Sub-10 nm strontium titanate nanocubes highly dispersed in non-polar organic solvents†
Kyoichi
Fujinami
ab,
Kiyofumi
Katagiri
*a,
Jumpei
Kamiya
a,
Tadashi
Hamanaka
c and
Kunihito
Koumoto
*ad
aDepartment of Applied Chemistry, Graduate School of Engineering, Nagoya University, Furo-cho, Chikusa-ku, Nagoya, 464-8603, Japan. E-mail: katagiri@apchem.nagoya-u.ac.jp; koumoto@apchem.nagoya-u.ac.jp; Fax: +81-52-789-3201; Tel: +81-52-789-3330
bTokuyama Corporation, Shibuya-ku, Tokyo, Japan
cJapan Fine Ceramics Center (JFCC), 2-4-1 Mutsuno, Atsuta-ku, Nagoya, 456-8587, Japan
dCREST, Japan Science and Technology Agency, Tokyo, Japan
First published on 16th September 2010
Abstract
Strontium titanate (SrTiO3) nanoparticles with well defined cubic shape and sub-10 nm size that are highly dispersible in non-polar organic solvents were successfully synthesized by hydrothermal (HT) processing. Water-soluble titanium complexes and strontium hydroxide were employed as precursors. When the HT process was carried out without oleic acid, the SrTiO3 particles obtained were relatively large and aggregated. However, SrTiO3 nanocubes that are highly dispersible in hexane were obtained via the HT process using oleic acid and hydrazine.
Perovskite-type mixed-metal oxide materials have been extensively studied because of their wide applications as catalytic, oxygen-transport, ferroelectric, piezoelectric, and dielectric materials.1–3 Strontium titanate (SrTiO3) is a typical perovskite-type mixed-metal oxide that is capable of tunable chemical and physical properties by altering its composition. For example, it is easily converted into an n-type semiconductor at room temperature by either reduction or doping. For that reason, SrTiO3 has been also investigated as a photocatalytic4 and thermoelectric materials.5–7 There is a need to refine the preparation methods for these materials since these properties depend mainly on particle size, morphology, purity, stoichiometry and homogeneity. Consequently, development of new synthesis techniques for SrTiO3 materials is essential from the viewpoint of practical applications. The traditional procedures for the preparation of alkaline earth titanate materials require high temperature heat treatment for crystallization that leads to extensive aggregation and formation of large particles. A variety of liquid phase chemical processes, the conventional hydrothermal (HT) method,8,9 solvothermal methods,10–13 the sol–gel method14 and the oxalate method,15 have been reported in the literature. Of these methods, the HT process is a promising technique for obtaining SrTiO3, and does not require high temperature calcination. However, there are several problems in the conventional HT process. In particular, the size and morphology of particles cannot be adequately controlled, reactants such as organic solvent and chloride are harmful for the environment, and heat treatment is necessary. A liquid phase process without organic solvents and/or halides is important from the viewpoint of green sustainable chemistry. A synthetic process at low temperature has advantages not only for low energy consumption but also for preparation of organic–inorganic hybrid materials. In addition, the HT process is well known as a versatile method for preparing colloidal metal oxide nanocrystals with controlled shape and size.16–21 Ohara and Adschiri et al. reported successful preparation of Fe2O3 and CeO2 nanocubes via a supercritical HT process in which long chain fatty acids were used as surface modifiers.18,19 The nanocubes obtained were covered with hydrophobic organic chains, thus transparent dispersions of the nanocubes in organic solvents were obtained. However, supercritical conditions at high temperature (400 °C) were required in this system, and preparation of nanocrystals of perovskite-type mixed metal oxides with controlled shape has not been reported. Although Chan-Park and co-workers reported oleic acid-capped SrTiO3 particles that are highly dispersible in organic solvents, the particles had non-characteristic shape.20
In the present study, we have developed a novel process for the synthesis of highly dispersible SrTiO3 nanocrystals with cubic-shape. A conventional HT process which does not require supercritical conditions was adopted, and a water-soluble titanium complex and strontium hydroxide octahydrate were employed as precursors of SrTiO3. Since both the water-soluble titanium complex and strontium hydroxide octahydrate can be dissolved in water under the conditions used in this study, it was easy to utilize an HT process and high quality products were obtained. Oleic acid and hydrazine were used as additives to control the size and morphology of the particles and to improve the dispersibility. Commercially available titanium bis(ammonium lactate)dihydroxide (TALH) solution was employed as the water-soluble titanium complex. In a typical synthesis, aqueous solutions of TALH and strontium hydroxide octahydrate were mixed and the pH of the solution adjusted to 13.5. The Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sr molar ratio was fixed at 1
Sr molar ratio was fixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Hydrazine and oleic acid were added to an autoclave containing the aqueous solution of TALH and strontium hydroxide octahydrate, and the autoclave was heated in an oven at 120 or 200 °C for 24 h. After the HT reaction, the products were isolated from the reaction mixture by centrifugation. The SrTiO3 particles obtained were characterized by powder X-ray diffraction (XRD), dynamic light scattering (DLS), FT-IR spectroscopy, and transmission electron microscopy (TEM). The details of the preparation process and characterization of SrTiO3 particles are given in the ESI†.
1. Hydrazine and oleic acid were added to an autoclave containing the aqueous solution of TALH and strontium hydroxide octahydrate, and the autoclave was heated in an oven at 120 or 200 °C for 24 h. After the HT reaction, the products were isolated from the reaction mixture by centrifugation. The SrTiO3 particles obtained were characterized by powder X-ray diffraction (XRD), dynamic light scattering (DLS), FT-IR spectroscopy, and transmission electron microscopy (TEM). The details of the preparation process and characterization of SrTiO3 particles are given in the ESI†.
Fig. 1 shows typical XRD patterns of SrTiO3 particles prepared by HT treatment at 120 or 200 °C with or without oleic acid. The pattern of SrTiO3 particles prepared at 120 °C without oleic acid shows good crystallinity with diffraction peaks that correspond to the peaks of the cubic perovskite SrTiO3 structure (JCPDS: no. 35-0734, a = 0.3905 nm) (Fig. 1a). No impurity phase was observed in the XRD pattern, and the purity of the SrTiO3 was thought to be sufficiently high. The full width at half maximum (FWHM) of the diffraction peaks of the XRD pattern was quite small, indicating that the crystalline size of the SrTiO3 was relatively large and not nanoscale. These results imply that SrTiO3 particles can be obtained from TALH and strontium hydroxide by the conventional HT method, but it is difficult to obtain nanosize particles by that method. Diffraction peaks assignable to the cubic perovskite SrTiO3 structure were not found in the XRD pattern of the product prepared at 120 °C in the presence of oleic acid (Fig. 1b). The halo pattern indicates that the product was amorphous, hence the addition of oleic acid suppressed crystallization of SrTiO3. HT synthesis at a higher temperature (200 °C) was investigated for the reaction mixture containing oleic acid. The XRD pattern revealed diffraction peaks indexed to the cubic perovskite SrTiO3 structure (Fig. 1c). The FWHM of the peaks was relatively broad by comparison with that of Fig. 1a, indicating nanoscale crystallization of SrTiO3 in this system. The average crystalline size evaluated from FWHM of the (200) diffraction peak using Scherrer's equation was ca. 10 nm. It was thus found that both reaction temperature and presence of oleic acid played important roles in the formation of SrTiO3 by hydrothermal processing.
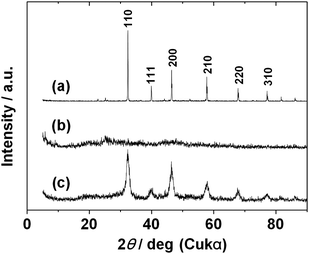 | ||
| Fig. 1 XRD patterns of samples synthesized by HT process: (a) without oleic acid at 120 °C; (b) with oleic acid at 120 °C; and (c) with oleic acid at 200 °C. | ||
Photographic images of dispersions of the SrTiO3 particles are shown in Fig. 2. Hexane was employed as a non-polar medium for the dispersions. A turbid dispersion was observed for the SrTiO3 particles prepared at 120 °C without oleic acid (Fig. 2a). These particles easily precipitated from unstirred solution. By contrast, a transparent dispersion was obtained for SrTiO3 particles prepared at 200 °C with oleic acid (Fig. 2b). The dispersion was stable for several months without precipitation, aggregation or gelation. These results indicate that the SrTiO3 particles prepared using oleic acid were hydrophobic as a result of their surfaces being capped with oleic acid. Consequently, these particles were highly dispersible in hydrophobic organic solvents such as hexane. In addition, their sizes were nanometre scale. The particle size estimated by DLS was 20.4 nm (in hexane), indicating that the particles can disperse in non-polar solvents without aggregation, to give a transparent dispersion. Chan-Park et al. have also reported oleic acid-capped SrTiO3 particles.20 However, their images of transparent dispersions in toluene showed a yellow-brown color, whereas we have obtained colorless transparent dispersions. The SrTiO3 and oleic acid starting materials were colorless, and thus SrTiO3 particles with much higher purity have been obtained in our system.
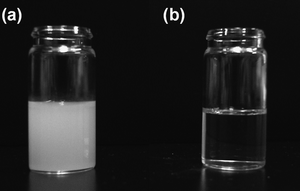 | ||
| Fig. 2 Photographic image of SrTiO3 particle dispersions prepared without oleic acid at 120 °C (a) and with oleic acid at 200 °C (b). | ||
Oleic acid is one of the versatile candidates as a surfactant acts for good dispersion of the inorganic nanoparticles in non-polar inorganic solvents. FT-IR spectroscopy was performed to reveal the interaction of oleic acid and SrTiO3 nanoparticle surface. Fig. 3 shows FT-IR spectra of SrTiO3 particles prepared at 200 °C with oleic acid and oleic acid itself. Bands in the 2840–2970 cm−1 region are attributable to the symmetric and asymmetric stretching of CH2 groups and the terminal CH3 group. The weak band at 3010 cm−1, assignable to the olefinic C–H stretching vibration, is also observed.21 The strong peak at 1708 cm−1 found in the spectrum of oleic acid itself, associated with free C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration, is almost absent in the spectrum of SrTiO3 particles prepared at 200 °C with oleic acid. In contrast, two characteristic bands at 1549 and 1435 cm−1 are apparent in the spectrum of SrTiO3 particles that are due to the COO− asymmetric and symmetric stretching vibrations, respectively, of carboxylate anions complexed with surface metal centers of the SrTiO3 particles.22–24 This is the result of reactions forming chemical bonds between the nanocrystalline surface of SrTiO3 and the organic ligand molecules in the HT reaction condition, which are essential for the perfect dispersion of SrTiO3 nanoparticles in organic solvents.
O stretching vibration, is almost absent in the spectrum of SrTiO3 particles prepared at 200 °C with oleic acid. In contrast, two characteristic bands at 1549 and 1435 cm−1 are apparent in the spectrum of SrTiO3 particles that are due to the COO− asymmetric and symmetric stretching vibrations, respectively, of carboxylate anions complexed with surface metal centers of the SrTiO3 particles.22–24 This is the result of reactions forming chemical bonds between the nanocrystalline surface of SrTiO3 and the organic ligand molecules in the HT reaction condition, which are essential for the perfect dispersion of SrTiO3 nanoparticles in organic solvents.
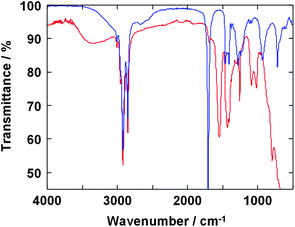 | ||
| Fig. 3 FT-IR spectra of SrTiO3 nanoparticle prepared with oleic acid at 200 °C (red line) and oleic acid itself (blue line). | ||
Fig. 4 shows TEM images of the SrTiO3 particles obtained under different conditions. It is clear that the size and morphology of the two samples were quite different. Large (200–500 nm) crystalline particles were observed for the SrTiO3 prepared without oleic acid (Fig. 4a). This feature was well-corresponded to the result of XRD measurement, shown in Fig. 1a. The sample synthesized in the presence of oleic acid, on the other hand, had the appearance of nanocubes with an average diameter of 9.0 nm (Fig. 4b). Their size was also corresponded to the estimate from Scherrer's equation and DLS measurement. Several preparations of SrTiO3 nanoparticles have been reported, but those materials did not show such a regular cubic shape in sub-10 nm range.20,25–28 The single-crystallinity of SrTiO3 nanocubes was confirmed using a high-resolution (HR) TEM (Fig. 4c). The electron diffraction pattern also revealed the single-crystallinity of SrTiO3 nanocubes (Fig. 4d).
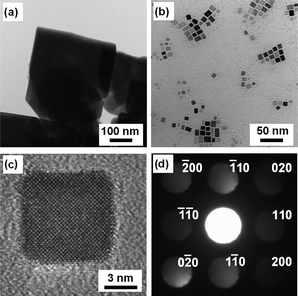 | ||
| Fig. 4 TEM images of SrTiO3 particles prepared: (a) without oleic acid at 120 °C and (b) with oleic acid at 200 °C. HR-TEM image (c) and electron diffraction pattern (d) of SrTiO3 particles prepared with oleic acid at 200 °C. | ||
The nanocubes prepared in the present study did not aggregate, due to the surface coating of oleic acid. Aggregation of the particles is a universal problem in the field of nanopowders, and this cohesion-preventive effect by surface coating is one solution to that problem. Usually, the growth of alkaline earth titanate nanoparticles under hydrothermal/solvothermal conditions is attributed to the in situ reaction mechanism8,28 or the dissolution–precipitation reaction mechanism.12,13,28 Both TALH and strontium hydroxide octahydrate can be dissolved in water under the conditions of this study, thus the reaction can be controlled more precisely since it proceeds in a homogeneous system. As a result, high quality SrTiO3 nanocubes have been obtained by this newly developed synthesis method.
Another key point of this study is the role of oleic acid as an additive.17,18,29,30 From the HT synthesis using oleic acid, perfectly cubic-shaped SrTiO3 nanoparticles can be obtained. Because of the equilibrium between crystallographic habit growth and preferential dissolution of high-energy faceted edges, the dominant morphology is spherical10,12,30 or quasi-cubic shape9,11,13 with rounded corners, for the usual alkaline earth titanate particles synthesized by the hydrothermal/solvothermal method. By contrast, the morphology of the SrTiO3 particles obtained in this study was a beautiful cubic form whose corners were sharp right angles. This means that dissolution of the particle surfaces, even corners, was suppressed by capping the surfaces of the particles with the hydrophobic tails of oleic acid molecules. As a result, growth of the particles occurred in a similar fashion to the crystallographic habit growth.
There are three types of interactions among the nanoparticles: (1) London–van der Waals force, (2) magnetic force and (3) reacting force of the electric double layer. The attractive forces (1 and 2) between the particles must be counteracted by the repulsive force (3) to make the particles exist stably in the solvent. However, this type of repulsive force is very weak. Generally, to synthesize particles that are well dispersed in a solvent, nanoparticles should be given a surface modification to make them absorb a layer of surfactant on the surface. The space hindrance repulsive force from the surfactant can thereby overcome the affinities between the particles.29 Since the surfaces of the particles were covered with oleic acid, particles were located at a constant distance and did not aggregate.
Hydrazine has an important role on the control of cubic shape of SrTiO3. In the absence of hydrazine, SrTiO3 nanoparticles without cubic shape were obtained even when the HT synthesis was carried out in the presence of oleic acid (figure not shown). Hydrazine was often employed for the HT process as an agent for maintaining the pH of the reaction solutions under basic condition since ammonium ions are generated by thermal decomposition of hydrazine. However, the pH value of the reaction solution after HT process in the presence of hydrazine was almost same as that in the absence of hydrazine. Therefore, it seems that hydrazine does not act as a pH maintaining agent in this case. The mechanism for cubic shape generation and the substantial role of hydrazine are now under investigation.
In summary, highly dispersible SrTiO3 nanocubes were successfully synthesized via the HT process using an aqueous solution containing a water-soluble titanium complex and strontium hydroxide octahydrate. Oleic acid acts as a surface-modifying additive that enables control of the particle size and enhancement of the dispersibility. SrTiO3 nanocubes with sub-10 nm size were obtained by the HT process at 200 °C in the presence of oleic acid. The SrTiO3 nanocubes were perfectly dispersed in the non-polar solvent hexane. The dispersion was optically transparent and stable for several months. These nanocubes capped with organic chains have the potential to form closed-packed superlattices by self-assembly. Accordingly, they should find applications in areas such as electronics, photonics and catalysis. Due to the convenience of the process and the availability of the chemicals employed, this procedure is expected to be applicable to a variety of metal oxide nanocrystals with controlled shape and high dispersibility. In addition, the capping agent, oleic acid, may be removed by photo-irradiation since SrTiO3 has a photocatalytic property. Therefore, there is a possibility that SrTiO3 nanocubes which are dispersible in polar solvents, including water, can also be obtained. These approaches are currently under investigation in our laboratory.
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (Grant-in-Aid for Young Scientists (A), 20686046) and the JGC-S Scholarship Foundation. This work was also partly supported by the Core Research for Evolutional Science and Technology (CREST) program of the Japan Science and Technology Agency (JST) and the Knowledge Cluster Initiative Tokai Region Nanotechnology Manufacturing Cluster.
Notes and references
- C. D. Chandler, C. Roger and M. J. Hampden-Smith, Chem. Rev., 1993, 93, 1205 CrossRef CAS.
- M. A. Pena and J. L. G. Fierro, Chem. Rev., 2001, 101, 1981 CrossRef CAS.
- A. S. Bhalla, R. Guo and R. Roy, Mater. Res. Innovations, 2000, 4, 3 Search PubMed.
- L. Chen, S. Zhang, L. Wang, D. Xue and S. Yin, J. Cryst. Growth, 2009, 311, 746 CrossRef CAS.
- S. Ohta, T. Nomura, H. Ohta, M. Hirano, H. Hosono and K. Koumoto, Appl. Phys. Lett., 2005, 87, 092108 CrossRef.
- H. Ohta, S.-W. Kim, Y. Mune, T. Mizoguchi, K. Nomura, S. Ohta, T. Nomura, Y. Nakanishi, Y. Ikuhara, M. Hirano, H. Hosono and K. Koumoto, Nat. Mater., 2007, 6, 129 CrossRef CAS.
- H. Muta, K. Kurosaki and S. Yamanaka, J. Alloys Compd., 2005, 392, 306 CrossRef CAS.
- S. Wada, T. Suzuki and T. Noma, J. Ceram. Soc. Jpn., 1995, 103, 1220 CAS.
- T. Yan, X.-L. Liu, N.-R. Wang and J.-F. Chen, J. Cryst. Growth, 2005, 281, 669 CrossRef CAS.
- X. Wei, G. Xu, Z. Ren, Y. Wang, G. Shen and G. Han, J. Am. Ceram. Soc., 2008, 91, 315 CAS.
- S. Wada, A. Nozawa, M. Ohno, T. Tsurumi and Y. Kuroiwa, Key Eng. Mater., 2009, 388, 111 CrossRef CAS.
- Q. Liu, Z. Yan, G. Sun and W. Zheng, Chem. Lett., 2007, 458 CrossRef CAS.
- B. Hou, Z. Li, Y. Xu, D. Wu and Y. Sun, Chem. Lett., 2005, 1040 CrossRef CAS.
- V. Somani and S. J. Kalita, J. Electroceram., 2007, 18, 57 CrossRef CAS.
- S. Wada, M. Narahara, T. Hoshina, H. Kakemoto and T. Tsurumi, J. Mater. Sci., 2003, 38, 2655 CrossRef CAS.
- T. Taniguchi, K. Nakagawa, T. Watanabe, N. Matsushita and M. Yoshimura, Cryst. Growth Des., 2008, 8, 3725 CrossRef CAS.
- T. Taniguchi, T. Watanabe, N. Sakamoto, N. Matsushita and M. Yoshimura, J. Phys. Chem. C, 2009, 113, 839 CrossRef CAS.
- T. Mousavand, S. Takami, M. Umetsu, S. Ohara and T. Adschiri, J. Mater. Sci., 2006, 41, 1445 CrossRef CAS.
- J. Zhang, S. Ohara, M. Umetsu, T. Naka, Y. Hatakeyama and T. Adschiri, Adv. Mater., 2007, 19, 203 CrossRef CAS.
- Q. J. Cai, Y. Gan, M. B. Chan-Park, H. B. Yang, Z. S. Lu, C. M. Li, J. Guo and Z. L. Dong, Chem. Mater., 2009, 21, 3153 CrossRef CAS.
- E. Pretsch, P. Bühlmann and C. Affolter, Structure Determination of Organic Compounds—Tables of Spectral Data, Springer-Verlag, New York, 3rd edn, 2003 Search PubMed.
- P. J. Thistlethwaite and M. S. Hook, Langmuir, 2000, 16, 4993 CrossRef CAS.
- M. Nara, H. Torii and M. Tasumi, J. Phys. Chem., 1996, 100, 19812 CrossRef CAS.
- Y. G. Aronoff, B. Chen, G. Lu, C. Seto, J. Schwartz and S. L. Bernasek, J. Am. Chem. Soc., 1997, 119, 259 CrossRef CAS.
- Y. Gan, Q. J. Cai, C. M. Li, H. B. Yang, Z. S. Lu, C. Gong and M. B. Chan-Park, ACS Appl. Mater. Interfaces, 2009, 1, 2230 Search PubMed.
- X. Wei, Z. Ren, C. Xu, G. Shen and G. Han, J. Am. Ceram. Soc., 2008, 91, 3795 CrossRef CAS.
- J. O. Eckert, Jr, C. C. Hung-Houston, B. L. Gersten, M. M. Lencka and R. E. Riman, J. Am. Ceram. Soc., 1996, 79, 2929.
- F. A. Rabuffetti, H.-S. Kim, J. A. Enterkin, Y. Wang, C. H. Lanier, L. D. Marks, F. A. Poeppelmeier and P. C. Stair, Chem. Mater., 2008, 20, 5628 CrossRef CAS.
- H. Yan, J. Zhang, C. You, Z. Song, B. Yu and Y. Shen, Mater. Chem. Phys., 2009, 113, 46 CrossRef CAS.
- S. Sun, H. Zeng, D. B. Robinson, S. Raoux, P. M. Rice, S. X. Wang and G. Li, J. Am. Chem. Soc., 2004, 126, 273 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Preparation procedure and characterization of SrTiO3. See DOI: 10.1039/c0nr00543f |
| This journal is © The Royal Society of Chemistry 2010 |
