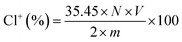Open Access Article
Open Access ArticleStudies of PET nonwovens modified by novel antimicrobials configured with both N-halamine and dual quaternary ammonium with different alkyl chain length†
Hsiu-Wen Chiena,
Ying-Yuan Chenb,
Yen-Lun Chenb,
Chi-Hui Cheng *c and
Jui-Che Lin
*c and
Jui-Che Lin *b
*b
aDepartment of Chemical and Material Engineering, National Kaohsiung University of Science and Technology, Kaohsiung, Taiwan
bDepartment of Chemical Engineering, National Cheng Kung University, Tainan, Taiwan. E-mail: jclin@mail.ncku.edu.tw
cDepartment of Paediatrics, Chang Gung University, Chang Gung Memorial Hospital, Taoyuan, Taiwan. E-mail: pedneph.cheng@msa.hinet.net
First published on 4th March 2019
Abstract
This work describes the synthesis of novel antimicrobial agents consisting of N-halamine and dual quaternary ammonium with different alkyl chain lengths and their antimicrobial applications for PET nonwovens. The antimicrobial agents were grafted onto PET nonwovens via esterification with a crosslinker, 1,2,3,4-butanetetracarboxylic acid (BTCA). The cyclic amide structure in the antimicrobial agents could be easily converted to N-halamine after immersion in a diluted chlorine bleach solution. Variations in surface chemical composition of the modified PET nonwovens were examined by XPS. Antimicrobial activities of the nonwovens/fabrics were tested against S. aureus (Gram-positive) and E. coli (Gram-negative) strains. Systematic investigation showed the antibacterial activities were dependent upon the alkyl chain length. The synergism of N-halamine and dual quaternary ammonium could lead to significant antimicrobial activity with inactivation of up to 90% of S. aureus and E. coli after 10 minute contact. This work suggested that the novel composite biocides with N-halamine and dual quaternary ammonium groups and the associated surface modification methods could be of use for further developing antimicrobial nonwoven applications.
Introduction
An antibacterial surface can effectively prevent the initial attachment of bacteria to surfaces of devices and subsequently avoid infections. In the past, a simple design to add soluble and low molecular weight antimicrobial agents to existing restorative materials would exhibit inhibitory effects against bacteria by release of agents in a wet environment.1,2 However, such an approach has not been well accepted from the clinical point of view, as the release of agents results in a limited period of effectiveness over time, toxicity to the environment and possible antimicrobial resistance in bacteria with repeated overuse.3 Thus, it is necessary to develop immobilized bactericides on the surface of devices.Among the numerous antibacterial agents, the N-halamine and quaternary ammonium compounds have attracted great attention as effective antibacterial agents used in different fields.4–7 N-Halamines are one of antimicrobial agents have created a lot of interests in application of antimicrobial textiles due to their long-term stabilities, weak toxicities to humans, and antimicrobial activities against a broad spectrum of microorganisms including Gram-negative and Gram-positive bacteria. An N-halamine compound can be defined as a compound containing one or more nitrogen–halogen covalent bonds that is usually formed by halogenation of imide, amide, or amine groups. An N-halamine has biocidal properties thanks to the oxidation state of halide atoms in chloramine (![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) N–Cl).8–10 Other common antibacterial agent, such as quaternary ammonium salts containing with alkyl chain are widely used in antibacterial applications since the cationic charges in the structures attract cell membrane surface through ionic interaction, then disrupt the structure of cellular membranes resulting in the leakage of intracellular components and consequent cell death.11–13
N–Cl).8–10 Other common antibacterial agent, such as quaternary ammonium salts containing with alkyl chain are widely used in antibacterial applications since the cationic charges in the structures attract cell membrane surface through ionic interaction, then disrupt the structure of cellular membranes resulting in the leakage of intracellular components and consequent cell death.11–13
To bond the antibacterial agents to surfaces of devices, various methods have been reported, such as chemical grafting,14 layer-by-layer assembly deposition15 or surface polymerization.16,17 In general, the strategies are developing methacrylate monomers with pendant bactericide moieties which are subsequently polymerized or copolymerized with other monomers. For example, Cerkez et al. reported a vinyl N-halamine monomer, hydantoin acrylamide, was copolymerized with silane and epoxide group-containing monomers, and the resultant copolymers were coated onto cotton fabric through hydrolysis of alkoxy groups with formation of silyl ether bonding and opening of the epoxide ring, respectively.14 Zhang et al. synthesized a quaternary ammonium compound, 2-dimethyl-2-hexadecyl-1-methacryloxyethyl ammonium bromide (DEHMA), which was copolymerized with acrylic acid (AA) onto polyester (PET) fibres via EB irradiation process.16 The grafted fibres were further soaked in AgNO3 solution to load silver ions for significantly enhancing the antibacterial efficiency.16
Recently, the synergetic antimicrobial activity via two different bactericides rendered different antimicrobial mechanism have attracted more attention.18–20 Chen et al. showed the cellulose fibres initially grafted with polyquaternarized ammonium then followed by halamine functionality end attachment exhibited antimicrobial capability.18 Hu et al. rather used two different monomers that carry quaternarized ammonium and halamine chemical configuration, respectively, to modify the cellulose fibres for antimicrobial application.19 Ning et al., instead, prepared various antimicrobial small molecules by incorporating quaternary ammonium and long alkyl chain into N-halamine antimicrobial agents.20 In addition, the antimicrobial efficacy were evaluated by either direct microbial solution contact with the biocides (i.e. not immobilized onto any substrate)20 or by immersing the grafted fibres into plenty of microbial solutions.18,19
Considering the rise of antimicrobial agent resistance, the development of new antibacterial agents with improved biocidal functions is required for the nonwovens used biomedical applications, namely PET nonwoven. In this study, the PET nonwoven fibres were grafted with novel “composite” biocides combining “dual” quaternary ammonium with different alkyl chain length and N-halamine along the same alkyl chain that are not reported before. The modified surfaces were further characterized by XPS. The wettability (water absorption capability) and biocidal efficacies were examined by the American Association of Textile Chemists and Colourists (AATCC) methods. Most importantly, the modified AATCC antimicrobial testing method employed, in which a limited amount of bacterial suspension was added and sandwiched in between two pieces of nonwovens, is more similar to the real application scenario than those reported in earlier investigations for the grafted cellulose fibres.18,19
Experimental
Materials
PET nonwoven with fibre density of 50 g m−2 was purchased from Web-Pro Co., Ltd, Taiwan. 5,5-Dimethylhydantoin (DMH), N,N,N′,N′-tetramethylethylenediamine (TMEDA), 1,2-epoxyoctane, 1,3-dibromopropane, 1,2,3,4-butanetetracarboxylic acid (BTCA), and starch from potato powder were purchased from Sigma-Aldrich. 2-Bromoethanol and sodium hypophosphite monohydrate were purchased from Alfa Aesar. All other chemicals were purchased from Sigma-Aldrich unless specified otherwise. The starch solution contained with 6% (w/v) starch, 3% (w/v) BTCA and 1.5% (w/v) NaH2PO2.Staphylococcus aureus (ATCC 21351) and Escherichia coli (ATCC 23501) was received from Food Industry Research and Development Institute (Hsinchu, Taiwan). Trypticase soy agar contained 15 g L−1 agar (BD, USA) with 15 g L−1 tryptone (BD, USA), 5 g L−1 soy peptone (HiMedia Laboratories, US), and 5 g L−1 NaCl. Bacteria suspension buffer was prepared by 0.85% NaCl, pH 7.
Synthesis of antimicrobial agents
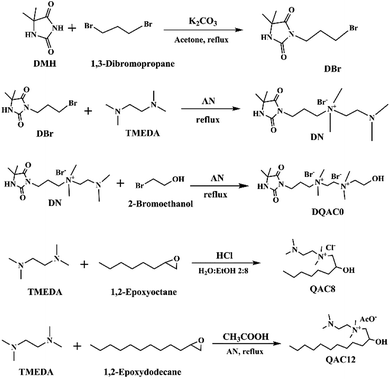
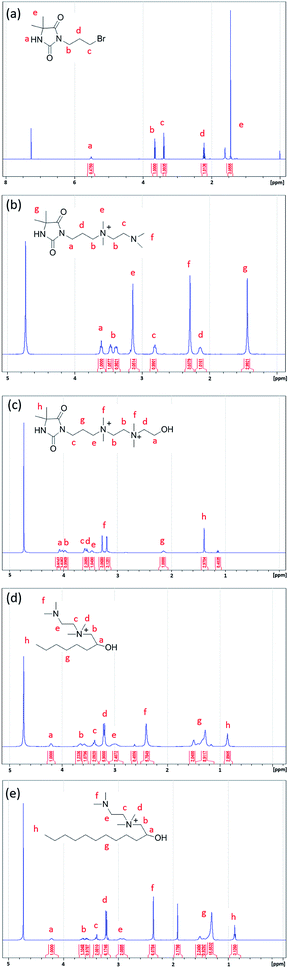
Three antimicrobial agents including DQAC0, QAC8 and QAC12 were synthesized. DQA0 is incorporating of dual quaternary ammonium with N-halamine. QAC8 is incorporating of dual quaternary ammonium with octane (C8) tail while QAC12 is incorporating of dual quaternary ammonium with dodecane (C12) alkyl end. The structure of these antimicrobial agent and procedure of synthesis are described in Scheme 1.Synthesis of 3-(3-bromopropyl)-5,5-dimethylimidazolidine-2,4-dione (DBr) was performed with DMH (0.01 mol) and 1,3-dibromopropane (0.01 mol) in 170 mL acetone with 10 g K2CO3 in a round-bottom flask for reaction of 18 h. After reaction, K2CO3 and the part of solvent and were removed by filtration and rotary vacuum evaporation, respectively. Next, the residue was purified by column chromatography eluting. The final product was dried and analysed. The 1H NMR (500 MHz CDCl3, δ): 5.49–5.60 (s, 1H, NCONHC), 3.60–3.70 (s, 2H, NCH2CH2), 3.35–3.45 (s, 2H, CH2CH2Br), 2.20–2.30 (s, 2H, CH2CH2CH2), 1.40–1.50 (s, 6H, C(CH3)2) (Fig. 1a).
Synthesis of 3-(4,4-dimethyl-2,5-dioxoimidazolidin-1-yl)-N-(2-(dimethylamino)ethyl)-N,N-dimethylpropan-1-aminium bromide (DN) was mixing DBr (0.008 mol) and TMEDA (0.04 mol) in 25 mL acetonitrile (AN). The mixture was heated to reflux for 24 h. After removing the solvent under a reduce pressure, the excess TMEDA was removed in an oven at 40 °C overnight. After that, diethyl ether was added to the solution and stirred to produce white crystals. The powder was dissolved in H2O and then precipitated with diethyl ether for several times. The white powder product was dried and analysed. The 1H NMR (500 MHz D2O, δ): 3.55–3.68 (s, 2H, –NCH2CH2), 3.35–3.50 (d, 4H, CH2(CH3)2N+CH2), 3.10–3.20 (s, 6H, CH2(CH3)2N+CH2), 2.78–2.90 (s, 2H, CH2CH2N(CH3)2), 2.25–2.35 (s, 6H, CH2–CH2–N(CH3)2), 2.08–2.20 (s, 2H, CH2CH2CH2), 1.40–1.53 (s, 6H, C(CH3)2) (Fig. 1b).
Synthesis of N1-(3-(4,4-dimethyl-2,5-dioxoimidazolidin-1-yl)propyl)-N2-(2-hydroxyethyl)-N1,N1,N2,N2-tetramethylethane-1,2-diaminium bromide (DQAC0) was mixing DN (0.005 mol) and 2-bromoethanol (0.0075 mol) in 15 mL acetonitrile. The mixture was refluxed for 24 h. After reaction, the plenty of diethyl ether was added to the solution and stirred to produce white crystals. The powder was repeatedly dissolved in ethanol and then precipitated with diethyl ether for several times. The white powder product was dried and analysed. The 1H NMR (500 MHz D2O, δ): 4.08–4.10 (s, 2H, CH2CH2OH), 3.93–4.06 (d, 4H, (CH3)2N+CH2CH2(CH3)2N+), 3.50–3.68 (s, 4H, NCH2CH2, (CH3)2N+CH2CH2(OH)), 3.40–3.50 (s, 2H, CH2CH2(CH3)2N+), 3.10–3.35 (d, 12H, (CH3)2N+CH2CH2(CH3)2N+), 2.08–2.20 (s, 2H, CH2CH2CH2), 1.38–1.50 (s, 6H, C(CH3)2) (Fig. 1c).
The N-(2-(dimethylamino)ethyl)-2-hydroxy-N,N-dimethyloctan-1-aminium chloride (QCA8) was synthesized by a reaction of TMEDA (0.05 mol) with 1,2-epoxyoctane (0.01 mol) and HCl (0.01 mol) in 60 mL of 80% ethanol aqueous solution at 50 °C for 4 h. After the solution was removed by rotary evaporation, diethyl ether was added to the solution and stirred to produce white crystals. The powder was repeatedly dissolved in H2O and then precipitated with diethyl ether for several times to obtain a purified product. The white powder product of QCA8 was dried and analysed. The 1H NMR (500 MHz D2O, δ): 4.18–4.30 (s, 1H, CH2CH(OH)CH2), 3.50–3.75 (s, 2H, (CH3)2N+CH2CH(OH)), 3.35–3.45 (s, 2H, CH2CH2(CH3)2N+), 3.10–3.30 (s, 6H, CH2(CH3)2N+CH2), 2.88–3.10 (s, 2H, CH2CH2N(CH3)2), 2.30–2.50 (s, 6H, (CH3)2NCH2), 1.10–1.60 (m, 10H, CH3(CH2)5CH(OH)), 0.81–0.91 (s, 3H, CH3(CH2)5CH(OH)) (Fig. 1d).
The N-(2-(dimethylamino)ethyl)-2-hydroxy-N,N-dimethyldodecan-1-aminium acetate (QCA12) was synthesized by a reaction of TMEDA (0.05 mol) with 1,2-epoxydodecane (0.01 mol) and CH3COOH (0.01 mol) in 25 mL of acetonitrile. The mixture was heated to reflux for 24 h. After removing solvent by rotary evaporation, the powder was repeatedly dissolved in H2O and then precipitated with diethyl ether for several times to obtain a purified product. The white powder product of QCA12 was dried and analysed. The 1H NMR (500 MHz D2O, δ): 4.10–4.30 (s, 1H, CH2CH(OH)CH2), 3.50–3.75 (s, 2H, (CH3)2N+CH2CH(OH)), 3.35–3.45 (s, 2H, CH2CH2(CH3)2N+), 3.10–3.30 (s, 6H, CH2(CH3)2N+CH2), 2.88–3.10 (s, 2H, CH2CH2N(CH3)2), 2.30–2.50 (s, 6H, (CH3)2NCH2), 1.10–1.60 (m, 18H, CH3(CH2)9CH(OH)), 0.81–0.91 (s, 3H, CH3(CH2)5CH(OH)) (Fig. 1e).
The yields for DBr, DN, DQAC0, QAC8 and QAC12 were 63.5%, 95.1%, 90.6%, 87.7%, and 81.4%, respectively.
Preparation of starch-pretreated PET nonwovens
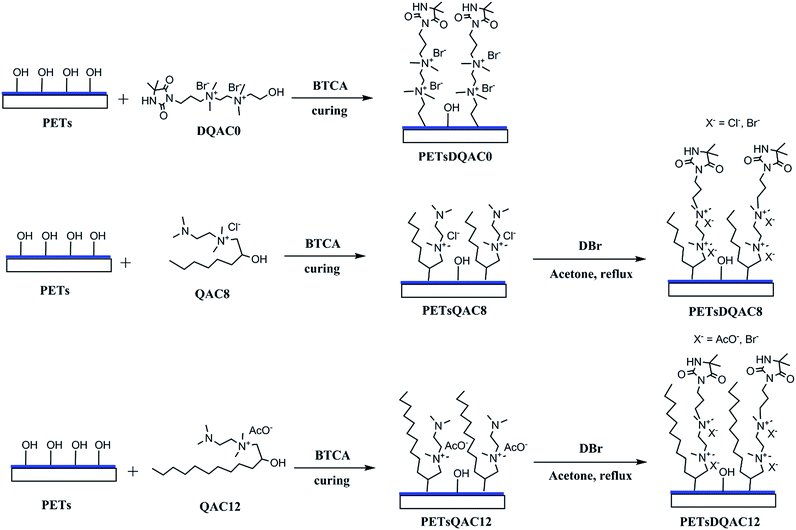
PET nonwovens were first cut into 1.5 × 1.5 cm2 and rinsed in 50% ethanol aqueous under ultrasonic condition. The hydrolysis of PET nonwoven fibre was then obtained by immersing in 1 N NaOH at 70 °C for 10 min. The PET nonwovens were rinsed with excess deionized water and 1% acetic acid to remove NaOH and dried at 50 °C for 24 h. The hydrolysed PET nonwovens were further immersed in starch/BTCA/NaH2PO2 solution (6 wt%/3 wt%/1.5 wt%) at 90 °C for 15 min and cured at 170 °C for 5 min to graft starch on fibres. The starch pretreated PET nonwoven, called PETs, was finally washed in hot water at 70 °C for 1 h to remove residue.Surface modification of PETs nonwovens with different antimicrobial agents
The procedure of surface modification with antimicrobial agents was shown in Fig. 2. The antimicrobial agent solution contains 10% (w/v) antimicrobial agent (DQAC0, QAC8 and QAC12), 10% (w/v) BTCA and 2.5% (w/v) NaH2PO2, respectively. The PETs nonwovens were immersed in antimicrobial agent solution at 90 °C for 5 min and curing at 170 °C for 10 min. The antimicrobial agent modified PETs nonwoven were rinsed with deionized water several times and sonicated for 15 min.The PETs nonwovens treated with QAC8 and QAC12 were further refluxed in 10 mL acetone with excess of DBr for 48 h. After reaction, the nonwovens fibres were washed with plenty of acetone to remove residue and air-dried for further testing.
Chlorination and analytical titration
The DQAC0, DQAC8 and DQAC12 grafted PETs nonwovens were chlorinated by exposure to a diluted bleach solution with 10% sodium hypochlorite at pH 7 for 1 h. The chlorinated nonwovens were washed with a plenty of distilled water and dried at 50 °C for 1 h to remove free chlorine from the surface of the nonwovens.The oxidative chlorine loading (Cl+%) in the nonwovens were determined by the iodometric/thiosulfate titration method.21 The Cl+(wt%) in the nonwovens was calculated according to the following equation:
Nonwoven fabrics characterization
The surface composition measurement was performed by X-ray photoelectron spectroscopy (XPS or ESCA) (PHI Quantera SXM, USA). The X-ray source was monochromatic Al-Kα (hν = 1486.6 eV), and the take-off angle was set at 45°. The high-resolution spectra were deconvoluted by mixing the Gaussian–Lorentzian functions using the free software program, XPSPEAK. Quantification of the element was performed on the peak areas of the C 1s, O 1s, N 1s and Cl 2p multiplex.22American Association of Textile Chemists and Colourists (AATCC) 39-1980 test method was designed to measure the wettability of the nonwovens by measuring the time it takes a drop of water placed on the fabric surface to be completely absorbed into the fabric.23 The wettability of the nonwovens was assessed by a 10 μL drop of water placed on the nonwovens. Time was recorded until the water drop absorbs completely.
Evaluation of antimicrobial activity
To mimic the antimicrobial nonwovens' applications in hospital setting, the antimicrobial activity of the modified PET nonwovens was evaluated by using a modified AATCC 100 Test Method in which the PET nonwoven fibres were challenged with Staphylococcus aureus (ATCC 21351) and Escherichia coli (ATCC 23501).24 The bacteria were suspended in saline at pH 7 to a final concentration of about 106 CFU mL−1. Then 25 μL of the bacterial suspension was added and “sandwiched in between two pieces of nonwovens” (1.5 × 1.5 cm2). After different periods of contact times (3 min and 10 min), nonwovens were vortexed in 5 mL of 0.001 N sodium thiosulfate solution for 5 min. SEM analyses on the vortexed nonwovens were performed to ensure the removal of adhered microbial. Serial dilutions of the vortexed solutions were prepared and 100 μL of dilutions were placed on sterile Trypticase soy agar plates. After incubation at 37 °C for 24 h, the number of bacterial colonies on plate was counted to get the corresponding concentration of living bacteria.The antimicrobial efficacy of the modified PET nonwovens was also evaluated after repeatedly washing in a beaker (50 × 70 mm2) containing 100 mL water, 2 wt% neutral detergents, and 1 wt% sand at 40 °C with stirring speed of 200 rpm for 10 min. After each cycle of washing, the modified PET nonwovens was exposed to a diluted bleach solution with 10% sodium hypochlorite for rechlorination. The rechlorinated PET nonwoven fibres were then challenged with S. aureus and E. coli to examine the antimicrobial efficiency.
Statistical analysis
The data were reported as means ± standard deviation (SD). The statistical analyses between different groups were determined using Student's t-test. Probabilities with p ≤ 0.05 were considered as significant difference. All statistical analyses were performed using the GraphPad Instat 3.0 program (GraphPad Software, USA).Results and discussion
Characterization of modified PET samples
PET is a linear polyester widely used in textile because of ease of processing, reduced weight, plasticity, and low cost. Despite many excellent properties of PET, the lack of reactive groups is the major disadvantage for textile fibre production. Thus, it is necessary to impart desired properties by introducing specific functional groups on the surface of PET. Hydrolysis of PET is a common approach to yield a surface mixture of hydroxyl and carboxylic acid groups.25 However, the density of hydroxyl functional group is not enough for further modification. In this context, we used a hydrolysed PET as a base to create a polyol surface via a chemical method with the use of starch and BTCA as finishing chemicals. The esterification between hydroxyl groups of starch and hydrolysed PET, and carboxylic acid groups of BTCA acts as crosslinking under a heat to form a polyol thin film on the surface. The characteristics of the starch modified PET fibres (PETs) were examined by XPS to confirm the increase of surface oxygen content (Table 1). Similar results were obtained in the C 1s high resolution scan. Fig. 3 shows a peak was detected at 286.5 eV which can be attributed to the C*–O in the starch, further confirming the polyol crosslinking. The notion of N1s on the PETs was likely due to the low level of protein within the potato powder starch or the adsorption of adventitious N atoms prior the ESCA measurement.| Sample | Atomic percentage (%) | ||
|---|---|---|---|
| C 1s | O 1s | N 1s | |
| PET | 70.1 | 29.9 | <0.1 |
| PETs | 58.8 | 38.5 | 2.8 |
| PETsDQAC0 | 65.5 | 31.6 | 3.0 |
| PETsDQAC8 | 62.0 | 32.2 | 5.8 |
| PETsDQAC12 | 62.4 | 32.4 | 5.2 |
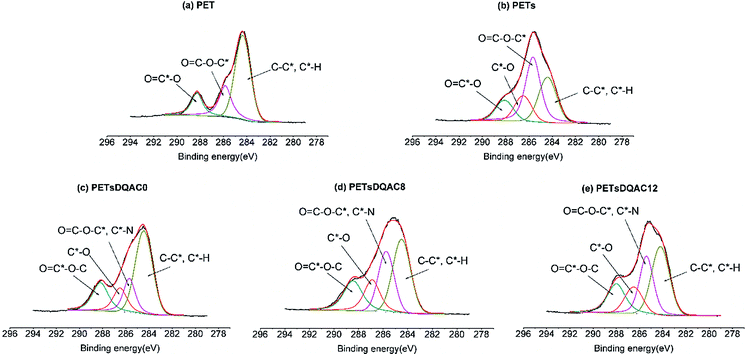 | ||
| Fig. 3 The high-resolution XPS spectra of the C 1s region of (a) PET nonwovens grafted with (b) starch, starch plus (c) DQAC0, (d) DQAC8, (e) DQAC12. | ||
After the treatment, three antimicrobial agents, including DQAC0, DQAC8 and DQAC12 were immobilized on the PETs surface. As expected, the nitrogen contents on the PETsDQAC0, PETsDQAC8 and PETsDQAC12 surfaces were higher than those on the PETs surface due to the additional immobilization of antimicrobial agents with hydantoin and quaternary ammonium structure (Table 1). However, the nitrogen contents on PETsDQAC0 (3.0%) was less than those on PETsDQAC8 (5.8%) and PETsDQAC12 (5.2%). This was likely resulted from easier surface migration for the DQAC8 and DQAC12 which carries longer alkyl chain (i.e. more hydrophobic structure) under the vacuum condition (i.e. hydrophobic environment) in which XPS analysis was performed.
After chlorination of DQACx (x = 0, 8 and 12), the content of oxidative chlorine in the bulk was iodometrically titrated. The oxidative chlorinated content (wt%) was 0.15%, 0.093%, and 0.053% for the PETsDQAC0, PETsDQAC8, and PETsDQAC12, respectively. In contrast, the surface chlorine contents (atomic%) evaluated by XPS were quite different from the bulk ones. The surface chlorine content on PETsDQAC0, PETsDQAC8, and PETsDQAC12 as determined by the XPS were 0.45 wt%, 1.08 wt% and 1.22 wt%, respectively (Fig. 4). These results suggested that the chlorination of PETsDQAC8 and PETsDQAC12 were probably dominated on the surface rather than in the bulk, likely resulted from the longer alkyl chain associated (see discussions in the following wettability analyses).
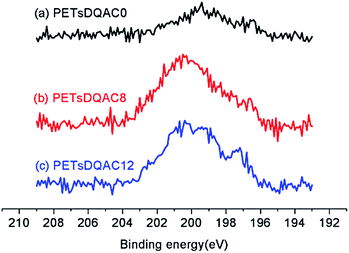 | ||
| Fig. 4 The high-resolution XPS spectra of the Cl 2p region of chlorinated PETsDQAC0, PETsDQAC8 and PETsDQAC12 nonwovens. | ||
The representative SEM micrographs for the pristine non-treated PET nonwoven and the one modified by DQAC0 (PETsDQAC0) were shown in ESI Fig. 1.† There is no distinct variation in surface morphology after the antimicrobial surface grafting reaction.
The liquid absorption properties of fabrics are critical not only to the success of wet processes such as dyeing, printing and finishing, but also to the performance of products such as performance clothing, disposable hygiene materials and medical products.23 According to the wettability results summarized in Table 2, we observed that the PET nonwovens had a strong hydrophobic characteristic, while the PETs nonwovens had a more hydrophilic character due to polyol crosslinking. After grafting with antibacterial agents, the PETsDQAC0 showed the most hydrophilic character, as a result of the “dual” quaternary ammonium in the chemical structure, while the PETsQAC12 had a strongest hydrophobic character, resulting from the long alkyl chains (C12) within the chemical configuration. With a shorter alkyl chain length (C8), the PETsDQAC8 showed an intermediate hydrophilicity as compared to the other two, more hydrophilic as compared to PETsDQAC12 while only slightly more hydrophobic than the PETsDQAC0. An increase in the alkyl chain lengths of these novel antimicrobials containing halamine plus dual quaternary ammonium configuration results in an increase in the surface hydrophobicity, and further resulted in poor wetting of the nonwovens. This surface hydrophobicity finding could be further correlated with the oxidative chlorine content noted by the iodometrically titration mentioned earlier. The aqueous bleach solutions would become more difficult to pass through the hydrophobic nonwovens and lead to lower active chlorine content in the PETsDQAC8 and PETsDQAC12 nonwovens. Thus, the oxidative chlorine in PETsDQAC12 showed the lowest content as compared to that in PETsDQAC0 and PETsDQAC8.
Antibacterial efficacy
The antimicrobial efficacy of three compounds that can be synthesized prior to be immobilized onto the PET nonwovens, namely DQAC0, QAC8 and QAC12, were examined with free bacterial suspension. All these three showed antimicrobial effect as expected due to the halamine and quaternary ammonium structure within the molecule configuration.In order to simulate future antimicrobial nonwovens applications in hospital (e.g. disposable hospital apparels or dividing curtains within ward), the antimicrobial efficacy of the modified PET nonwovens was evaluated by a modified AATCC 100 Test Method in which bacterial suspension was added and “sandwiched in between two pieces of nonwovens” instead of placing the inoculated fabrics under the favourable conditions of nutrients and temperatures as conventional AATCC 100 Test Method suggests.
Due to a poor wetting of PETsDQAC12, only PETsDQAC0 and PETsDQAC8 were evaluated their antimicrobial efficiency. Fig. 5 shows the biocidal efficacy of the nonwovens challenged with S. aureus (Gram-positive) and E. coli (Gram-negative) bacteria after 10 min of contact. The PET nonwoven was used as the control. No antimicrobial efficacy was noted for the control against both bacteria. The PETs showed 75% reduction for S. aureus, while only 22% reduction for E. coli. All of the DQAC0 and DQAC8 grafted samples provided an up to 90% reduction for the S. aureus (Fig. 5a). In addition, the antibacterial efficiencies were even better on the chlorinated samples as compared to the non-chlorinated ones. These findings could be attributed to the formation of nitrogen-chloride bonds (i.e. N-halamine functionalities) from the nitrogen-hydrogen ones after chlorination, becoming the strong oxidizing agents against bacteria.
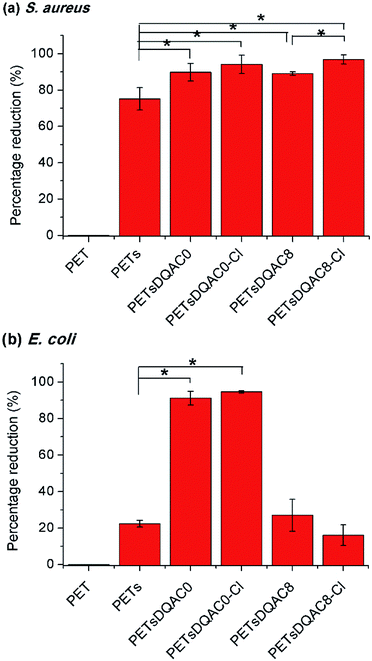 | ||
| Fig. 5 Antimicrobial efficiency of modified nonwoven against S. aureus (a) and E. coli (b) after 10 min of contact time. n = 3, value = mean ± standard deviation. *Represent p < 0.05. | ||
As compared to the reduction of S. aureus, the E. coli was significantly reduced on PETsDQAC0 and its chlorinated one (Fig. 5b). However, there was only about 20% reduction in E. coli for PETsDQAC8 and its chlorinated one. Previous studies in Mathias's group showed that the antibacterial activity against S. aureus increased with an increase in the alkyl chain length for antimicrobial with quaternary ammonium group, whereas the antibacterial activity against E. coli decreased with the increasing alkyl chain length.26 The different biocidal efficacies between Gram-positive and Gram-negative bacterial are the results of their different structures of bacterial membranes.26,27
In order to further examine the biocidal efficacy after chlorination, the PETsDQA0 was challenged with both bacteria with a shorter contact of 3 min. Fig. 6a shows there was 65% and 90% reduction on the non-chlorinated and chlorinated one against S. aureus, respectively. In addition, 30% and 85% reduction on the non-chlorinated and chlorinated one against E. coli, respectively, were noted (Fig. 6b).
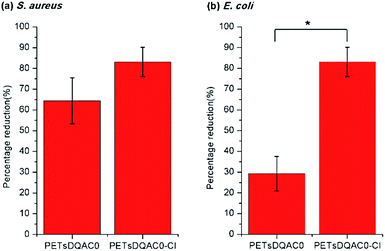 | ||
| Fig. 6 Antimicrobial efficiency of the DQAC0 modified nonwoven against (a) S. aureus and (b) E. coli after 3 min of contact time. n = 3, value = mean ± standard deviation. *Represent p < 0.05. | ||
All these results indicated the N-halamine can further enhance the antimicrobial efficacies by acting synergistically with quaternary ammonium functionalities. Nevertheless, the enhancement was dependent upon the microbial contact duration as well as the type of microbial tested.
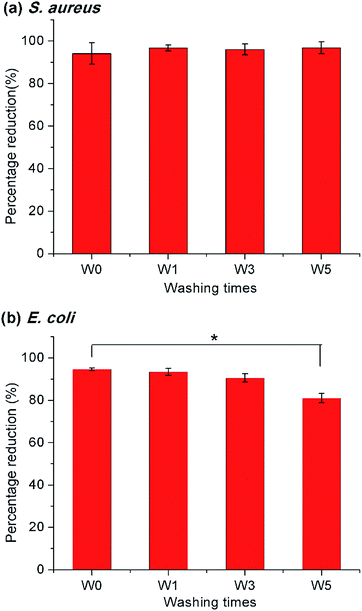
To examine the practical applicability of the modified PET nonwoven, the PETsDQAC0 was undergone repeated washing and chlorination. The antimicrobial testing showed that over 90% reduction for the S. aureus after 5 times of washing and chlorination cycles (Fig. 7a), whereas the antibacterial activity against E. coli slightly decreased after 5 times of washing and chlorination. After washing and chlorination for 5 cycles, the reduction of E. coli dropped to 81% (Fig. 7b). In spite of this, the antibacterial activity maintained on a high level. The results indicated that the surface modification remained stable after repeated washing and chlorine content could be restored through rechlorination. Henceforth, the novel “composite” antimicrobials synthesized here could be of potential for antimicrobial applications in nonwovens.
Conclusions
The antimicrobial agents consisting of cyclic amide group and “dual” quaternary ammonium with different alkyl chain lengths, including C0, C8, and C12, were successfully synthesized and grafted onto the starch-pretreated PET nonwovens via esterification with BTCA. The cyclic amide structure could be easily converted to N-halamine after immersion in a diluted chlorine bleach solution. The change in chemical properties for the grafted PET nonwovens was confirmed by XPS. The antimicrobial efficacies for the grafted PET nonwovens were evaluated against S. aureus and E. coli. The effect of the oxidative chlorine on antibacterial efficiency was also investigated. The DQAC0 on the PET nonwovens exhibited a more hydrophilic characteristics, and a highest antimicrobial activity against both S. aureus and E. coli as compared to the DQAC8 and DQAC12 grafted ones, either before or after chlorination. Systematic investigation on the DQAC0 provided a capable idea as an antibacterial agent for the nonwovens/fabrics used biomedical applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the financial support from the Ministry of Science and Technology, Taiwan under Grant MOST 105-2221-E-006-254, MOST 106-2221-E-006-203-MY3, and MOST 103-2314-B-182-022-MY3. The financial support from the Chang Gung Memorial Hospital (CMRPG 3C1583, CMRPG 3D1283, CMRPG 3H0181) is also acknowledged.References
- L. Rizzello and P. P. Prompa, Chem. Soc. Rev., 2014, 43, 1501–1518 RSC
.
- M. Rai, A. Yadav and A. Gade, Biotechnol. Adv., 2009, 27, 76–83 CrossRef CAS PubMed
.
- E.-R. Kenawy, S. D. Worley and R. M. Broughton, Biomacromolecules, 2007, 8, 1359–1384 CrossRef CAS PubMed
.
- Y. Liu, X. Ren and T. S. Huang, Appl. Surf. Sci., 2014, 231–236 CrossRef CAS
.
- H. B. Kocer, I. Cerkez, S. D. Worley, R. M. Broughton and T. S. Huang, ACS Appl. Mater. Interfaces, 2011, 3, 3189–3194 CrossRef CAS PubMed
.
- J. Yue, P. Zhao, J. Y. Gerasimove, M. Lagemaat, A. Grotenhuis, M. Rustema-Abbing, H. C. Mei, H. J. Busscher, A. Herrmann and Y. Ren, Adv. Funct. Mater., 2015, 25, 6756–6767 CrossRef CAS
.
- R. Gharibi, H. Yeganeh and Z. Abdali, J. Mater. Sci., 2018, 53, 1581–1595 CrossRef CAS
.
- Z. Chen and Y. Sun, Ind. Eng. Chem. Res., 2006, 45, 2634–2640 CrossRef CAS PubMed
.
- F. Hui and C. Debiemme-Chouvy, Biomacromolecules, 2013, 14, 585–601 CrossRef CAS PubMed
.
- A. Dong, Y. J. Wang, Y. Gao, T. Gao and G. Gao, Chem. Rev., 2017, 117, 4806–4862 CrossRef CAS PubMed
.
- M. Tischer, G. Pradel, K. Ohlsen and U. Holzgrabe, ChemMedChem, 2012, 7, 22–31 CrossRef CAS PubMed
.
- X. Lv, C. Liu, S. Song, Y. Qiao, Y. Hu, P. Li, Z. Li and S. Sun, RSC Adv., 2018, 8, 2941–2949 RSC
.
- N. Wilkosz, D. Jamroz, W. Kopec, K. Nakai, S. I. Yusa, M. Wytrwal-Sarna, J. Bednar, M. Nowakowska and M. Kepczynski, J. Phys. Chem. B, 2017, 121, 7318–7326 CrossRef CAS PubMed
.
- I. Cerkez, H. B. Kocer, S. D. Worley, R. M. Broughton and T. S. Huang, React. Funct. Polym., 2012, 72, 673–679 CrossRef CAS
.
- I. Cerkez, H. B. Kocer, S. D. Worley, R. M. Broughton and T. S. Huang, Langmuir, 2011, 27, 4091–4097 CrossRef CAS PubMed
.
- S. Zhang, D. Huang, X. Ren and T. S. Huang, Mater. Sci. Eng., C, 2018, 85, 123–129 CrossRef CAS PubMed
.
- D. Roy, J. S. Knapp, J. T. Guthrie and S. Perrier, Biomacromolecules, 2008, 9, 91–99 CrossRef CAS PubMed
.
- X. Chen, Z. Liu, W. Cao, C. Yong and X. Xing, J. Appl. Polym. Sci., 2015, 132, 42702 Search PubMed
.
- B. J. Hu, X. Q. Chen, Y. Zuo, Z. L. Liu and X. D. Xing, J. Appl. Polym. Sci., 2014, 131 Search PubMed
.
- C. Ning, L. Li, S. Logsetty, S. Ghanbar, M. Guo, W. Ens and S. Liu, RSC Adv., 2015, 5, 93877–93887 RSC
.
- X. Ren, L. Kou, H. B. Kocer, C. Zhu, S. D. Worley, R. M. Broughton and T. S. Huang, Colloids Surf., A, 2008, 317, 711–716 CrossRef CAS
.
- S. C. Huang, C. H. Cheng, Y. Chiu, Y. C. Lin and J. C. Lin, Mater. Sci. Eng., C, 2017, 80, 584–593 CrossRef CAS PubMed
.
- K. P. M. Tang, C. W. Kan and J. T. Fan, Text. Prog., 2014, 46, 1–132 CrossRef
.
- X. Li, Y. Liu, J. Du, X. Ren and T. S. Huang, J. Eng. Fibers Fabr., 2015, 10, 147–154 CAS
.
- T. Brueckner, A. Eberl, S. Heumann, M. Rabe and G. M. Guebitz, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 6435–6443 CrossRef CAS
.
- B. Diman, M. O. Elasri and L. J. Mathias, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 5965–5973 CrossRef
.
- T. J. Piggot, D. A. Holdbrook and S. Khalid, J. Phys. Chem. B, 2011, 115, 13381–13388 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra00094a |
| This journal is © The Royal Society of Chemistry 2019 |