymer segments with greater hydrophobicity originating from the difference in the mode of adsorption of the polymers and the contribution of secondary interactions such as solvophobic interactions between IL-LBL and aqueous LBL.
![FTIR spectra of (PEI/PSS)20 multilayers prepared from (a) [EMIm][EtSO4] and (b) aqueous solutions. Signal positions typical for the imidazolium cation including aromatic C–H (3150, 3110 cm−1) and ring (1570, 1450 cm−1) stretchings are depicted as dashed lines.](/image/article/2010/NR/b9nr00333a/b9nr00333a-f3.gif) | ||
| Fig. 3 FTIR spectra of (PEI/PSS)20 multilayers prepared from (a) [EMIm][EtSO4] and (b) aqueous solutions. Signal positions typical for the imidazolium cation including aromatic C–H (3150, 3110 cm−1) and ring (1570, 1450 cm−1) stretchings are depicted as dashed lines. | ||
As is common to all LBL studies, the surface morphology and topology of the LBL films was characterized by AFM and SEM imaging. The first PEI layer displayed a very uniform morphology on a Si wafer [Fig. 4(a)]. Upon the deposition of the PSS layer, aggregates from tens of nanometres to 500 nm in lateral dimensions and several nanometres in height were observed [Fig. 4(b)], which correlates well with the strong screening of ionic interactions in polyelectrolytes in ILs. One should consider the possibility of two cases: (1) The PSS layer forms a continuous layer with some aggregates, and (2) the PSS film is limited only to the islands observed in the AFM. Without specific nanoscale topology of a component, characteristic, for instance, for nanoscale colloids, it is actually quite difficult to distinguish between these two cases. These data obtained for SWNTs (see below) indicate that the first case is more likely. Notably, LBL growth can occur regardless of whether the PSS film is continuous or not.61 As the layer number increased, the substrate was fairly uniformly covered by the polyelectrolytes as can be seen for the surface morphology of a (PEI/PSS)20 film [Fig. 4(c)]. Despite the high-contrast AFM appearance, the (PEI/PSS)20 film was rather smooth, exhibiting surface roughness about 3.7 nm.
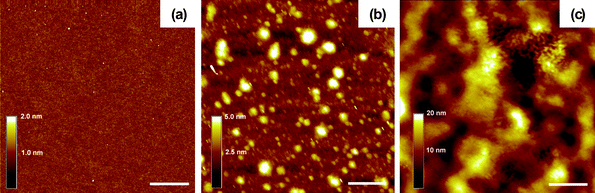 | ||
| Fig. 4 Tapping-mode AFM images of (a) a single PEI film, (b) a (PEI/PSS)1 bilayer and (c) a (PEI/PSS)20 film. Scale bar = 1 μm. | ||
Since PSS could be dissolved in [EMIm][EtSO4], we reasoned that PSS-stabilized SWNTs might also form stable dispersions in [EMIm][EtSO4]. Although ILs have been reported to show a great affinity to SWNTs, giving composite gels,53 the PSS modification was necessary to obtain a dispersion/solution rather than a gel, which is necessary for LBL assembly. Indeed, the PSS-modified SWNTs were dispersed in the IL, forming a stable homogeneous mixture. Interestingly, SWNTs in the IL showed a better resolved absorption profile and electronic transition bands than the same nanotubes in water (Fig. 5). The imidazolium cations in the IL are likely to facilitate the debundling of SWNTs by the specific interactions with the SWNTs that leads to sharper UV–vis spectral features.53
![Absorption spectra of SWNT-PSS in water (broken line) and in [EMIm][EtSO4] (solid line). The concentration of SWNTs is 0.025 mg mL−1 and the optical path length is 5 mm.](/image/article/2010/NR/b9nr00333a/b9nr00333a-f5.gif) | ||
| Fig. 5 Absorption spectra of SWNT-PSS in water (broken line) and in [EMIm][EtSO4] (solid line). The concentration of SWNTs is 0.025 mg mL−1 and the optical path length is 5 mm. | ||
PVA was chosen as an LBL partner for SWNT-PSS because this polymer has demonstrated successful multilayer assembly with SWNT-PSS in the past.62–64 It was also important to demonstrate the general nature of LBL deposition from ILs by using not only charged but also neutral polymers, which can take advantage of secondary interactions (i.e. all except covalent and electrostatic bonds). Fig. 6(a) shows a continuous decrease of optical transmittance from the UV to the near-infrared region, demonstrating successful LBL accumulation of SWNTs on a substrate upon repetition of the LBL cycles in a similar manner to an aqueous system.62 Each spectrum displays a structured profile originated from well-exfoliated SWNTs similar to the observed UV–vis spectrum of SWNT dispersions in [EMIm][EtSO4] (Fig. 5). The [PVA/(SWNT-PSS)]10 multilayer film on a CaF2 substrate was also characterized by XPS measurements. A bare CaF2 substrate was used as a reference. Besides substrate-related signals (F and Ca), C1s and O 1s signals were observed for the CaF2 reference [Fig. 7(b)], which originate from contamination of the sample under ambient condition. Both spectra for CaF2 and [PVA/(SWNT-PSS)]10 LBL films showed a similar background at around 400 eV, where the N 1s peak should appear, indicating the [PVA/(SWNT-PSS)]10 film is also free from IL contamination. Although S can be differentiated and quantified as a component of PSS [Fig. 7(a)], it is difficult to tell the component ratio of the film due to the presence of C 1s and O 1s signals from the background. The S 2p peak and a shoulder on the C 1s peak above 285 eV clearly indicate the presence of PSS and alcohol (i.e., PVA), respectively, in the film.
![(a) Transmission spectra of [PVA/(SWNT-PSS)]n multilayers on a glass slide. Spectra were measured after each SWNT-PSS deposition. The spectrum of glass slide was subtracted from each spectrum. (b) Thickness growth curve of [PVA/(SWNT-PSS)]n multilayer films measured by ellipsometry.](/image/article/2010/NR/b9nr00333a/b9nr00333a-f6.gif) | ||
| Fig. 6 (a) Transmission spectra of [PVA/(SWNT-PSS)]n multilayers on a glass slide. Spectra were measured after each SWNT-PSS deposition. The spectrum of glass slide was subtracted from each spectrum. (b) Thickness growth curve of [PVA/(SWNT-PSS)]n multilayer films measured by ellipsometry. | ||
![Core level spectra of O 1s, N 1s, C 1s and S 2p for (a) [PVA/(SWNT-PSS)]10 multilayers on CaF2 and (b) the bare CaF2 substrate.](/image/article/2010/NR/b9nr00333a/b9nr00333a-f7.gif) | ||
| Fig. 7 Core level spectra of O 1s, N 1s, C 1s and S 2p for (a) [PVA/(SWNT-PSS)]10 multilayers on CaF2 and (b) the bare CaF2 substrate. | ||
AFM and SEM imaging of a single bilayer of PVA/(SWNT-PSS) film (Fig. 8) confirm that nanotubes are present in a debundled form. The height of each nanotubes was 0.9–1.2 nm, which agrees very well with the expected diameter of individual SWNTs. Irregularly shaped nearly round objects of tens of nanometres in diameter were observed in both AFM and SEM images, and are attributed to carbonaceous and/or metal impurities from the original SWNT product (up to 30%), which could not be removed by rinsing with acetonitrile, unlike the aqueous system.62
![(a) AFM image of a single bilayer of the PVA/(SWNT-PSS) film. (b) SEM image of a [PVA/(SWNT-PSS)]10 film.](/image/article/2010/NR/b9nr00333a/b9nr00333a-f8.gif) | ||
| Fig. 8 (a) AFM image of a single bilayer of the PVA/(SWNT-PSS) film. (b) SEM image of a [PVA/(SWNT-PSS)]10 film. | ||
In contrast to the (PEI/PSS)n LBL system, an initial lag period was observed for multilayer accumulation as indicated by ellipsometry measurements of [PVA/(SWNT-PSS)]n [Fig. 6(b)]. The film thickness showed linear growth after the deposition of 3 bilayers and an average thickness increment of 0.85 nm for [PVA/(SWNT-PSS)]n when n > 4. Regardless of a relatively slow growth of [PVA/(SWNT-PSS)]n, a well-networked structure of nanotubes forms after only a few LBL cycles and the film becomes conductive (Fig. 9). An extensive 3D network is necessary for the formation of efficient charge-transfer pathways since the electrical conductivity of the SWNT-polymer composite film is dominated by charge percolation. For n = 3 [PVA/(SWNT-PSS)]n, the film had density of SWNT sufficient to reach the percolation threshold, giving a surface resistivity of about 4.5 MΩ sq−1 which can be converted to a conductivity of 10 S m−1 by using the value of film thickness estimated by ellipsometry [Fig. 6(b)]. As more LBL layers were added, the conductivity increased to the order of 102 S m−1. Overall, the conductivity trace resembles that of film thickness [Fig. 6(b)] with the inflection point corresponding to the end of the lag period discussed above. After that, the dependence of the conductivity on the number of deposited layers plateaus at 10 deposition cycles. Such behavior is expected because the conductivity parameter (unlike total nominal resistance) reflects mostly the film structure rather than the amount of conducting material deposited. Similar trends and conductivity plateaus could also be observed in water-based LBL coatings of SWNTs.62
![Electrical conductivity of [PVA/(SWNT-PSS)]n LBL film.](/image/article/2010/NR/b9nr00333a/b9nr00333a-f9.gif) | ||
| Fig. 9 Electrical conductivity of [PVA/(SWNT-PSS)]n LBL film. | ||
The conductivity in the range of 102 S m−1 with optical transmittance as high as 98% at 600 nm is comparable to those of PVA/(SWNT-PSS) LBL composites prepared by aqueous systems62 and to the coatings from reduced graphene films,65,66 which are considered to be one of the most promising materials for electronics. It is not as high as the conductivity of the best examples of SWNT films, i.e. 103–104 S m−1.62 Note however, that no additional SWNT doping was applied in this study, which typically increases conductivity by at least 1 order of magnitude. We do not think that the observed conductivity is the limit for LBL deposition in ILs and a substantial increase can be achieved by the optimization of deposition conditions as we did for SWNTs previously.62 For many traditional electronic applications for instance, field-effect transistors, sensing, and memory chips, only a few LBL cycles (fewer than 10) are likely to be needed. The use of SWNTs as intermediate layers facilitating charge transfer in optoelectronic devices and solar cells can also be envisioned. For some other applications, such as SWNT-based supercapacitors, a greater number of layers would be needed, which it is certainly possible to deposit. LBL deposition from IL solvents make it easy to control preparation of such layers on electronics-grade organic or inorganic substrates without damaging them, which is always a great concern in respect to aqueous solutions.
Conclusions
Non-aqueous LBL deposition from IL solutions was demonstrated for multiple LBL components, which include positive and negatively charged polyelectrolytes, a neutral polymer, and carbon nanotubes. These results clearly indicate that ILs could be potentially used as an alternative for water as a solvent in LBL depositions. What is also significant is that ILs67,68 are considered to be “green” solvents reducing the use of VOCs, allowing the tradition of the environmental benignity of water-based LBL assembly to continue. Coatings with electrical performance parameters suitable for some electronic and optoelectronic applications were obtained in this entirely non-aqueous system, which would greatly extend the usability of LBL assembly for device manufacturing applications.The use of ILs and the benefits of replacement of water are certainly not limited to electronic devices. A huge variety of combinations of ionic components would enable us to further expand the diversity of materials and applications of LBL films. For the future extension of this study one could consider that water-sensitive active chemicals could also be impregnated inside the films and could be possibly released out by the triggering of an electric current. It should also be noted here that special IL-soluble polymers, such as cellulose,51 could be introduced to assemble with SWNTs to improve the tribological properties of conductive coatings, which is part of our current endeavors.
Acknowledgements
The authors would like to express their appreciation to AFOSR, ONR, and DARPA for continuous support of this project.References
- G. Decher, Science, 1997, 277, 1232 CrossRef CAS.
- J. H. Fendler, Chem. Mater., 1996, 8, 1616 CrossRef CAS.
- P. T. Hammond, Adv. Mater., 2004, 16, 1271 CrossRef CAS.
- (a) K. Ariga, J. P. Hill and Q. Ji, Phys. Chem. Chem. Phys., 2007, 9, 2319 RSC; (b) K. Ariga, J. P. Hill, M. V. Lee, A. Vinu, R. Charvet and S. Acharya, Sci. Technol. Adv. Mater., 2008, 9, 014109 CrossRef.
- X. Zhang, H. Chen and H. Zhang, Chem. Commun., 2007, 1395 RSC.
- G. Decher, J. D. Hong and J. Schimitt, Thin Solid Films, 1992, 210–211, 831 CrossRef.
- Y. Lvov, G. Decher and H. Möhwald, Langmuir, 1993, 9, 481 CrossRef CAS.
- W. Chen and T. J. McCarthy, Macromolecules, 1997, 30, 78 CrossRef CAS.
- Y. Lvov, K. Ariga, M. Onda, I. Ichinose and T. Kunitake, Colloids Surf., A, 1999, 146, 337 CrossRef CAS.
- S. S. Shiratori and M. F. Rubner, Macromolecules, 2000, 33, 4213 CrossRef CAS.
- S. Srivastava and N. A. Kotov, Acc. Chem. Res., 2008, 41, 1831 CrossRef CAS.
- N. A. Kotov, I. Dekany and J. H. Fendler, J. Phys. Chem., 1995, 99, 13065 CrossRef CAS.
- Y. Lvov, K. Ariga, I. Ichinose and T. Kunitake, Langmuir, 1996, 12, 3038 CrossRef CAS.
- F. Caruso, R. A. Caruso and H. Möhwald, Science, 1998, 282, 1111 CrossRef CAS.
- T. Cassagneau, J. H. Fendler and T. E. Mallouk, J. Am. Chem. Soc., 1998, 120, 7848 CrossRef CAS.
- S. Vial, I. Pastoriza-Santos, J. Perez-Juste and L. M. Liz-Marzan, Langmuir, 2007, 23, 4606 CrossRef CAS.
- Y. Lvov, K. Ariga, I. Ichinose and T. Kunitake, J. Am. Chem. Soc., 1995, 117, 6117 CrossRef CAS.
- P. He, N. Hu and J. F. Rusling, Langmuir, 2004, 20, 722 CrossRef CAS.
- Y. Lvov, G. Decher and G. Sukhorukov, Macromolecules, 1993, 26, 5396 CrossRef CAS.
- Y. Lvov, H. Haas, G. Decher, H. Möhwald, A. Mikhailov, B. Mtchedlishvily, E. Morgunove and B. Vainstein, Langmuir, 1994, 10, 4232 CrossRef CAS.
- J. Lang and M. H. Lin, J. Phys. Chem. B, 1999, 103, 11393 CrossRef CAS.
- X. Qiu, S. Leporatti, E. Donath and H. Möhwald, Langmuir, 2001, 17, 5375 CrossRef CAS.
- N. A. Kotov, J. Dekany and J. H. Fendler, Adv. Mater., 1996, 8, 637 CrossRef CAS.
- A. A. Mamedov, N. A. Kotov, M. Prato, D. M. Guldi, J. P. Wicksted and A. Hirsch, Nat. Mater., 2002, 1, 190 CrossRef CAS.
- M. Sano, A. Kamino, J. Okamura and S. Shinkai, Nano Lett., 2002, 2, 531 CrossRef CAS.
- D. Li, M. B. Müller, S. Gilje, R. B. Kaner and G. G. Wallace, Nat. Nanotechnol., 2008, 3, 101 CrossRef CAS.
- W. Stockton and M. F. Rubner, Macromolecules, 1997, 30, 2717 CrossRef CAS.
- L. Wang, Z. Wang, X. Zhang, J. Shen, L. Chi and H. Fuchs, Macromol. Rapid Commun., 1997, 18, 509 CrossRef CAS.
- H. Lee, L. J. Kepley, H. G. Hong and T. E. Mallouk, J. Am. Chem. Soc., 1988, 110, 618 CrossRef CAS.
- Y. Shimazaki, M. Mitsuishi, S. Ito and M. Yamamoto, Langmuir, 1997, 13, 1385 CrossRef CAS.
- G. K. Such, J. F. Quinn, A. Quinn, E. Tjipto and F. Caruso, J. Am. Chem. Soc., 2006, 128, 9318 CrossRef CAS.
- I. Ichinose, H. Senzu and T. Kunitake, Chem. Mater., 1997, 9, 1296 CrossRef CAS.
- J. I. Anzai, Y. Kobayashi, N. Nakamura, M. Nishimura and T. Hoshi, Langmuir, 1999, 15, 221 CrossRef CAS.
- S. L. Clark and P. T. Hammond, Langmuir, 2000, 16, 10206 CrossRef CAS.
- T. Serizawa, S. Kamimura, N. Kawanishi and M. Akashi, Langmuir, 2002, 18, 8381 CrossRef CAS.
- J. T. Stricker, A. D. Gudmundsdóttir, A. P. Smith, B. E. Taylor and M. F. Durstock, J. Phys. Chem. B, 2007, 111, 6322 CrossRef CAS.
- A. A. Argun, A. J. Nathan and P. T. Hammond, Adv. Mater., 2008, 20, 1539 CrossRef CAS.
- O. N. Oliveira, A. Riul and V. B. P. Leite, Braz. J. Phys., 2004, 34, 73 CAS.
- L. Wang, Y. Fu, Z. Wang, Y. Fan and X. Zhang, Langmuir, 1999, 15, 1360 CrossRef CAS.
- E. Hao and T. Lian, Langmuir, 2000, 16, 7879 CrossRef CAS.
- E. Hao and T. Lian, Chem. Mater., 2000, 12, 3392 CrossRef CAS.
- T. Serizawa, K. Hamada, T. Kitayama, N. Fujimoto, K. Hatada and M. Akashi, J. Am. Chem. Soc., 2000, 122, 1891 CrossRef CAS.
- V. K. Kamineni, Y. Lvov and T. A. Dobbins, Langmuir, 2007, 23, 7423 CrossRef CAS.
- M. S. Akhter and S. M. Alawi, Colloids Surf., A, 2003, 219, 281 CrossRef CAS.
- V. I. Pârvulescu and C. Hardacre, Chem. Rev., 2007, 107, 2615 CrossRef CAS.
- X. Han and D. W. Armstrong, Acc. Chem. Res., 2007, 40, 1079 CrossRef CAS.
- T. L. Greaves and C. J. Drummond, Chem. Soc. Rev., 2008, 37, 1709 RSC.
- C. Wakai, A. Oleinikova and H. Weingärtner, J. Phys. Chem. B, 2005, 109, 17028 CrossRef CAS.
- N. Winterton, J. Mater. Chem., 2006, 16, 4281 RSC.
- T. Ueki and M. Watanabe, Macromolecules, 2008, 41, 3739 CrossRef CAS.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974 CrossRef CAS.
- C. Jiang, X. Wang, R. Gunawidjaja, Y.-H. Lin, M. K. Gupta, D. L. Kaplan, R. R. Naik and V. V. Tsukruk, Adv. Funct. Mater., 2007, 17, 2229 CrossRef CAS.
- T. Fukushima, A. Kosaka, Y. Ishimura, T. Yamamoto, T. Takigawa, N. Ishii and T. Aida, Science, 2003, 300, 2072 CrossRef CAS.
- J. Cho, K. Char, J.-D. Hong and K.-B. Lee, Adv. Mater., 2001, 13, 1076 CrossRef CAS.
- P. A. Chiarelli, M. S. Johal, J. L. Casson, J. B. Roberts, J. M. Robinson and H.-L. Wang, Adv. Mater., 2001, 13, 1167 CrossRef CAS.
- P. Snedden, A. I. Cooper, K. Scott and N. Winterton, Macromolecules, 2003, 36, 4549 CrossRef CAS.
- S. Vozar, Y.-C. Poh, T. Serbowicz, M. Bachner, P. Podsiadlo, M. Qin, E. Verploegen, N. A. Kotov and A. J. Hart, Rev. Sci. Instrum., 2009, 80, 023903 CrossRef.
- C. B. Bucur, Z. Sui and J. B. Schlenoff, J. Am. Chem. Soc., 2006, 128, 13690 CrossRef CAS.
- M. Elżbieciak, S. Zapotoczny, P. Nowak, R. Krastev, M. Nowakowska and P. Warszyński, Langmuir, 2009, 25, 3255 CrossRef CAS.
- M. R. Böhmer, O. A. Evers and J. M. H. Scheutjens, Macromolecules, 1990, 23, 2288 CrossRef CAS.
- J. W. Ostrander, A. A. Mamedov and N. A. Kotov, J. Am. Chem. Soc., 2001, 123, 1101 CrossRef CAS.
- B. S. Shim, Z. Tang, M. P. Morabito, A. Agarwal, H. Hong and N. A. Kotov, Chem. Mater., 2007, 19, 5467 CrossRef CAS.
- B. S. Shim, P. Podsiadlo, G. D. Lilly, A. Agarwal, J. Lee, Z. Tang, S. Ho, P. Ingle, D. Paterson, W. Lu and N. A. Kotov, Nano Lett., 2007, 7, 3266 CrossRef CAS.
- B. S. Shim, J. Zhu, E. Jan, K. Critchley, S. Ho, P. Podsiadlo, K. Sun and N. A. Kotov, ACS Nano, 2009, 3, 1711 CrossRef CAS.
- G. Eda, G. Fanchini and M. Chhowalla, Nat. Nanotechnol., 2008, 3, 270 CrossRef CAS.
- X. L. Li, G. Y. Zhang, X. D. Bai, X. M. Sun, X. R. Wang, E. Wang and H. J. Dai, Nat. Nanotech., 2008, 3, 538 CrossRef CAS.
- R. D. Rogers and K. R. Seddon, Science, 2003, 302, 792 CrossRef.
- M. J. Earle and K. R. Seddon, Pure Appl. Chem., 2000, 72, 1391 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Aggregation of PEI and PSS in [EMIm][EtSO4], detailed FTIR data, water-contact angle for (PEI/PSS)10 multilayers, and XPS survey spectra. See DOI: 10.1039/b9nr00333a |
| This journal is © The Royal Society of Chemistry 2010 |
