Preparation of thermoplastic polyurethanes using in situ generated poly(propylene carbonate)-diols
Sang Hwan
Lee
,
Anish
Cyriac
,
Jong Yeob
Jeon
and
Bun Yeoul
Lee
*
Department of Molecular Science and Technology, Ajou University, Suwon 443-749, South Korea. E-mail: bunyeoul@ajou.ac.kr; Fax: +82-31-219-2394; Tel: +82-31-219-1844
First published on 7th March 2012
Abstract
Low-molecular-weight poly(propylene carbonate)s bearing –OH groups at both ends (PPC-diols) are prepared by feeding protic chain-transfer agents (1,2-propanediol, terephthalic acid, 2,6-naphthalenedicarboxylic acid, and phenylphosphonic acid) in the CO2/propylene oxide copolymerization catalyzed by a highly active Salen–Co(III) complex tethered by four quaternary ammonium salts. The generated low-molecular-weight PPC-diols are used in situ for the formation of thermoplastic polyurethanes through subsequent feeding of diisocyanates (4,4′-methylenebis(phenyl isocyanate), 1,4-phenylene diisocyanate, and toluene 2,4-diisocyanate). The formation of polyurethanes is confirmed by 1H NMR spectroscopy and GPC studies. By varying the structure of the fed diisocyanate and chain-transfer agent, the glass transition temperature of the polyurethane can be tuned in the range 40–60 °C. A high glass transition temperature of up to 60 °C, which is 20 °C higher than that of high-molecular-weight PPC itself (40 °C), is attained when 2,6-naphthalenedicarboxylic acid (as the chain-transfer agent) and 4,4′-methylenebis(phenyl isocyanate) are employed. In addition, flame-retarding polyurethanes are generated by using an organophosphorus-based chain-transfer agent.
Introduction
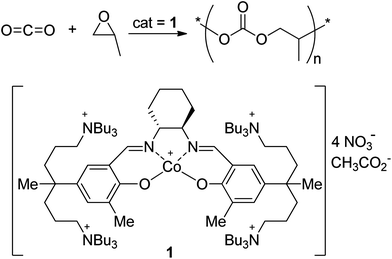
Polyurethanes (PUs), which are formed by the reaction of diisocyanate or polyisocyanate with a low-molecular-weight diol or polyol, are bulk polymers, the annual production of which exceeds 10 million tons. Typical applications of these polymers are in foams, adhesives and coatings, and synthetic fibers. The low-molecular-weight diols most commonly used in PU manufacture are polyether-diols, which are prepared by the ring-opening polymerization of epoxide such as propylene oxide. Polyester-based diols are also used in industry. Another attractive class of diols is poly(alkylene carbonate)-diols.1,2 The merits of PUs prepared using poly(alkylene carbonate)-diols are good hydrolytic stability compared with polyester-based polyurethanes, improved antistatic effect, and improved biocompatibility.3,4 The poly(alkylene carbonate)-diol is prepared by the condensation reaction of an α,ω-alkanediol (such as 1,6-hexanediol) with diethyl carbonate or ethylene carbonate.5,6 However, both components needed in the preparation of poly(alkylene carbonate)-diol are expensive, so its usage is limited in industry.Poly(alkylene carbonate) can be prepared by CO2/epoxide copolymerization (Scheme 1), although the structure is different from those prepared by the condensation reaction of 1,6-hexanediol with diethyl carbonate.7–11 The catalytic system for the CO2/epoxide copolymerization has been improved since its first discovery by Inoue.12–14 Recently, a highly efficient catalyst (1) was discovered, which shows a high turnover frequency (TOF, 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1), producing a high-molecular-weight polymer (Mn up to 300
000 h−1), producing a high-molecular-weight polymer (Mn up to 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) with high selectivity (>99%).15,16 Catalyst 1 can be prepared on a large scale.17,18 A pilot plant for a continuous process was constructed for the commercialization of poly(propylene carbonate) (PPC) with 1, and various chain architectures were realized to widen its potential applications.19,20
000) with high selectivity (>99%).15,16 Catalyst 1 can be prepared on a large scale.17,18 A pilot plant for a continuous process was constructed for the commercialization of poly(propylene carbonate) (PPC) with 1, and various chain architectures were realized to widen its potential applications.19,20
 | ||
| Scheme 1 | ||
When a protic compound such as an alcohol or carboxylic acid is deliberately added in the CO2/epoxide copolymerization, rapid reversible chain-transfer reactions occur between the chain-growing anionic site and the protic compound, resulting in uniform chain growth from all the added protic molecules with chain end that finishes with an –OH group. When the chain-transfer agent is a diol or di(carboxylic acid), the generated polymer is the poly(alkylene carbonate)-diol. The chain length is controlled by the amount of added protic compound. This kind of polymerization is generally termed “immortal polymerization”.21 In fact, immortal polymerization was realized in the CO2/propylene oxide (PO) copolymerization catalyzed by zinc-based bimetallic catalysts for the preparation of a low-molecular-weight poly(propylene carbonate)-diol (PPC-diol).22 However, the low-molecular-weight polymer is easily degraded to propylene carbonate, possibly due to the facile back-biting reaction in the presence of the catalyst residue.23,24 Immortal polymerization was also reported with the Salen–Co(III) catalyst in the presence of an alcohol compound.25,26 Catalyst 1 also works very well in the presence of a sufficiently high amount of alcohol or carboxylic acid, allowing precise control of the molecular weight, the variation of chain topology, and the formation of various types of block copolymers.18,27 In this work, we demonstrate the formation of a variety of thermoplastic PUs in a one-pot process directly after CO2/PO copolymerization using various chain-transfer agents. A drawback of PPC itself is its low thermal stability, i.e., an inappropriate glass transition temperature (Tg) of 40 °C.28,29 One of the PUs prepared in this work shows a high Tg of 60 °C. A nonflammable PU is also prepared in this work.
Experimental section
General remarks
CO2 gas (99.999% purity) was dried through storage in a column of molecular sieves 3A at a pressure of 30 bar. Propylene oxide (PO) was dried by stirring over CaH2, and was then vacuum-transferred to a reservoir. The 1H NMR (400 MHz) spectra were recorded on a Varian Mercury Plus 400 instrument. The gel permeation chromatograms (GPCs) were obtained in THF at 35 °C using a Waters Millennium apparatus with polystyrene standards. The Tg data were determined from a second heating at a heating rate of 10 °C min−1 on DSC (differential scanning calorimetry) using a Thermal Analysis Q10 instrument. Catalyst (1) was synthesized by the previously reported method.18Typical procedure for CO2/PO copolymerization and formation of polyurethane
A bomb reactor was assembled inside a glove box after charging with catalyst 1 (6.0 mg, 3.6 μmol), PO (10.4 g, 179 mmol), and terephthalic acid (238 mg, 1.43 mmol). 4,4′-Methylenebis(phenyl isocyanate) (MDI) (358 mg, 1.43 mmol) was dissolved in PO (10.4 g, 180 mmol) and added to a reservoir placed at the top of the bomb reactor. After being charged with CO2 gas (25 bar pressure, room temperature), the reactor was immersed in an oil bath at 70 °C. The solution temperature reached ∼70 °C in 50 min, and the pressure started to drop. The polymerization was performed for 1 h excluding the heating time, and a pressure drop of 3–4 bar was observed. In a separate experiment, formation of a negligible amount of polymer was observed during the heating time of 50 min. The reactor was cooled to room temperature through immersion in an ice bath. The diisocyanate solution in the reservoir at the top of the reactor was charged by opening the valve under the CO2 pressure. The reactor was again immersed in the oil bath (70 °C) and stirred for 1 h. After the reactor was cooled to room temperature using an ice bath, the CO2 gas was released. The viscous polymer solution was filtered through a short pad of silica gel and washed with methylene chloride (10 mL), and a colorless solution was obtained. All volatiles were removed using a rotary evaporator to obtain a solid residue. 1H NMR spectroscopy was used to calculate the selectivity and to identify the carbonate and urethane linkages. In the 1H NMR spectra, propylene carbonate was observed with less than 10% selectivity; this was removed completely by evacuation in a vacuum oven at 150 °C for several hours.Results and discussion
The low-molecular-weight PPC-diols were prepared by the addition of a protic compound (HO–A–OH) such as 1,2-propanediol (2 in Chart 1), terephthalic acid (3), 2,6-naphthalenedicarboxylic acid (4), or phenylphosphonic acid (5) in the CO2/PO copolymerization catalyzed by 1 (Scheme 2).18 The [HO–A–OH]/[1] ratio was fixed to 400. In this immortal polymerization, the polymer chains grow uniformly not only from all the –OH groups in the system but also from the four nitrate anions and the acetate anion pertaining to 1. The polymer chains grown from the nitrate or acetate anions bear a nitrate or acetate moiety at one end, while the other bears a hydroxyl (–OH) group. The polymer chains grown from HO–A–OH bear –OH groups at both ends, that is, they are PPC-diol chains. Under the condition of [HO–A–OH]/[1] = 400, the chains grown from HO–A–OH overwhelm those grown from the nitrate or acetate anions, and so most of the chains are PPC-diol chains. In this copolymerization, in the presence of a significant amount of HO–A–OH, the attained turnover frequency (TOF) is ∼10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1 (∼610 g PPC/g cat h), which is roughly half that attained in the absence of HO–A–OH (∼20
000 h−1 (∼610 g PPC/g cat h), which is roughly half that attained in the absence of HO–A–OH (∼20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). Water acts as a catalyst poison, and some residual water in the protic chain-transfer agent may be responsible for this lowering of the activity. When [HO–A–OH]/[1] is increased to 450, the TOF is lowered further from 10
000). Water acts as a catalyst poison, and some residual water in the protic chain-transfer agent may be responsible for this lowering of the activity. When [HO–A–OH]/[1] is increased to 450, the TOF is lowered further from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 8900 h−1 (Table 1, entries 2 and 3). The measured molecular weight of the PPC-diol deviates slightly from the calculated absolute value (= {102.13 × TON}/{[HO–A–OH]/[1] + 5}); this is due to the use of the polystyrene standard in the molecular weight measurement (entry 1).18
000 to 8900 h−1 (Table 1, entries 2 and 3). The measured molecular weight of the PPC-diol deviates slightly from the calculated absolute value (= {102.13 × TON}/{[HO–A–OH]/[1] + 5}); this is due to the use of the polystyrene standard in the molecular weight measurement (entry 1).18
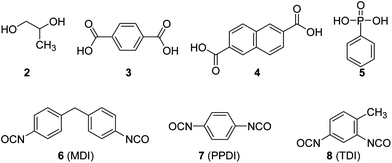 | ||
| Chart 1 Chemical structures of the chain transfer agents and diisocyanates. | ||
 | ||
| Scheme 2 | ||
| Entry | A(OH)2 | B(NCO)2 | [A(OH)2]/[1] | TON | Conversion (%) | Selectivity (%)b | Calculated Mn of PPC-diolc | Measured Mn of PUd | PDI | T g e/°C |
|---|---|---|---|---|---|---|---|---|---|---|
a
CO
2
/PO copolymerization conditions: PO (10.4 g, 180 mmol), 1 (6.0 mg, 3.6 μmol), [PO]/[1] = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, CO2 (30 bar), temperature (70 °C), 1 h.
b Selectivity for formation of polymer over propylene carbonate.
c Calculated using the equation of “Mn = {TON × 102.13}/{[HO–A–OH]/[1] + 5}”.
d Determined by GPC using a polystyrene standard.
e Glass transition temperature measured by DSC.
f The measured molecular weight of the PPC-diol. 000, CO2 (30 bar), temperature (70 °C), 1 h.
b Selectivity for formation of polymer over propylene carbonate.
c Calculated using the equation of “Mn = {TON × 102.13}/{[HO–A–OH]/[1] + 5}”.
d Determined by GPC using a polystyrene standard.
e Glass transition temperature measured by DSC.
f The measured molecular weight of the PPC-diol.
|
||||||||||
| 1 | 2 | — | 400 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
21 | 94 | 2600 | 4100f | 1.05 | 28 |
| 2 | 2 | 6 | 400 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
20 | 95 | 2500 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
4.25 | 47 |
| 3 | 2 | 6 | 450 | 8900 | 18 | 96 | 2000 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
3.68 | 49 |
| 4 | 2 | 7 | 400 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
22 | 93 | 2700 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
4.07 | 44 |
| 5 | 2 | 8 | 400 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
27 | 92 | 3400 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
4.67 | 40 |
| 6 | 3 | 6 | 400 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
27 | 97 | 3400 | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
3.51 | 49 |
| 7 | 3 | 7 | 400 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
22 | 95 | 2700 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
3.48 | 47 |
| 8 | 3 | 8 | 400 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
24 | 94 | 3000 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
3.91 | 44 |
| 9 | 4 | 6 | 400 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
26 | 93 | 3300 | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
3.69 | 50 |
| 10 | 4 | 6 | 500 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
20 | 91 | 2100 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
3.11 | 52 |
| 11 | 4 | 6 | 600 | 7400 | 15 | 96 | 1300 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
4.83 | 60 |
| 12 | 4 | 7 | 400 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
26 | 95 | 3300 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
3.57 | 45 |
| 13 | 4 | 8 | 400 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
23 | 93 | 2900 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
3.74 | 43 |
| 14 | 5 | 6 | 400 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
23 | 97 | 2900 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
3.57 | 44 |
| 15 | 5 | 7 | 400 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
24 | 96 | 3000 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
2.58 | 41 |
| 16 | 5 | 8 | 400 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
28 | 92 | 3500 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.93 | 41 |
In the previous preliminary studies, thermoplastic PUs were prepared after isolating PPC-diol in a CO2/epoxide copolymerization carried out in the presence of a chain-transfer agent.18 An equimolar amount of diisocyanate and PPC-diol was mixed in THF solution, and the two components were reacted at 100 °C after the removal of THF. Through this method, an increase in Mn from 2200 of PPC-diol to 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 was observed because of the formation of urethane linkages. However, the molecular weight was not sufficiently high, possibly because of stoichiometry imbalance between the –OH and –NCO groups. In this kind of step reaction polymerization, the stoichiometry balance between the two reacting functional groups should be controlled precisely in order to obtain a high-molecular-weight polymer. Contamination by water may break the stoichiometry balance, resulting in the formation of unsatisfactorily low-molecular-weight PUs and, furthermore, causing variation in the molecular weight by batch.
000 was observed because of the formation of urethane linkages. However, the molecular weight was not sufficiently high, possibly because of stoichiometry imbalance between the –OH and –NCO groups. In this kind of step reaction polymerization, the stoichiometry balance between the two reacting functional groups should be controlled precisely in order to obtain a high-molecular-weight polymer. Contamination by water may break the stoichiometry balance, resulting in the formation of unsatisfactorily low-molecular-weight PUs and, furthermore, causing variation in the molecular weight by batch.
In this work, the urethane linkage is formed without isolation of the PPC-diol as was disclosed in the previous work.30 Because water acts as a catalyst poison in the CO2/epoxide copolymerization, the copolymerization conditions are severely anhydrous. So, we can eliminate the possibility of water contamination if we carry out the urethane-bond-forming reaction directly after the CO2/epoxide copolymerization without the isolation step of PPC-diol. After the CO2/epoxide copolymerization, a PO solution with equimolar amounts of diisocyanate is fed in one pot using the CO2 pressure, and the solution is further stirred at 70 °C for 1 h under the pressure of CO2. At a later stage in the urethane bond formation, CO2/epoxide copolymerization does not occur; this is inferred from the observation that no pressure drop occurs, and there is no increase in the weight of the isolated polymer. Typically, the formation of a urethane linkage requires a catalyst such as dibutyltin dilaurate or an amine base, e.g., 1,4-diazabicyclo[2.2.2]octane.31,32 It has been reported that an increase in the amine basicity results in an increase in the rate of urethane bond formation.33 At the end of the CO2/epoxide copolymerization, catalyst 1 becomes a cobalt complex possessing five alkoxide or carbonate anions, which are derived from the four nitrate and acetate anions. That is, at the end of the copolymerization, the polymerization solution containing the PPC-diol is basic, which may facilitate the formation of urethane linkages. When [A(OH)2]/[1] = 500, the mole ratio of alkoxide anion per –OH group on PPC-diol is 0.5 mol%, sufficiently high to catalyse the urethane-bond formation. After PU formation, the catalyst residue is efficiently removed by filtration through a short pad of silica gel, as in the preparation of PPC itself. The yellow catalyst residue is collected on the top layer of the silica gel pad, while the polymers pass through it, giving a colorless polymer solution. PPC is easily depolymerized to propylene carbonate in the presence of catalyst residue, but is thermally stable up to 190 °C once the catalyst has been removed completely.24 PUs prepared in this way are also thermally stable up to 190 °C, as inferred from the intact GPC curves after thermal treatment.
Actually, a high-molecular-weight polyurethane is formed in this way; the low-molecular-weight PPC-diol (measured Mn, 4100), which is prepared by feeding 1,2-propanediol (2), becomes a high molecular weight PU (Mn, 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800; Mw, 161
800; Mw, 161![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) upon feeding with 4,4′-methylenebis(phenyl isocyanate) (MDI, 6) in the second stage (entries 1 and 2 in Table 1 and Fig. 1). Formation of low-molecular-weight PPC-diol in the feeding of chain transfer agents was well established and the molecular-weight distribution of the formed PPC-diol is very narrow (Mw/Mn, 1.05).18,27 However, the molecular-weight distribution of the formed PU is rather broad (Mw/Mn, 4.25). When 1,4-phenylene diisocyanate (PPDI, 7 in Chart 1) is fed instead of MDI (entry 4), the molecular weight is still fairly high (Mn, 28
000) upon feeding with 4,4′-methylenebis(phenyl isocyanate) (MDI, 6) in the second stage (entries 1 and 2 in Table 1 and Fig. 1). Formation of low-molecular-weight PPC-diol in the feeding of chain transfer agents was well established and the molecular-weight distribution of the formed PPC-diol is very narrow (Mw/Mn, 1.05).18,27 However, the molecular-weight distribution of the formed PU is rather broad (Mw/Mn, 4.25). When 1,4-phenylene diisocyanate (PPDI, 7 in Chart 1) is fed instead of MDI (entry 4), the molecular weight is still fairly high (Mn, 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900; Mw, 118
900; Mw, 118![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), but not as high as that of the PU formed with MDI feeding. Through the addition of toluene 2,4-diisocyanate (TDI) (entry 5), the molecular weight is further decreased (Mn, 23
000), but not as high as that of the PU formed with MDI feeding. Through the addition of toluene 2,4-diisocyanate (TDI) (entry 5), the molecular weight is further decreased (Mn, 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700; Mw, 110
700; Mw, 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). The lower molecular weights may be attributed to the lower reactivity of PPDI and TDI compared with MDI. During the urethane-bond-forming step, some part of the polymer sticks to the reactor wall and cannot participate in further reaction, consequently resulting in some broadness and fluctuation in the data of molecular weight distribution.
000). The lower molecular weights may be attributed to the lower reactivity of PPDI and TDI compared with MDI. During the urethane-bond-forming step, some part of the polymer sticks to the reactor wall and cannot participate in further reaction, consequently resulting in some broadness and fluctuation in the data of molecular weight distribution.
 | ||
| Fig. 1 GPC curve (A, entry 1) of PPC-diol prepared using 1,2-propanediol as a chain transfer agent and those of PUs prepared by feeding TDI (B, entry 5), PPDI (C, entry 4), and MDI (D, entry 2). | ||
The Tg of the low-molecular weight PPC-diol is 28 °C, but this changes to 47 °C after urethane bond formation with MDI (6); this Tg is higher than that of high-molecular-weight PPC itself (40 °C). One of the drawbacks of PPC in terms of finding broader applications is its inappropriately low Tg of 40 °C. Not only by the addition of more 1,2-propanediol, but also by lowering the TON, PPC-diol of lower molecular weight can be generated (calculated absolute Mn, 2600), from which PU containing higher proportion of rigid MDI units can be prepared (entry 3). The PU generated here exhibits a high Tg value of 49 °C. When PPDI is fed instead of MDI under otherwise identical conditions, a slightly lower Tg of 44 °C is observed for the generated PU (entry 4). The lower Tg of 44 °C compared with that of the PU prepared using MDI (47 °C) is due to a small quantity of rigid benzene rings being contained in the PU prepared using PPDI. When TDI is employed in urethane bond formation, there is no increase in Tg (40 °C) compared with the high-molecular-weight PPC itself (entry 5).
With the aim of increasing the Tg value of PU, terephthalic acid (3) bearing a rigid benzene ring is employed as the chain-transfer agent (entries 6–8). The same trend is observed as in the urethane-linkage-formation reaction from the PPC-diol prepared using 1,2-propanediol. The PU prepared by feeding MDI shows the highest molecular weight (Mn, 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800; Mw, 171
800; Mw, 171![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and the highest Tg (49 °C). Using PPDI and TDI, PUs of lower molecular weight are generated (Mn, 21
000) and the highest Tg (49 °C). Using PPDI and TDI, PUs of lower molecular weight are generated (Mn, 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), and the Tg values (47 and 44 °C, respectively) are not as high as that of the PU obtained using MDI.
000), and the Tg values (47 and 44 °C, respectively) are not as high as that of the PU obtained using MDI.
Fig. 2 shows the 1H NMR spectra of PUs prepared by reacting MDI (A, entry 6) or PPDI (B, entry 7) and PPC-diol prepared by feeding terephthalic acid as a chain-transfer agent. In the spectrum of A, the signal at 8.10 ppm is assigned to the aromatic protons on the terephthalic acid, while the two signals at 7.29 and 7.09 ppm are assigned to the aromatic protons on MDI. The integration values of the three signals are almost the same, in agreement with the stoichiometric balance between the terephthalic acid and MDI. The broad signal at 6.85 ppm can be assigned to N–H. The signals at 5.23–4.90 ppm are assigned to the methine (CH(CH3)) of PO, while those at 4.52–4.00 ppm are due to the methylene (CH2) of PO. The integration value ratio between these PO-related signals and the terephthalic-acid- or MDI-related signals is also in agreement with the calculated values. That is, the integration value ratio between the signals at 8.10 and 5.23–4.90 should be 4.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33.5 by calculation, and the measured ratio is 4.0
33.5 by calculation, and the measured ratio is 4.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 in the spectrum shown in Fig. 2(A). In the spectrum of Fig. 2(B), the PPDI signal is observed at 7.33 ppm, for which the integration value is also almost the same as that of the terephthalic acid signal at 8.10 ppm.
35 in the spectrum shown in Fig. 2(A). In the spectrum of Fig. 2(B), the PPDI signal is observed at 7.33 ppm, for which the integration value is also almost the same as that of the terephthalic acid signal at 8.10 ppm.
 | ||
| Fig. 2 1H NMR spectra of PUs prepared by reacting MDI (A, entry 6) or PPDI (B, entry 7) with PPC-diol prepared by feeding terephthalic acid as a chain transfer agent. | ||
With the aim of increasing Tg further, 2,6-naphthalenedicarboxylic acid (4) bearing a more rigid naphthalene ring is employed as the chain-transfer agent (entries 9–13). However, Tg is not increased significantly by employing 2,6-naphthalenedicarboxylic acid instead of terephthalic acid under identical conditions (entry 6 versus 9). By preparing a lower-molecular-weight PPC-diol through the addition of more 2,6-naphthalenedicarboxylic acid, a PU containing more 2,6-naphthalenedicarboxylate and MDI units is obtained, which shows a high Tg of 60 °C (entry 11). Fig. 3 shows the DSC thermograms of poly(propylene carbonate) itself (A) and of PUs prepared by reacting MDI (B and C, entries 9 and 11, respectively) with PPC-diol prepared by feeding 2,6-naphthalenedicarboxylic acid as the chain-transfer agent.
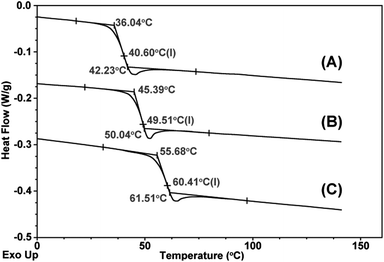 | ||
| Fig. 3 DSC thermograms of poly(propylene carbonate) (A) and of PUs prepared by reacting MDI (B and C, entries 9 and 11, respectively) with PPC-diol prepared by feeding 2,6-naphthalenedicarboxylic acid as the chain-transfer agent. | ||
With the aim of preparing a flame-retarding PU, phenylphosphonic acid (5) was employed as the chain-transfer agent (entries 14–16).33 Again, the PU prepared by feeding MDI shows a higher molecular weight (Mn, 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700; Mw, 80
700; Mw, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200) and higher Tg (44 °C) than those obtained by feeding PPDI and TDI (Mn, 21
200) and higher Tg (44 °C) than those obtained by feeding PPDI and TDI (Mn, 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000; Tg, 41 °C). The PU prepared using phenylphosphonic acid is not flammable because of the presence of flame-retarding phosphorus atoms.34 The polymer sample was ignited with a gas-lighter, but the flame was quenched within a few seconds when the gas-lighter was removed (Fig. 4). After quenching of the fire, the burned part of the polymer was covered with a residual black coating. The mechanism of this flame retardation has been already proposed.35 The organophosphorus compound is changed to phosphoric acid during burning, and then the polymer is esterified and dehydrated to form a protective black char layer that prevents the polymer from coming into contact with the flame and oxygen.
000; Tg, 41 °C). The PU prepared using phenylphosphonic acid is not flammable because of the presence of flame-retarding phosphorus atoms.34 The polymer sample was ignited with a gas-lighter, but the flame was quenched within a few seconds when the gas-lighter was removed (Fig. 4). After quenching of the fire, the burned part of the polymer was covered with a residual black coating. The mechanism of this flame retardation has been already proposed.35 The organophosphorus compound is changed to phosphoric acid during burning, and then the polymer is esterified and dehydrated to form a protective black char layer that prevents the polymer from coming into contact with the flame and oxygen.
 | ||
| Fig. 4 Flammability of PU prepared by reacting PPDI with the PPC-diol prepared using phenylphosphonic acid as the chain-transfer agent (entry 15). The pictures were taken at 0.8 second intervals from a video clip. | ||
Conclusion
Low-molecular-weight PPC-diols were prepared by employing various protic chain-transfer agents in the CO2/PO copolymerization catalyzed by a highly active Salen–Co(III) catalyst tethered by quaternary ammonium salts. The low-molecular-weight PPC-diols were applied to the formation of TPU by in situ reaction with diisocyanate. After the polymerization, the catalyst was completely removed by passing the polymer solution through a short pad of silica gel. The formation of polyurethanes was confirmed by 1H NMR spectroscopy and GPC studies. By incorporating aromatic units in the polymer backbone of the PUs, their thermal properties can be improved. Glass transition temperatures of up to 60 °C were attained; this is 20 °C higher than that of PPC itself. In addition, by using an organophosphorus compound bearing –OH as the chain-transfer agent, a non-flammable PU was obtained.Acknowledgements
This work was supported by the Inter-Metropolitan Cooperation Development funded by the Presidential Committee on Regional Development and by the National Emergency Management Agency.Notes and references
- J. C. Salamone, Polymeric Materials Encyclopedia, CRC Press, 1996 Search PubMed.
- N. Cao, M. Pegoraro, F. Severini, L. Di Landro, G. Zoia and A. Greco, Polymer, 1992, 33, 1384–1390 CrossRef CAS.
- M. Szycher, D. Dempsey and A. Edwards, US Pat., 5863627, 1999.
- M. O. Myers, US Pat., 4931486, 1990.
- P. A. Gunatillake, G. F. Meijs, S. J. McCarthy, R. Adhikari and N. Sherriff, J. Appl. Polym. Sci., 1998, 69, 1621–1633 CrossRef CAS.
- R. A. Grey, US Pat., 5171830, 1992.
- G. W. Coates and D. R. Moore, Angew. Chem., Int. Ed., 2004, 43, 6618–6639 CrossRef CAS.
- D. J. Darensbourg, Chem. Rev., 2007, 107, 2388–2410 CrossRef CAS.
- G. A. Luinstra, Polym. Rev., 2008, 48, 192–219 CrossRef CAS.
- M. R. Kember, A. Buchard and C. K. Williams, Chem. Commun., 2011, 47, 141–163 RSC.
- S. Klaus, M. W. Lehenmeier, C. E. Anderson and B. Rieger, Coord. Chem. Rev., 2011, 255, 1460–1479 CrossRef CAS.
- S. Inoue, H. Koinuma and T. Tsuruta, J. Polym. Sci., Part B: Polym. Lett., 1969, 7, 287 CrossRef CAS.
- S. I. Vagin, R. Reichardt, S. Klaus and B. Rieger, J. Am. Chem. Soc., 2010, 132, 14367–14369 CrossRef CAS.
- K. Nakano, K. Kobayashi and K. Nozaki, J. Am. Chem. Soc., 2011, 133, 10720–10723 CrossRef CAS.
- S. Sujith, J. K. Min, J. E. Seong, S. J. Na and B. Y. Lee, Angew. Chem., Int. Ed., 2008, 47, 7306–7309 CrossRef CAS.
- S. J. Na, S. Sujith, A. Cyriac, B. E. Kim, J. Yoo, Y. K. Kang, S. J. Han, C. Lee and B. Y. Lee, Inorg. Chem., 2009, 48, 10455–10465 CrossRef CAS.
- J. Min, J. E. Seong, S. J. Na, A. Cyriac and B. Y. Lee, Bull. Korean Chem. Soc., 2009, 30, 745–748 CrossRef CAS.
- A. Cyriac, S. H. Lee, J. K. Varghese, E. S. Park, J. H. Park and B. Y. Lee, Macromolecules, 2010, 43, 7398–7401 CrossRef CAS.
- A. Cyriac, S. H. Lee and B. Y. Lee, Polym. Chem., 2011, 2, 950–956 RSC.
- J. E. Seong, S. J. Na, A. Cyriac, B.-W. Kim and B. Y. Lee, Macromolecules, 2010, 43, 903–908 CrossRef CAS.
- S. Inoue, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 2861–2871 CrossRef CAS.
- J. Kuyper, P. W. Lednor, G. A. Pogany, EP Pat., 222453, 1987.
- B. Liu, L. Chen, M. Zhang and A. Yu, Macromol. Rapid Commun., 2002, 23, 881–884 CrossRef CAS.
- J. K. Varghese, S. J. Na, J. H. Park, D. Woo, I. Yang and B. Y. Lee, Polym. Degrad. Stab., 2010, 95, 1039–1044 CrossRef CAS.
- K. Nakano, T. Kamada and K. Nozaki, Angew. Chem., Int. Ed., 2006, 45, 7274–7277 CrossRef CAS.
- S. D. Allen, G. W. Coates, A. E. Cherian, C. A. Simoneau, A. A. Gridnev and J. J. Farmer, US Pat., Application 2010–994544, 2010.
- S. H. Lee, A. Cyriac, J. Y. Jeon and B. Y. Lee, Clean Technology, 2011, 17, 244–249 Search PubMed.
- K. Nakano, S. Hashimoto, M. Nakamura, T. Kamada and K. Nozaki, Angew. Chem., Int. Ed., 2011, 50, 4868–4871 CrossRef CAS.
- B. Y. Lee and A. Cyriac, Nat. Chem., 2011, 3, 505–507 CrossRef CAS.
- A. Cyriac, S. H. Lee, J. K. Varghese, J. H. Park, J. Y. Jeon, S. J. Kim and B. Y. Lee, Green Chem., 2011, 13, 3469–3475 RSC.
- A. Farkas and K. G. Flynn, J. Am. Chem. Soc., 1960, 82, 642–645 CrossRef CAS.
- M. Ratier, D. Khatmi and J. G. Duboudin, Appl. Organomet. Chem., 1992, 6, 293–296 CrossRef CAS.
- G. Wegener, M. Brandt, L. Duda, J. Hofmann, B. Klesczewski, D. Koch, R.-J. Kumpf, H. Orzesek, H.-G. Pirkl, C. Six, C. Steinlein and M. Weisbeck, Appl. Catal., A, 2001, 221, 303–335 CrossRef CAS.
- E. D. Weil and S. Levchik, J. Fire Sci., 2004, 22, 25–40 CrossRef CAS.
- B. Martel, J. Appl. Polym. Sci., 1988, 35, 1213–1226 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
