Open Access Article
Open Access ArticleOrganosolv biorefinery: resource-based process optimisation, pilot technology scale-up and economics
Giorgio
Tofani
 *a,
Edita
Jasiukaitytė-Grojzdek
*a,
Edita
Jasiukaitytė-Grojzdek
 a,
Miha
Grilc
a,
Miha
Grilc
 ab and
Blaž
Likozar
ab and
Blaž
Likozar
 *acde
*acde
aDepartment of Catalysis and Chemical Reaction Engineering, National Institute of Chemistry, Hajdrihova 19, SI-1001 Ljubljana, Slovenia. E-mail: Giorgio.Tofani@ki.si; Blaz.Likozar@ki.si
bUniversity of Nova Gorica, Vipavska 13, SI-5000 Nova Gorica, Slovenia
cPulp and Paper Institute, Bogišićeva 8, 1000 Ljubljana, Slovenia
dFaculty of Polymer Technology, Ozare 19, SI-2380 Slovenj Gradec, Slovenia
eFaculty of Chemistry and Chemical Technology, University of Ljubljana, Večna pot 113, SI-1000 Ljubljana, Slovenia
First published on 21st November 2023
Abstract
This tutorial review aims to describe the status of the scaling up of organosolv treatment. It is a process where various lignocellulosic materials are fractionated, selective depolymerization mechanisms are catalyzed, and their main components (polysaccharides, lignin and extractives) can be extracted, separated and isolated using liquid organic solvents such as alcohols, ketones and proton-donating acid molecules. Organosolv fractionation can be applied to several renewable biomasses, allows the production of pure species systems to prepare valuable chemicals, polymers and biomaterial compositions with a related environmental impact, lower than that of classical industrial plants, and optimizes the resource carbon efficiency. However, the high energy consumption for the recovery after dissolution, input costs and feedstock flexibility robustness are slowing down the piloting of commercial operations. As a critical indicator evaluation, a summary of reasons why engineering organosolv is still extremely interesting, together with an overview of the most important organosolv technologies, describing current equipment scale range economics, limitations and market research opportunities, is presented in detail. A variety of sources (wood, straw, bagasse, wastes…), media (water, methanol, ethanol, formates, acetates…) and products (biogas, bioethanol, (nano)cellulose, glucose, furans…) are comparatively benchmarked. Existing (model) validated, demonstrational or patented configurations are collected, listing strengths as well as challenges.
1. Introduction
Over the years, academic institutions, research centres and industries have been concentrating on finding green and sustainable technologies and raw materials to replace fossil resources to generate energy, chemicals, and materials (i.e., plastics), reducing environmental pollution and developing a real and practicable circular economy. A valuable alternative is lignocellulosic biomass, mainly composed of wood and grass, and the resulting wastes generated by industrial production and end-life products, such as recycled paper and agricultural residues. These sources of lignocellulose are mainly used to produce energy, sugars and paper products.1–6Lignocellulosic biomasses are available in high quantities on the Earth (annual output of around 170 billion tons per year (ref. 7)), and their use shows a low carbon footprint.8,9 However, lignocellulose is not beneficial only to produce commodities such as sugars. The reason is correlated with the heterogeneous composition of such a kind of biomass. It contains lignin (a crosslinked phenolic polymer), cellulose (a linear polymer composed of glucose), hemicellulose (a branched heteropolymer composed of different sugar units) and extractives (a heterogeneous mixture of various compounds such as fatty acids and rosin). These biopolymers and chemicals can be recovered and used to prepare a wide range of biomaterials and high-value chemicals.10–15
The first and primary step to separate the different components of lignocellulose is the pulping process to break the biomass's rigid structure. The most common methods are the ones used already in the paper industry, called kraft and sulphite processes.16 However, these treatments were designed to obtain cellulose fibres for papermaking, ignoring the loss and degradation of lignin and hemicelluloses. Moreover, lignin can be removed by acid and enzymatic treatments during the production of sugars.2 Lignin and hemicelluloses can be recovered from the resulting waste streams, but their quality is low due to the high depolymerisation rate and contamination by, for example, sulphur and the high content of salts. Therefore, the development of biorefineries for the appropriate processing of lignocellulosic raw materials is crucial to achieve a circular economy. They will offer a more eco-sustainable solution with a fractionation process designed with the target of extraction and valorisation of all the components of lignocellulose, looking to have a “zero-waste” approach. All the streams generated must be considered a valuable source of chemicals, energy and biomaterials.
In this manuscript, the focus will be on the organosolv process. It is a fractionation treatment where organic solvents such as ethanol, acetone, formic acid and acetic acid are combined or not with water and catalysts (i.e., sulfuric acid) at temperatures above 100 °C to separate lignin, cellulose and hemicellulose.17 Afterwards, the biopolymers are recovered by filtration, precipitation and evaporation processes. In particular, it was found that the organosolv processes allow the extraction of lignin having higher purity than lignin obtained using other pulping processes.18 The reasons why the organosolv process is highly interesting are described in the next section.
Before continuing in this description of the organosolv process, it is essential to underline that the fractionation processes where ionic liquids and deep eutectic mixtures are used as solvents were not considered in order to separate “classical” organic solvents from “designer” solvents as described in the literature.19,20
2. Why is organosolv a valuable process?
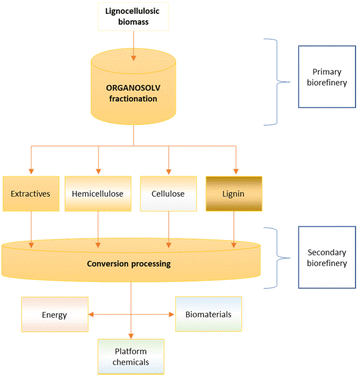
The possibility to select the organic solvents, typically available in the academic and industrial sectors, and tune the reaction conditions, such as time, temperature and the ratio of organic solvent/water, allows using the organosolv process on different types of lignocellulosic biomasses such as wood (e.g. bark, sawdust and logs), grass (i.e. Miscanthus giganteous), agricultural residues (e.g. rice husk and corn stover) and waste paper (e.g. cardboard).17,19–21A list of lignocellulosic biomasses treated using organosolv fractionation is reported in Table 1. The main advantage of this methodology is that all the main components from lignocellulose, extractives, hemicelluloses, cellulose and lignin can be extracted and valorised to produce chemicals and biomaterials. In particular, highly pure lignin can be isolated. The classical methods (kraft and sulphite processes) strongly modify the chemical structure of the lignin by condensation reactions and insertion of sulphur.16 Soda and hydrolysis processes show higher contents of ashes and sugars in the isolated lignin, reducing the purity.18 Consequently, the organosolv process can be used to develop biorefineries using it as a fractionation stage. A simplified scheme of the organosolv process applied in a biorefinery is shown in Fig. 1.| Lignocellulosic source | Organosolv solvent | Extractive applications | Hemicellulose applications | Cellulose applications | Lignin applications | Ref.e | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a It is not specified, but Fraunhofer CBP technology is based on ethanol/water organosolv process. b No other information is provided. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Corn stover | Gama-butyrolactone/water aluminium sulfate octadecahydrate | — | — | — | — | 10 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Old corrugated cardboard | Formic acid/water | — | — | Dissolving pulp | — | 21 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bark of Norway spruce | Ethanol/water, sulfuric acid | — | — | — | — | 22 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spent coffee grounds | Methanol/hexane, sulfuric acid | Fatty acid methyl esters | Biogas | Biogas | — | 23 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Exhausted olive pomace | Ethanol/water, sulfuric acid | Omega-3 fatty acids | — | — | — | 24 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Beechwood | Ethanol/water | — | Isobutanol | — | — | 25 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wheat straw | Ethanol/water, sodium hydroxide | — | Biofilm | Nanocellulose biofilm | — | 26 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sitka spruce wood | Ethanol/water, sulfuric acid | — | Ethyl glycosides | Glucose | — | 27 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Debarked beech wood | Ethanol/water, sulfuric acid | — | Furfural | — | — | 28 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sugarcane bagasse | Ethanol/water, sulfuric acid | — | — | Glucose | — | 29 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sugarcane bagasse | Glycerol/water, sulfuric acid | — | — | Bioethanol | — | 30 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hardwood | 1,4-Butylene glycol/water, 1-butyl-3-methylimidazolium hydrogen sulfate as catalyst | — | — | Cellulose Nanofibrils biofilm | — | 31 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kenaf fibres | Acetic acid/water, hydrogen peroxide | — | — | Nanocellulose | — | 32 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bark-free birch woodchips | Ethanol/water | — | — | Nanocellulose | — | 33 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Eucalyptus hardwood | Gama-valerolactone/water or ethanol/water with sulfuric acid | — | — | Cellulose nanocrystals | — | 34 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Rice Straw | Ethanol/water, organic acid | — | — | Butyl Glucosides | — | 35 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wood chips | Methanol/water | — | — | Carboxymethylcellulose | — | 36 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wood chips | Ethanol/water, sulfuric acid | — | — | Dissolving pulp | — | 37 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aspen wood chips | Ethanol/water, hydrochloric acid | — | — | — | Aerogels | 38 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Beechwood | Lignin, Fraunhofer CBPa | — | — | — | Coating material | 39 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Beechwood, Japanese knotweed | Ethanol/water, sulfuric acid | — | — | — | Barrier coating material | 40 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Beechwood | Lignin, Fraunhofer CBPa | — | — | — | Tissue engineering | 41 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Corn stover | Ethanol/water, sulfuric acid or formic acid or sodium hydroxide | — | Sugars | Sugars | Nanoparticles | 42 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wood chips | Ethanol/water | — | — | — | Flotation collector | 43 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Wheat straw, spruce wood, beech wood | Ethanol/water | — | — | — | Nanoparticles | 44 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Beechwood | Lignin, Fraunhofer CBPa | — | — | — | Adhesive | 45 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cornstalk | Formic acid/acetic acid/water | — | — | — | Adhesive | 46 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Beechwood chips | Ethanol/water, sulfuric acid | — | — | — | Adhesive | 47 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Beechwood | Lignin, Fraunhofer CBPa | — | — | — | Composite for 3D printing | 48 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oil palm empty fruit bunch | Formic acid/water | — | — | — | Composite for 3D printing | 49 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Southern yellow pine | Ethanol/water, sulfuric acid | — | — | — | Stereolithography | 50 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hybrid poplar chips | Butanol/water, acetic acid | — | — | — | Self-healing polymer | 51 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bamboo | Acetic acid/water | — | — | — | Self-healing polymer | 52 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscanthus X giganteus | Ethanol/water | — | — | — | Antioxidant | 53 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| n.d. | Lignin, Chemical point UGb | — | — | — | Oxidant inhibitor | 54 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Corncob | Ethanol/water | — | — | — | Antioxidant | 55 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| n.d. | Lignin, South China University of Technologyb | — | — | — | UV-absorber | 56 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| n.d. | Lignin, Shanfeng Co. Ltdb | — | — | — | Metal biosorbent | 57 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| n.d. | Lignin, Shanfeng Co. Ltdb | — | — | — | Metal biosorbent | 58 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aleppo pine, Eucalyptus globulus | Ethanol/water, sulfuric acid | — | — | — | Stabilisers for cellulose nitrate | 59 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Coconut shells | Acetone/water, inorganic acids | — | — | — | Flame retardant resin | 60 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Exhausted olive pomace | Ethanol/water, sulfuric acid | — | — | — | Rigid polyurethane foam | 61 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spruce wood | Ethanol/water, sulfuric acid | — | — | — | Antimicrobial | 62 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Banana peels | Acetic acid/water, hydrochloric acid | — | — | — | Antioxidant, Antimicrobial | 63 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poplar wood | Methanol/dioxane | — | — | — | Biofilm | 64 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Regarding the components that can be extracted and used to prepare high-value chemicals and biomaterials, this manuscript focuses on the main four: extractives, hemicelluloses, cellulose and lignin.
2.1. Extractives
Extractives are a mixture of various chemicals (phenolic compounds, fats, waxes, terpenes and terpenoids), which, by definition, are soluble in organic solvents. The extractives are the less developed family of chemicals studied after organosolv treatment because they are generally separated by a pre-treatment.22 However, two recent publications studied the recovery of extractives from spent coffee grounds and exhausted olive pomace, using the organosolv process by the application of a solvent mixture composed of methanol/hexane or ethanol/water in the presence of sulfuric acid, which found use as the catalyst. Afterwards, the extractives were converted to fatty acids (fatty acids methyl esters and omega-3 fatty acids).23,242.2. Hemicelluloses
More studies were done on the valorisation of hemicelluloses.Hemicellulose is a branched polysaccharide mainly composed of xylose, arabinose, glucose, mannose, galactose, and rhamnose. This polysaccharide can be extracted from different lignocellulosic biomasses (i.e. wood and straw) using various organosolv methods; the most described process consists of using an ethanol/water solution in the presence or not of acid and alkali catalysts such as sulfuric acid and sodium hydroxide. The extracted hemicelluloses were further modified using biological and chemical methods in order to obtain iso-butanol,25 as a component for biofilms,26 ethyl glycosides27 and furfural for resin applications.28
2.3. Cellulose
The organosolv fractionation also allows cellulose recovery, a linear polysaccharide composed of glucose units. Cellulose can be extracted from different lignocellulosic biomasses, such as wood, straw and cardboard, by organosolv methods. Various organosolv processes were studied to recover cellulose, such as aqueous solutions containing organic acids (formic acid and acetic acid), alcohols (ethanol, methanol and glycerol) and alternative solvents (gamma-valerolactone). Acid or alkali catalysts are also added to enhance the fractionation efficiency. Currently, the main application of organosolv cellulose consists in its fermentation to produce commodities such as sugars and biofuels.29,30 However, high-value chemicals and biomaterials can also be obtained. The main studies focus on generating cellulose nanofibrils (CNF),31 nanocellulose,32 cellulose nanocrystals (CNCs),33,34 butyl glucosides,35 carboxymethylcellulose36 and dissolving pulp.372.4. Lignin
Lignin is the most studied component extracted from lignocellulosic biomass using organosolv processes. It is a highly branched and heterogeneous macromolecule composed of phenyl propane units. Lignin can be extracted from cardboard, grass, wood, grass, cornstalk, stover, and other agricultural residues using different organosolv fractionations. These methods include using solutions composed of organic acids (formic acid and acetic acid), alcohols (ethanol, methanol and butanol) and ketones such as acetone. Adding acid (both organic and inorganic) or alkali catalysts is also considered to boost fractionation yields. The interest in organosolv lignin comes from its superior purity over the other technical types.18 For this reason, organosolv lignin is under study for the preparation of added-value materials such as aerogels,38 coating materials,39,40 tissue engineering,41 nanoparticles,42–44 adhesives,45–47 bio-composites for 3D-printing,48–50 recyclable and self-healing polymers,51,52 antioxidant agents,53–55 UV-absorber agents,56 metal absorbers for wastewater treatment,57,58 stabilisers of cellulose nitrate,59 flame retardant resins,60 polyurethane foams,61 antimicrobial agents62,63 and bio-films.643. Organosolv processes over the years
Due to their great potential, several organosolv processes have been developed over the years, and novel solvents are under study. They can be classified into four categories based on the type of solvents used: alcohol, ketone, organic acid, and hybrid processes. Below, the main organosolv processes are briefly described. The processes are also reported in Table 2.| Name | Class | Solvent | Conditions | Ref. |
|---|---|---|---|---|
| Alcell | Alcohol organosolv | Aqueous ethanol | 180–200 °C, 29–31 bar, pH 4 | 65 and 66 |
| Organocell | Alcohol organosolv | Aqueous methanol | (1) pH = 4–6, 200 °C, 40 bar (2) pH = 8–12 170 °C | 67–69 |
| Lignol | Alcohol organosolv | Aqueous ethanol | Alcell-like conditions with Sulfuric acid pH 2–3 | 70 and 71 |
| ASAM | Alcohol organosolv | Aqueous methanol or ethanol (>30%) | pH above 13, 170–180 °C, anthraquinone as catalysts, sulphite as delignification agent | 72–74 |
| Fraunhofer Centre | Alcohol organosolv | Aqueous ethanol (90%) | Alcell-like conditions, 200 °C, 40 bar | 75 and 76 |
| Glycell | Alcohol organosolv | Glycerol | Sulfuric acid as catalyst, 130–160 °C, 2–5 bar | 77 and 78 |
| Fabiola | Ketone organosolv | Aqueous acetone | Sulfuric acid as catalyst, 140 °C, 15 bar | 79–81 |
| SEW or AVAP ® | Alcohol organosolv | Aqueous ethanol | Sulphur dioxide at 130–160 °C | 82–84 |
| Vertoro B.V (Goldilocks®) | Alcohol organosolv | Methanol | Sulfuric acid as catalyst, 160–200 °C, 15 bar | 85–87 |
| AST | Alcohol organosolv | Butanol | Sulfuric acid as catalyst, 180 °C | 51 and 88 |
| Acetosolv | Organic acid organosolv | Acetic acid 85% | Sulfuric acid as catalyst, 200 °C, 20 bar | 90–92 |
| Acetocell | Organic acid organosolv | Acetic acid 85% | 200 °C, 20 bar | 90–92 |
| Formacell | Organic acid organosolv | Acetic acid 85%, Formic acid 10% | Acetosolv-like conditions | 93 and 94 |
| Milox | Organic acid organosolv | Aqueous formic acid | (1) 120 °C (2) addition of performic acid | 95 and 96 |
| Formico | Organic acid organosolv | Aqueous formic acid (>40%) | 130–170 °C | 97 and 98 |
| CIMV | Organic acid organosolv | Acetic acid/formic acid/water mixture (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) 20) |
110 °C | 99–101 |
| LignoFibre | Organic acid organosolv | Acetic acid (80%) | Phosphinic acid (3.5%) as catalyst, 150 °C | 102 and 103 |
| Bloom | Hybrid organosolv | Dioxane | Chloric acid as catalyst and formaldehyde as stabiliser, 80–100 °C | 104–106 |
The Alcell treatment is one of the oldest organosolv processes developed.65 In this case, aqueous ethanol (around 50%) is used as the solvent operating at about 180–200 °C and a pressure of 29–31 bars.66 The pH is around 4 without adding acid or alkali compounds due to the formation of acetic acid during biomass fractionation. Currently, operational plants are not reported. A scheme of the Alcell process is reported in Fig. 2.
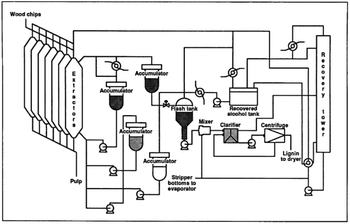 | ||
| Fig. 2 The Alcell process (reproduced from ref. 65 with permission from Tappi J., copyright 1991). | ||
The organocell method is a two-stage process where aqueous methanol (around 50%) is used as the solvent. In the first stage, the “acid stage”, the biomass is treated in the solvent having pH = 4–6, at around 200 °C and 40 bars. In the second stage, called the “alkaline stage”, sodium hydroxide with fresh solvent is added, and the temperature is kept at around 170° with a pH between 8 and 12.67,68 Currently, operational plants are not reported. A scheme of the organocell process is reported in Fig. 3.69
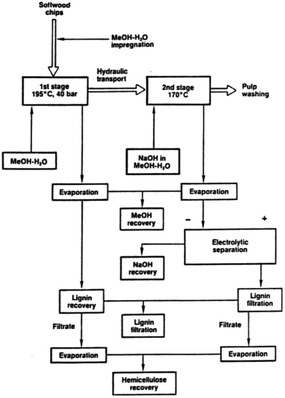 | ||
| Fig. 3 The organocell process (reproduced from ref. 69 with permission from Tappi J., copyright 1989). | ||
The lignol process is an ethanol–water treatment derived from the Alcell method. The main difference is the addition of an inorganic acid (e.g. sulfuric acid) to keep the pH between 2 and 3.70,71 Currently, a pilot plant is operational. A description is reported in the next section. A scheme of the lignol process is shown in Fig. 4.
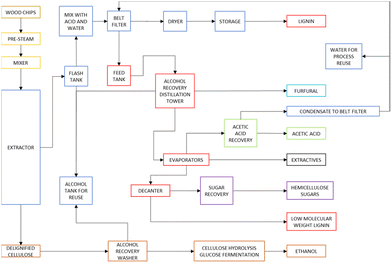 | ||
| Fig. 4 The Lignol process (image redrawn and modified from ref. 70). | ||
The alkali–sulphite–anthraquinone–methanol (ASAM) process is derived from alkaline sulphite pulping where anthraquinone is used as the delignification catalyst but methanol (around 30%) is applied as the co-solvent together with water. The working temperature is about 170–180 °C, and the pH is above 13.72,73 A variation using ethanol instead of methanol was also studied.74 Currently, operational plants are not reported. A scheme of the ASAM process is reported in Fig. 5.
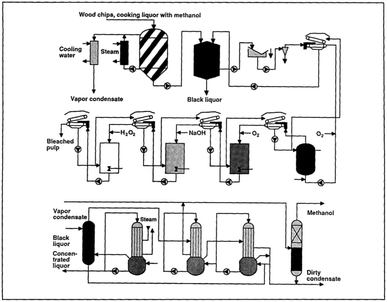 | ||
| Fig. 5 The ASAM process (reproduced from ref. 72 with permission from Tappi J., copyright 1991). | ||
The Fraunhofer Centre developed an alcohol organosolv process using aqueous ethanol (around 90%) at 200 °C and 40 bars. This treatment can be considered an Alcell-derived process.75,76 Currently, a pilot plant is operational. A description is reported in the next section. A scheme of the process is shown in Fig. 6.
 | ||
| Fig. 6 Example of the Fraunhofer Centre organosolv process (reproduced with permission and courtesy from Fraunhofer CBP, copyright: © Fraunhofer CBP). | ||
The Australian company Leaf Resources Ltd has ownership of the Glycell™ process.77,78 It consists of an organosolv fractionation composed of glycerol as a solvent in the presence of sulfuric acid as a catalyst. The operating temperature is around 130–160 °C and pressure is 2–5 bars. Currently, a pilot-scale plant is operational. A description is reported in the next section.
The Netherlands Organization for Applied Scientific Research (TNO) developed an organosolv process called Fabiola. In this treatment, aqueous acetone (around 50%) is used as a solvent, in the presence of sulfuric acid as the catalyst, at 140 °C and under 15 bars.79–81 Currently, operational plants are not reported, but studies for the scale-up are ongoing. A scheme of the Fabiola process is reported in Fig. 7.
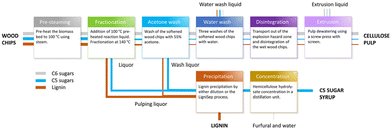 | ||
| Fig. 7 The Fabiola process (reproduced from ref. 79 with permission from the authors. Published by American Chemical Society, copyright 2022). | ||
The SO2–Ethanol–Water (SEW) or American Value-Added Pulping (AVAP®) is an ethanol-based organosolv process operating in the presence of sulphur dioxide at 130–160 °C.82–84 Currently, operational plants are not reported. The main limitation is the use of toxic gas. A scheme of the SEW process is reported in Fig. 8.
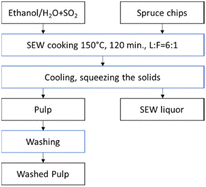 | ||
| Fig. 8 The SEW process (image redrawn and modified from ref. 83). | ||
The Vertoro B.V. company developed a patented process to obtain a crude liquid lignin oil useful as a chemical platform (Goldilocks®). The lignocellulosic biomass is processed using methanol as the solvent and sulfuric acid as a catalyst at 160–200 °C and under around 15 bars.85,86 Currently, operational plants are not reported, but studies for the scale-up are ongoing. A scheme of the process is reported in Fig. 9.87
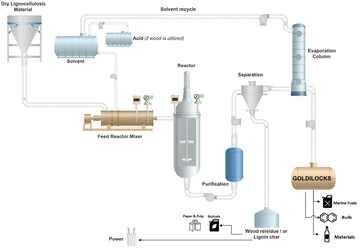 | ||
| Fig. 9 The Vertoro B.V. process (reproduced from ref. 87 with permission from Vertoro B.V., copyright: 2021). | ||
The American Science and Technology Corporation (AST) is currently using butanol as a solvent in the presence of sulfuric acid as a catalyst. The optimal temperature is 180 °C.51,88 Now, a semi-continuous plant is operational. A scheme of the AST process is reported in Fig. 10. A homogeneous solution of butanol and water in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio is formed due to the operative temperature being above 120 °C.88,89 At the end of the fractionation, the solvent is transferred to a tank where a split between the organic layer and the aqueous layer is possible, allowing the separation and recycling of the organic phase.
1 ratio is formed due to the operative temperature being above 120 °C.88,89 At the end of the fractionation, the solvent is transferred to a tank where a split between the organic layer and the aqueous layer is possible, allowing the separation and recycling of the organic phase.
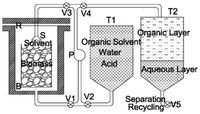 | ||
| Fig. 10 The AST process (reprinted with permission from ref. 51. Copyright 2019 American Chemical Society.). | ||
Acetosolv and Acetocell processes are two organosolv treatments that use acetic acid (85%) as the solvent operating at around 200 °C and under 20 bars.90,91 The difference is that an acid catalyst (e.g. sulfuric acid) is present in the Acetosolv process.92 Currently, operational plants are not reported.
The Formacell treatment is a derived Acetosolv process where a mixture of formic acid and acetic acid is used instead of acetic acid alone. Typically the solvent is 85% acetic acid, 10% formic acid and 5% water.93,94 Currently, operational plants have yet to be reported.
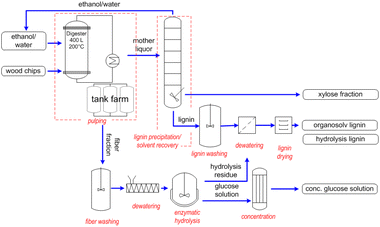
Chempolis Oy developed a process called Milox, where formic acid is used as a solvent operating at around 120 °C. In the second step, performic acid is added to enhance the delignification.95,96 Currently, operational plants are not reported.
Chempolis Oy also developed a second organosolv process called Formico. In this technology, formic acid is the main component in the biosolvent and the main delignification agent having a concentration of at least 40% with an operative temperature of 130–170 °C.97,98 Currently, a pilot plant is operational, and studies for a further scale-up are ongoing. A description is reported in the next section. A scheme of the possible production concept using the Formico process is reported in Fig. 11. Together with sulphur-free lignin and ethanol, Formico allows the extraction and isolation of sugar-based products. Alternatively, cellulose can be used for papermaking or textile applications.
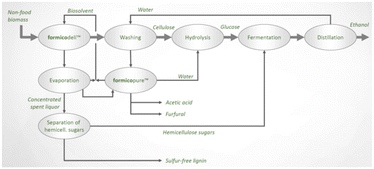 | ||
| Fig. 11 The Formico process (reproduced with permission and courtesy from Chempolis Oy, copyright: 2022). | ||
The Compagnie Industrielle de la Matière Végétale (CIMV) developed an organosolv process based on an acetic acid/formic acid/water mixture (typically 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) working at around 110 °C.99–101 Currently, a pilot plant is operational. A description is reported in the next section. A scheme of the process is reported in Fig. 12.
20) working at around 110 °C.99–101 Currently, a pilot plant is operational. A description is reported in the next section. A scheme of the process is reported in Fig. 12.
 | ||
| Fig. 12 The CIMV process (reproduced from ref. 100 with permission from the Authors Published by Frontiers, copyright: 2020). | ||
The Technical Research Centre of Finland Ltd (VTT) developed an organosolv process called LignoFibre. This process consists of using acetic acid (80%) as a solvent in combination with phosphinic acid (3.5%), working at 150 °C.102,103 Currently, operational plants are not reported.
The Swiss start-up Bloom Biorenewables, created in 2019 as a spin-off from the École Polytechnique Fédérale de Lausanne, is working on the scale-up of an organosolv process. The biomass is treated using dioxane as a solvent, chloric acid as a catalyst and formaldehyde as a stabiliser to control the lignin depolymerisation. The typical temperature is around 80–100 °C.104–106 The main novelty of the reaction compared to the organosolv process described before is the use of formaldehyde as a protective agent of lignin, reducing the risk of condensation and modification reactions. Combined with low temperatures and elevated lignin solubility in dioxane, this process allows for the production of high-quality lignin. Currently, the process is developed at a laboratory scale (10 L).
The processes show similar operative conditions. Therefore, their scaling up, in section 4, is based on chemical and economic evaluations of the companies which are owners of the patents.
It is possible to provide an overview to differentiate the processes on the lignin structure depending on the process parameters. Organic and inorganic acids enhance the removal of lignin, breaking the lignin–carbohydrate bonds, but reduce the β-O-4 bonds. An increase in temperature (above 170–180°) decreases the molecular weight and increases the phenol content. The solvents modify the functional groups of lignin. For example, the lignin extracted with an organic acid presents a higher content of carboxylic groups. The lignin extracted using alcohol shows a higher content of hydroxyl groups.107
4. Scale-up
Despite the various studies and the high interest in the organosolv process, its commercial scale-up still needs to be achieved. The reason is correlated to the limitations described in section 5 – Challenges. Currently, four pilot plants are operational, three in Europe and one in North America.108 The current pilot-scale plants and future scale-up are summarised in Table 3.| Name | Organosolv class | Current lignin scale | Future lignin scale | Starting biomass | Ref. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Wood products. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lignol | Alcohol | 1.000 t per year | — | Wood | 108 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fraunhofer | Alcohol | 500 t per year | — | Hardwood | 108 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Glycell | Alcohol | 8.000 t per year![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
16.000 t per year![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
Softwood | 109 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CIMV | Organic acid | 1.000 t per year | — | Wheat straw, wood and bagasse | 108 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Formico | Organic acid | 1.000 t per year | 50.000 t per year (expected in 2027) | Wood, straw, grass and bagasse | 108 and 114 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AST | Alcohol | 26 t per year | — | Hardwood, softwood, and agricultural wastes | 110 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fabiola | Ketone | 460 L reactor | — | Wood chips | 79 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fraction project | Hybrid | — | Laboratory Scale (project started in 2021) | Agricultural residues and paper and pulp industry residues | 111 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bloom Biorenewables | Hybrid | — | 630 L reactor (Start-up started in 2019) | Mainly wood | 112 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Vertoro B.V. | Alcohol | — | Demo-plant (Project started in 2022) | Lignin, plants, and residues from the paper industry | 113 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
A plant based on Lignol technology is operational for generating ethanol and lignin (around 1.000 t per year) from softwood and hardwood.108 The supplier is Suzano company, born by merging Suzano Pulp and Paper and Fibria Innovation Inc. (owner of Lignol technology).17
The Fraunhofer Centre in Germany has a pilot plant based on its technology where ethanol, lignin (around 500 t per year) and xylose are produced starting from hardwood as the raw material.108
The Leaf Resources Ltd has a pilot scale plant based on the Glycell™ technology.77 Currently, the plant produces around 8000 t per year of wood resin and wood turpentine starting from pine. In 2022, it started rebuilding and upgrading the plant to reach a production of 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t per year of wood products.109
000 t per year of wood products.109
The CIMV has a plant in France based on their technology for producing lignin (around 1.000 t per year), cellulose, C5 sugars and silica. The biomasses used as starting material are wheat straw, wood and bagasse.108
Chempolys Oy, in collaboration with Fortum Oyj, developed a pilot plant based on Formico technology for producing ethanol, cellulose pulp, lignin (around 1.000 t per year), xylose and other biochemicals from different biomasses such as wood, straw, grass and bagasse.108
Together with these pilot plants, AST has an operational semi-continuous plant, with a reactor of around 7600 litres, for generating cellulose and lignin (around 26 t per year).110 However, it is used for scientific purposes.
Simultaneously, projects are ongoing to develop new organosolv methods and the process scale-up. The Fabiola process was studied during the European project UnRavel (no. 792004), which ended in May 2022. The process passed from a laboratory scale to a reactor of 460 litres, and the starting material was composed of wood chips.79
The European project Fraction (no. 101023202) involves developing a scalable organosolv process based on gamma-valerolactone (GVL). This project started in 2021 and will end in 2024. Currently, they are testing different types of biomasses, including agricultural residues and paper and pulp industry residues.111
The company Bloom Biorenewables is working on the scale-up of its patented process. Currently, they are studying to pass from the laboratory scale of a 10 L reactor to 630 L for a pilot plant. The most studied biomass for this process is wood.112
In 2022, Vertoro B.V. started a collaboration with the Swedish company Sekab to construct a demo plant for the scale-up of Goldilocks® technology using different biomasses such as lignin, plants and residues from the paper industry.113
In conclusion, Fortum Oyj and Chempolis Oy are working on the development of a European industrial plant with a capacity of organosolv lignin production of 50.000 t per year using Formico technology. The raw materials would be 300.000 t per year of straw. The plant is expected to be operational in 2027.114
5. Challenges
The first publications about the organosolv process are from the second half of 1980, but no commercial or industrial plants are currently operational. Over the years, several techno-economic and LCA analyses were carried out on the organosolv process to detect the critical issues from the economic and environmental points of view. Most of the techno-economic analyses show that the scaling up of the organosolv process is difficult due to the high costs related to the purchase of chemicals and the energy consumption caused by heating and solvent recycling. However, the life-cycle assessment (LCA) studies showed that the organosolv process is more environmentally friendly than classical pulping treatments.In order to summarise the types of organosolv processes studied, we tried to classify such kinds of fractionations to the closer known organosolv classes already described above. In Table 4, the techno-economic analysis and LCA studies are reported.
| Type of assessment | Organosolv process | Estimated scale | Limitations | Environmental advantages over other processes | Ref. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Review article. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Economic | Lignol-like | 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t per year Beechwood chips 000 t per year Beechwood chips |
Heat integrated biorefinery | — | 115 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Economic and Environmental | Lignol-like | 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t per year Beechwood chips 000 t per year Beechwood chips |
Influence of feedstocks price | Low environmental impact | 116 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Economica | Alcohol, ketone and organic acid organosolv | — | Price of organic solvents, energy consumption | — | 117 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Economic and Environmental | Lignol-like | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t per year dry wood 000 t per year dry wood |
High investment costs, yields of products | Low environmental impact | 118 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Economic | Lignol-like | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 kg h−1 wood 500 kg h−1 wood |
Energy consumption and products yields | — | 119 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Economic and Environmental | Lignol-like | 208![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 ton per year bio-jet (product) 900 ton per year bio-jet (product) |
High operational costs (solvent purchase and recycling) | Organosolv reduces GHG emissions | 120 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Environmental | Lignol-like | 83.3 t h−1 of dry wood | Energy consumption and price of products | — | 121 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Environmental | Lignol-like and CIMV-like | 1 kg of bioplastic as a functional unit | Final product price, energy consumption | Reduction of GHG | 122 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Economic | Lignol-like | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 t h−1 bagasse and trash 400 t h−1 bagasse and trash |
Low energy efficiency | — | 123 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Economic | Lignol-like | Softwood and hardwood for target production of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of dry lignin per year 000 tons of dry lignin per year |
Energy consumption, the value of the products | — | 124 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Economic and environmental | Lignol-like | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kg h−1 for 7920 h of corn stover 000 kg h−1 for 7920 h of corn stover |
High costs in utilities due to recycling processes and CO2 emissions, heat integration not possible | More pure lignin | 125 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Economic | Alcell-like | 10.000 t per year of walnut shells | Extraction limitations and energy required | — | 126 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Economica | Lignol-like and Alcell-like | — | High consumption of chemicals and energy for the recovery of solvents. High dependence on yield and value of products | — | 127 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Environmentala | Lignol-like | — | — | Environmentally friendly in terms of climate change impact | 128 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Economic | Alcell-like | 2000 t d−1 of eucalyptus logs | The process is profitable when high-value chemicals are produced (lignin polyols and platform chemicals vs. technical lignin, sugars and ethanol) | — | 129 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Economic | Alcell-like | 114 t h−1 of sugarcane bagasse | Economic point of view is the limitation of the process | — | 130 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Economic and Environmental | not reported | 500 t d−1 wood | Depending on the value of final products, CO2 production is comparable with that of other processes, but other pollutants were not studied. | — | 131 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Economic and environmental | Lignol-like | 40.000 t per year of wood chips | Economic point of view is the limitation of the process. Necessary for the production of high-value chemicals | Lower global warming potential (excluding biogenic carbon) | 132 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Economic and environmental | Fabiola | 300.000 t per year dry biomass | Economic point of view is the limitation of the process. Improvement with respect of ethanol process. | Improved environmental impact compared to the ethanol process | 133 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In 2016, Nitzsche et al. simulated, using Aspen Plus® software, the organosolv process (Lignol-like) of having 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t per year beech wood chips as feedstock. The target products were polymer-grade ethylene, organosolv lignin and fuel. The main conclusion is that the heat-integrated biorefinery concept is not profitable. Therefore, only highly valuable products sold at high prices can open to a cost-effective process.115
000 t per year beech wood chips as feedstock. The target products were polymer-grade ethylene, organosolv lignin and fuel. The main conclusion is that the heat-integrated biorefinery concept is not profitable. Therefore, only highly valuable products sold at high prices can open to a cost-effective process.115
Budzinski and Nitzsche simulated four organosolv biorefineries (Lignol-like) to compare their economic and environmental aspects. The feedstock is 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t per year of beech wood. The products are polymer-grade ethylene, organosolv lignin, the fuel hydrolysis lignin, biomethane, liquid “food-grade” carbon dioxide and anhydrous ethanol. Aspen Plus® v8.6 software was used for the simulations. All four biorefineries showed a lower environmental impact when compared with reference systems (currently available fossil-based technologies to provide the target products) used for comparisons. However, the economic profitability is strongly influenced by the costs of feedstocks and the price of the products.116
000 t per year of beech wood. The products are polymer-grade ethylene, organosolv lignin, the fuel hydrolysis lignin, biomethane, liquid “food-grade” carbon dioxide and anhydrous ethanol. Aspen Plus® v8.6 software was used for the simulations. All four biorefineries showed a lower environmental impact when compared with reference systems (currently available fossil-based technologies to provide the target products) used for comparisons. However, the economic profitability is strongly influenced by the costs of feedstocks and the price of the products.116
In 2017, Zhao et al. wrote a review of different organosolv fractionation pre-treatments for enzymatic saccharification of cellulose where solvents such as ethanol, acetone and acetic acid were applied. The authors say this process is still not competitive compared to conventional pre-treatments due to the cost of organic solvents and their recovery. The reduction of energy consumption for solvent recovery and the development of more high-value products are two crucial points to make the organosolv process profitable.117
In 2018, Moncada et al. studied the techno-economic and environmental aspects of C6-sugar production from spruce and corn, comparing organosolv (Lignol-like) and wet-milling technologies considering a plant capacity of around 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t of dry wood (feedstock) per year. Based on the results, the organosolv process looks more environmentally friendly and economically feasible but with higher investment costs than wet milling technology. Moreover, the authors report that the economics of the organosolv process is highly sensitive to the yields of lignin and sugars.118
000 t of dry wood (feedstock) per year. Based on the results, the organosolv process looks more environmentally friendly and economically feasible but with higher investment costs than wet milling technology. Moreover, the authors report that the economics of the organosolv process is highly sensitive to the yields of lignin and sugars.118
Gurgel da Silva et al. made a techno-economic analysis of an organosolv process (Lignol-like) to obtain technical lignin. It was observed that a high amount of energy is required to disrupt the lignocellulosic structure (1/3rd of the total production costs). Aspen Plus v8.0 simulated a process of 88.500 kg h−1 dry biomass considering wood as the feedstock. The authors conclude that to make the process economically sustainable, it is necessary to improve the energy-saving mechanisms and enhance the recovery of the products.119
Santos et al. present a techno-economic analysis and an environmental assessment of the production of bio jet fuel, acetic acid, furfural and succinic acid using a sugarcane-based biorefinery. Eight biomass pre-treatment technologies were considered, i.e. dilute acid, dilute acid + alkaline treatment, steam explosion, steam explosion + alkaline treatment, organosolv (Lignol-like), alkaline wet oxidation, liquid hot water and liquid hot water + alkaline treatment. Organosolv was one of the processes able to obtain the highest yield of fuel and reduce greenhouse gas (GHG) emissions, but, at the same time, required the highest operational costs (solvent purchase and recycling).120
Bello et al. studied the LCA of different lignocellulosic biorefinery scenarios for an integrated valorisation of residual beech wood chips using an organosolv process (Lignol-like). The plant capacity considered was 83.3 t h−1 of dry wood. The authors conclude that the optimisation of technologies to improve energy saving and the production of high-value products is crucial for the feasibility of the industrial process. However, more studies are necessary to evaluate the development of an integrated biorefinery.121
Patel et al. studied the production of bioplastics from lignocellulosic biomasses using steam explosion and organosolv (Lignol-like and CIMV-like) processes. The target products were C6–C5 sugars and lignin. Only organosolv provides high purity lignin. For this study, the calculation considered the production of 1 kg of bioplastic as a functional unit. In this case, the profitability of the organosolv process is sensitive to the final price of the products and the necessity of energy integration. The CIMV process shows an 8% lower GHG impact and higher yield of C5-sugars.122
In 2019, Nieder-Heitmann et al. simulated and compared, using Aspen Plus® software, the production of succinic acid and electricity from biomasses using different processes: dilute acid, alkaline, organosolv (Lignol-like), ammonia fibre expansion, steam explosion, and wet oxidation. In the organosolv process, the feedstock was (23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 t h−1 bagasse and trash). However, the organosolv process was not economically profitable together with the wet oxidation treatment due to limited energy efficiency.123
400 t h−1 bagasse and trash). However, the organosolv process was not economically profitable together with the wet oxidation treatment due to limited energy efficiency.123
Mesfun et al. assessed a techno-economic hybrid process (organosolv–steam explosion) using wood (hardwood and softwood) as biomass. The model was developed with the target production of 50.000 t per year of lignin. The organosolv is a Lignol-like treatment. However, it is an extremely energy-intensive process that does not resolve the energy requirements already present in the organosolv process without significant improvements in the quality of the products.124
Gurgel da Silva et al. compared different pre-treatment processes for the production of ethanol, studying the economic and environmental impacts. Diluted acid, liquid hot water, steam explosion, ammonia fibre explosion, and organosolv (Lignol-like) pre-treatments were considered. The target product is bioethanol. The process simulation was performed with the software Aspen Plus® v8.6, considering corn stover as feedstock (97.000 kg h−1 for 7920 h). The organosolv process showed the highest utility costs due to the recycling process and, consequently, the highest carbon dioxide emissions. Also, it is the only process where heat integration is not feasible. However, the organosolv process is the one able to extract pure lignin.125
In 2020, a thesis described the simulation of a large-scale organosolv process (Alcell-like, 10.000 t per year of walnut shells) using Aspen Plus® software. The author reports the lack of data due to the novelty of the process at this scale, causing difficulty in having accurate assumptions and simulations. The target product is organosolv lignin. The process is, at the moment, not economically feasible. The operating costs are currently too high due to the extraction limitations and energy required to recover the solvent. The options are to optimise the process (e.g. selecting a different organic solvent to boost the extraction and improve the solvent recovery system) and sell the lignin at higher prices.126
Soltanian et al. studied the exergetic aspects of the pre-treatment process (diluted acid, organosolv and steam explosion) for converting lignocellulose to fuels. Different organosolv processes, Lignol-like and Alcell-like, were investigated. For exergy aspects, the organosolv process was found to be less efficient than steam exploded treatment due to the high consumption of chemicals and energy for the recovery of solvents. Moreover, organosolv efficiency is highly dependent on the effectiveness of recovery of lignin and hemicelluloses. Therefore, improving the fractionation stage and the method to recover the solvents127 is necessary.
In 2021, Ryan and Yaseneva reported and compared in a review the LCA on different woody biomass treatments (e.g. organosolv, kraft pulping, and diluted acid) for its conversion to sugars. The results suggested that the organosolv process considered in the publication (Lignol-like) is the most environmentally friendly, particularly regarding climate change.128
Dornelles et al. studied the economic aspects to valorise the eucalyptus. The author found that focusing on lignin, especially polyols, and commercialised sugar is more profitable than preparing cellulosic chemicals. Also, this organosolv approach (Alcell-like) is more profitable than producing ethanol, sugar and technical lignin. In this case, a plant with a capacity of 2000 dry tons of eucalyptus logs per day was considered.129
Ospina-Varón et al. studied different pre-treatment processes: steam explosion, organosolv (Alcell-like) and hot water to obtain nanocellulose. Aspen Plus v10 was used as the simulation software. This work considered a feedstock flow of sugarcane bagasse of 114 t h−1. The results show that the organosolv process has the best technical performance, but its economic behaviour is its biggest disadvantage.130
Ouhimmou et al. used as a study case the forest industry in the Mauricie region (Canada). Different pre-treatments were considered: hot water extraction, fast pyrolysis, organosolv fractionation, and kraft pulping. The organosolv considered is not specified, but it was studied on a scale of 500 t d−1 of wood. In summary, the profitability of the organosolv process is strongly influenced by the type of products generated during the process. In addition, this study evaluated greenhouse gas (CO2) generation, showing that organosolv has a GHG generation comparable to other pre-treatments. However, replacing other common pollutants (such as sulphur) was not considered.131
In 2022, Zeilerbauer et al. simulated an organosolv biorefinery (Lignol-like, input 40.000 t per year of wood chips) to evaluate the techno-economic and LCA aspects. The biorefinery was compared with the process based on fossil resources to obtain the target products, lignin monomers, lignin oligomers and C6 sugars. The organosolv biorefinery provided a lower global warming potential (excluding biogenic carbon) than its fossil counterparts. However, the process is not profitable with prices based on fossil references. Therefore, it is necessary to obtain products having higher market demand/price.132
Keller et al. reported a technoeconomic and environmental assessment regarding the Fabiola process as part of the project UnRavel (no. 792004) considering different feedstocks (e.g. beech, birch and wheat straw) and the use of “ethanol organosolv” process as a reference. This report also studied the social impact of the organosolv processes considering aspects such as labour rights and safety. In this work, a plant with a capacity of 300.000 t per year of dry matter biomass was considered. The extracted products were valorised considering C5 fraction to produce xylonate, C6 fraction for acetone synthesis and lignin for the generation of polyols, fillers or combustion for energy reasons. The results of this work showed an enhancement of the Fabiola process with respect to the ethanol organosolv treatment in terms of environmental, economic and social impacts. In particular, the Fabiola process requires lower energy and solvent demand than the ethanol organosolv treatment. It is an improvement, but at the same time, the authors state that further steps are necessary to reach a satisfactory overall sustainability. The authors advise different actions such as the improvement of the ratio solvent/biomass, the optimisation of reactor design and the valorisation of the extracts for pharmaceutical and cosmetic applications.133
From the chemical point of view, the limitation in the use and recovery of organic solvents depends on the nature of the solvent. Methanol is not produced from a renewable resource and presents toxicity problems, ethanol presents limitations in its recovery due to the formation of azeotropes with water, acetone requires elevated operating pressures when high acetone volume fractions are present, and other organic solvents show boiling points higher than 100 °C (e.g. glycerol, acetic acid, formic acid and 1-butanol).134–136
Another limitation of the organosolv process is to find the conditions to extract at the same time all four main components, extractives, hemicelluloses, cellulose and lignin. If extractives, cellulose and lignin are quite chemically and thermally stable, they are not the same as hemicellulose, which can be depolymerised by temperature and chemicals used during organosolv fractionation, causing the formation of monosaccharides and by-products that require specific techniques to be isolated.28
6. Future opportunities
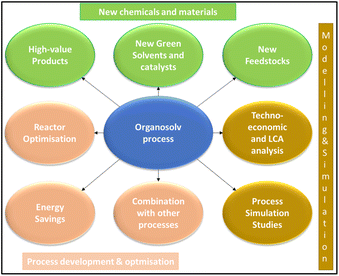
As described in the previous section, the scaling up of the organosolv process presents different limitations, notably the purchase of the solvent and the energy consumption for the disruption of the lignocellulosic structure and solvent recovery (Table 4). At the same time, this process shows several advantages concerning other pre-treatments, such as environmental impact, the highest purity of the resulting lignin and flexibility from the point of view of the types of biomasses used as feedstock and the operative parameters (e.g. 120–200 °C, 1–40 bars and type of solvent) that can be optimised (Tables 1 and 4). Consequently, the field for the organosolv process scaling up requires further research offering a wide range of opportunities for researchers such as organic chemists, inorganic chemists, and chemical engineers.An opportunity is the study of new green solvents. Several authors are working to find alternatives to the current organosolv processes, for example, the use of other organic solvents such as dimethyl carbonate mixed with ethylene glycol,137 ethyl lactate mixed with ethanol138 and MeTHF-3-one mixed with water.139 Already the use of acetone was an improvement with respect to ethanol,133 and the use of formic acid allows the scaling up of the Formico process at the industrial scale, which is expected in 2027. Moreover, the optimisation of the organosolv process is a crucial aspect, studying how to reduce the energy necessary for solvent recovery and lignin depolymerisation and isolation. For example, new catalysts can be used to improve fractionation,140 the process can be optimised by studying a reactor that improves the contact fibres-solvent, and different optimisation studies can be performed by the design of experiments and simulation models.141–143 The energy optimisation can also be achieved using alternative heating methods such as ultrasound, microwaves and electrical energy.135 Moreover, the use of the spent liquor before the stage of solvent recovery, or the use of biphasic systems to extract the chemicals from the organic solvent used for the fractionation can be considered.134 The use of the organosolv process in combination with other pre-treatments also has to be considered, such as ionsolv20 and liquid hot water processes.144 Also, the scaling up of the organosolv process using alternative feedstocks, such as waste paper,21 can be evaluated.
Of course, a constant study of LCA and techno-economic analysis are crucial to define the energy consumption and, in the suitable case, select the best location for the organosolv plant. In particular, more studies on organosolv processes based on organic acids are important because the main analyses are made on ethanol-based organosolv pre-treatments. In fact, the Lignol process is the most studied.
Another area of interest is the synthesis of new high-added-value products (chemical and biomaterials), having a high price, to make the organosolv process profitable.145,146 In this field, there will be interest in isolated lignin, polysaccharides and extractives. For example, lignins having a high content of carboxylic acids can be used for the preparation of polymers such as polyesters, and lignin with a high content of hydroxyl groups can be used for the preparation of polymers having covalent adaptable networks to improve the recycling of thermoset materials such as resins.147,148 It is important to underline that the preparation of such materials is possible thanks to the purity and limited dispersity of the organosolv lignin. Otherwise, the lignin must be purified and fractionated. Moreover, the depolymerised hemicelluloses streams can be valorised by separation using nano- and ultra-filtration149 or by modification in situ without purification stages for the production of valuable chemicals such as 5-hydroxymethylfurfural.150
A question that has also been answered is if the organosolv process allows the industries to produce specialties and high added value products and not commodities, do we need plants operating at high scales such as 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t per year?
000 t per year?
A summary of the most interesting research field regarding the improvement and development of the organosolv process is reported in Fig. 13.
7. Conclusions
In conclusion, the organosolv process presents several interesting aspects for the development of biorefineries. The main elements are flexibility because the process can be applied to different biomasses, the possibility of extraction and isolation of all the lignocellulosic components, such as highly pure lignin, cellulose, hemicelluloses and extractives, and the reduction of the environmental impact in comparison with traditional pulping processes. Several organosolv fractionation methods have been studied (i.e. Alcell and Acetosolv) over the years, and new processes are under study (i.e. Bloom and Fraction project). Currently, five pilot plants (500–1000 tons per year of lignin generated) are operational in Europe, North America and Oceania. The main solvents are ethanol, formic acid, and acetic acid in the presence or not of sulfuric acid. The commercialisation scale has still to be reached, but Fortum Oyj and Chempolis Oy are collaborating to achieve that goal. The limitations of the organosolv scale-up are mainly correlated with the energy consumption and the costs of the solvents. However, several aspects can be improved and optimised. The main fields are the study of new raw materials, the development of high value products in order to make the process more profitable, the optimisation of the entire process from the point of view of the reactor and energy optimisation and the simulation and modelling studies, in particular for techno-economic analysis and LCA. Therefore, the scaling up of organosolv fractionation offers several research opportunities for scientists from different areas, such as organic chemistry, inorganic chemistry, and chemical engineering.Author contributions
G.T and B.L. worked on the conceptualization. G.T. and E.J.G. prepared the draft of the manuscript, and B.L. and M.G. performed the final editing. The manuscript was written through the contributions of all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support provided by the Slovenian Research Agency (P2-0152 and J2-2492) and the European projects ESTELLA (GA ID: 101058371), HyPELignum (GA ID: 101070302) and GREEN-LOOP (GA ID: 101057765).References
- M. Boro, A. K. Verma, D. Chettri, V. K. Yata and A. K. Verma, Environ. Technol. Innovation, 2022, 28, 102679 CrossRef CAS.
- S. Sadula, N. Rodriguez Quiroz, A. Athaley, E. O. Ebikade, M. Ierapetritou, D. G. Vlachos and B. Saha, Green Chem., 2021, 23, 1200–1211 RSC.
- G. Tofani, I. Cornet and S. Tavernier, Chem. Pap., 2022, 76, 4351–4365 CrossRef CAS.
- G. Tofani, I. Cornet and S. Tavernier, Chem. Pap., 2021, 75, 5749–5758 CrossRef CAS.
- S. Balda, A. Sharma, N. Gupta, N. Capalash and P. Sharma, Biomass Convers. Biorefin., 2022 DOI:10.1007/s13399-022-02509-x.
- J. Leon, C. Aliaga, G. Boulougouris, M. Hortal and J. L. Marti, J. Cleaner Prod., 2015, 103, 301–308 CrossRef.
- X. Shen and R. Sun, Carbohydr. Polym., 2021, 261, 117884 CrossRef CAS PubMed.
- I. Vera, R. Hoefnagels, A. Kooij, C. Moretti and M. Junginger, Biofuels, Bioprod. Biorefin., 2020, 14, 198–224 CrossRef CAS.
- G. Velvizhi, C. Goswami, N. P. Shetti, E. Ahmad, K. Kishore Pant and T. M. Aminabhavi, Fuel, 2022, 313, 122678 CrossRef CAS.
- Y. Luo, M. Wei, B. Jiang, M. Zhang, Q. Miao, H. Fu, J. H. Clark and J. Fan, Green Chem., 2022, 24, 7429–7441 RSC.
- S. Rahmati, L. Atanda, M. Horn, K. D. Athukoralalage Don, J. Jimenez Forero, L. Moghaddam, D. Dubal, K. Ostrikov and W. O. S. Doherty, Green Chem., 2022, 24, 7410–7428 RSC.
- D. Tarasov, P. Schlee, A. Pranovich, A. Moreno, L. Wang, D. Rigo, M. H. Sipponen, C. Xu and M. Balakshin, Green Chem., 2022, 24, 6639–6656 RSC.
- Q. Chu, W. Tong, S. Wu, Y. Jin, J. Hu and K. Song, Green Chem., 2021, 23, 4074–4086 RSC.
- G. Tofani, J. de Nys, I. Cornet and S. Tavernier, Waste Biomass Valorization, 2021, 12, 503–514 CrossRef CAS.
- G. Tofani, I. Cornet and S. Tavernier, Biomass Convers. Biorefin., 2022, 12, 3409–3424 CrossRef CAS.
- D. Mboowa, Biomass Convers. Biorefin., 2021 DOI:10.1007/s13399-020-01243-6.
- A. A. Vaidya, K. D. Murton, D. A. Smith and G. Dedual, Biomass Convers. Biorefin., 2022, 12, 5427–5442 CrossRef CAS.
- A. E. Kazzaz and P. Fatehi, Ind. Crops Prod., 2020, 154, 112732 CrossRef.
- M. Galbe and O. Wallberg, Biotechnol. Biofuels, 2019, 12, 294 CrossRef.
- M. Chen, F. Malaret, A. E. J. Firth, P. Verdía, A. R. Abouelela, Y. Chen and J. P. Hallett, Green Chem., 2020, 22, 5161–5178 RSC.
- M. S. Jahan, M. M. Rahman and A. M. Sarkar, Cellulose, 2016, 23, 2039–2047 CrossRef CAS.
- B. Rietzler, M. Karlsson, I. Kwan, M. Lawoko and M. Ek, Biomacromolecules, 2022, 23, 3349–3358 CrossRef CAS PubMed.
- M. Lee, M. Yang, S. Choi, J. Shin, C. Park, S.-K. Cho and Y. M. Kim, Energies, 2019, 12, 2360 CrossRef CAS.
- A. Paz, A. Karnaouri, C. C. Templis, N. Papayannakos and E. Topakas, Waste Manage., 2020, 118, 435–444 CrossRef CAS PubMed.
- J. Lange, F. Müller, R. Takors and B. Blombach, J. Microb. Biotechnol., 2018, 11, 257–263 CAS.
- L. R. Mugwagwa and A. F. A. Chimphango, Int. J. Biol. Macromol., 2020, 143, 862–872 CrossRef CAS.
- F. P. Bouxin, S. D. Jackson and M. C. Jarvis, Bioresour. Technol., 2014, 151, 441–444 CrossRef CAS.
- J. Köchermann, J. Mühlenberg and M. Klemm, Ind. Eng. Chem. Res., 2018, 57, 14417–14427 CrossRef.
- A. A. Schmatz, F. Masarin and M. Brienzo, Bioenergy Res., 2022, 15, 1107–1115 CrossRef CAS.
- R. Terán Hilares, M. P. Swerts, M. A. Ahmed, L. Ramos, S. S. da Silva and J. C. Santos, Ind. Eng. Chem. Res., 2017, 56, 3833–3838 CrossRef.
- X. Zhao, F. Cheng, Y. Hu and R. Cheng, J. Nat. Fibers, 2021, 1–12 Search PubMed.
- V. A. Barbash, O. V. Yashchenko and V. O. Opolsky, Theor. Exp. Chem., 2018, 54, 193–198 CrossRef CAS.
- M. N. Muraleedharan, A. Karnaouri, M. Piatkova, M.-X. Ruiz-Caldas, L. Matsakas, B. Liu, U. Rova, P. Christakopoulos and A. P. Mathew, Int. J. Biol. Macromol., 2021, 183, 101–109 CrossRef CAS PubMed.
- R. Zhang and Y. Liu, Sci. Rep., 2018, 8, 16505 CrossRef PubMed.
- N. Aggarwal, P. Pal, N. Sharma and S. Saravanamurugan, ACS Omega, 2021, 6, 27247–27258 CrossRef CAS PubMed.
- M. Yáñez-S, B. Matsuhiro, S. Maldonado, R. González, J. Luengo, O. Uyarte, D. Serafine, S. Moya, J. Romero, R. Torres and M. J. Kogan, Cellulose, 2018, 25, 2901–2914 CrossRef.
- D. C. Martino, J. L. Colodette, R. Chandra and J. Saddler, Wood Sci. Technol., 2017, 51, 557–569 CrossRef CAS.
- P. Jõul, T. T. Ho, U. Kallavus, A. Konist, K. Leiman, O.-S. Salm, M. Kulp, M. Koel and T. Lukk, Materials, 2022, 15, 2861 CrossRef.
- S. Bergamasco, S. Tamantini, F. Zikeli, V. Vinciguerra, G. Scarascia Mugnozza and M. Romagnoli, Polymers, 2022, 14, 416 CrossRef CAS PubMed.
- G. Lavrič, A. Zamljen, J. Juhant Grkman, E. Jasiukaitytė-Grojzdek, M. Grilc, B. Likozar, D. Gregor-Svetec and U. Vrabič-Brodnjak, Polymers, 2021, 13, 4443 CrossRef PubMed.
- J. A. Menima-Medzogo, K. Walz, J. C. Lauer, G. Sivasankarapillai, F. R. Gleuwitz, B. Rolauffs, M.-P. Laborie and M. L. Hart, Biology, 2022, 11, 696 CrossRef CAS PubMed.
- Z.-H. Liu, N. Hao, S. Shinde, Y. Pu, X. Kang, A. J. Ragauskas and J. S. Yuan, Green Chem., 2019, 21, 245–260 RSC.
- K. Hrůzová, L. Matsakas, A. Sand, U. Rova and P. Christakopoulos, Bioresour. Technol., 2020, 306, 123235 CrossRef PubMed.
- J. Adamcyk, S. Beisl, S. Amini, T. Jung, F. Zikeli, J. Labidi and A. Friedl, Polymers, 2021, 13, 384 CrossRef CAS PubMed.
- G. Sivasankarapillai, E. Eslami and M.-P. Laborie, ACS Sustainable Chem. Eng., 2019, 7, 12817–12824 CrossRef CAS.
- S. Feng, T. Shui, H. Wang, X. Ai, T. Kuboki and C. C. Xu, Ind. Crops Prod., 2021, 161, 113225 CrossRef CAS.
- J. Saražin, A. Pizzi, S. Amirou, D. Schmiedl and M. Šernek, J. Renewable Mater., 2021, 9, 881–907 Search PubMed.
- V. Mimini, E. Sykacek, S. N. A. Syed Hashim, J. Holzweber, H. Hettegger, K. Fackler, A. Potthast, N. Mundigler and T. Rosenau, J. Wood Chem. Technol., 2019, 39, 14–30 CrossRef CAS.
- F. Ibrahim, D. Mohan, M. S. Sajab, S. B. Bakarudin and H. Kaco, Polymers, 2019, 11, 1544 CrossRef CAS PubMed.
- J. T. Sutton, K. Rajan, D. P. Harper and S. C. Chmely, Polymers, 2021, 13, 3473 CrossRef CAS PubMed.
- A. Ghosh, K. Kim, K. Rajan, C. C. Bowland, R. N. Gurram, R. W. Montgomery, A. Manesh, N. Labbé and A. K. Naskar, Ind. Eng. Chem. Res., 2019, 58, 20300–20308 CrossRef CAS.
- W. Huang, M. Wu, K. Rajan, Z. Wang and L. Zhou, Ind. Crops Prod., 2021, 159, 113062 CrossRef CAS.
- S. E. Klein, J. Rumpf, A. Alzagameem, M. Rehahn and M. Schulze, J. Coat. Technol. Res., 2019, 16, 1543–1552 CrossRef CAS.
- Y. Zhang, X. Liu, P. Apostolidis, R. Jing, S. Erkens, N. Poeran and A. Skarpas, Molecules, 2020, 25, 2455 CrossRef CAS PubMed.
- M. Michelin, A. M. Marques, L. M. Pastrana, J. A. Teixeira and M. A. Cerqueira, J. Food Eng., 2020, 285, 110107 CrossRef CAS.
- W. Huang, M. Wu, W. Liu, Z. Hua, Z. Wang and L. Zhou, Appl. Surf. Sci., 2019, 475, 302–311 CrossRef CAS.
- Z. Wang, W. Huang, P. Bin, X. Zhang and G. Yang, Int. J. Biol. Macromol., 2019, 126, 1014–1022 CrossRef CAS PubMed.
- Z. Chi, L. Hao, H. Dong, H. Yu, H. Liu, Z. Wang and H. Yu, Chem. Eng. J., 2020, 382, 122789 CrossRef CAS.
- M. Fodil Cherif, D. Trache, F. Benaliouche, A. F. Tarchoun, S. Chelouche and A. Mezroua, Int. J. Biol. Macromol., 2020, 164, 794–807 CrossRef CAS PubMed.
- S. J. Marciano, F. Avelino, L. R. R. da Silva, S. E. Mazzetto and D. Lomonaco, Biomass Convers. Biorefin., 2022, 12, 619–631 CrossRef CAS.
- A. Abid, N. Brosse, I. Ziegler-Devin and S. Gabsi, J. Polym. Res., 2020, 27, 266 CrossRef CAS.
- O. Gordobil, R. Herrera, M. Yahyaoui, S. İlk, M. Kaya and J. Labidi, RSC Adv., 2018, 8, 24525–24533 RSC.
- L. de Sousa Nascimento, F. I. D. da Mata Vieira, V. Horácio, F. P. Marques, M. F. Rosa, S. A. Souza, R. M. de Freitas, D. E. A. Uchoa, S. E. Mazzeto, D. Lomonaco and F. Avelino, Int. J. Biol. Macromol., 2021, 181, 241–252 CrossRef CAS PubMed.
- Q. Gu, T. L. Eberhardt, J. Shao and H. Pan, J. Appl. Polym. Sci., 2022, 139, 52003 CrossRef CAS.
- E. K. Pye and J. H. Lora, Tappi J., 1991, 74, 113–117 CAS.
- Alcell Technologies Inc., US005681427A, 1997.
- M. C. Schroeter, Tappi J., 1991, 74, 197–200 CAS.
- Organocell Gesellschaft fuer zellstoff und Umwelttechnik MBH, EP0498330A1, 1992.
- S. Aziz and K. Sarkanen, Tappi J., 1989, 72, 162–175 Search PubMed.
- X. Pan, C. Arato, N. Gilkes, D. Gregg, W. Mabee, K. Pye, Z. Xiao, X. Zhang and J. Saddler, Biotechnol. Bioeng., 2005, 90, 473–481 CrossRef CAS PubMed.
- Lignol Innovations LTD, A. Berlin, M. Y. Balakshin, R. MA, V. Maximenko Gutman and D. Ortiz, WO2011097720A1, 2011.
- N. P. Black, Tappi J., 1991, 74, 87–93 CAS.
- Austrian Energy & Environment SGP/Waagner-Biro GMBH, EP0538576A1, 1992.
- H. Kirçi, S. Bostançi and M. K. Yalinkiliç, Wood Sci. Technol., 1994, 28, 89–99 Search PubMed.
- B. Lesar, M. Humar, G. Hora, P. Hachmeister, D. Schmiedl, E. Pindel, M. Siika-aho and T. Liitiä, Eur. J. Wood Wood Prod., 2016, 74, 711–723 CrossRef CAS.
- P. Schulze, A. Seidel-Morgenstern, H. Lorenz, M. Leschinsky and G. Unkelbach, Bioresour. Technol., 2016, 199, 128–134 CrossRef CAS PubMed.
- I. Van Nieuwenhove, T. Renders, J. Lauwaert, T. De Roo, J. De Clercq and A. Verberckmoes, ACS Sustainable Chem. Eng., 2020, 8, 18789–18809 CrossRef CAS.
- Leaf Sciences Pty Ltd, EP3167112B1, 2021.
- A. T. Smit, M. Verges, P. Schulze, A. van Zomeren and H. Lorenz, ACS Sustainable Chem. Eng., 2022, 10, 10503–10513 CrossRef CAS.
- A. Smit and W. Huijgen, Green Chem., 2017, 19, 5505–5514 RSC.
- Stichting Energieonderzoek Centrum Nederland, Nederlandse Organisatie voor Toegepast-Natuurwetenschappelijk Onderzoek, TNO, n.d. US10793927B2, 2020 Search PubMed.
- M. Iakovlev and A. van Heiningen, J. Wood Chem. Technol., 2011, 31, 233–249 CrossRef CAS.
- E. Sklavounos, Conditioning of SO2-ethanol-water (SEW) spent liquor from lignocellulosics for ABE fermentation to biofuels and chemicals, Aalto University, 2014.
- A. Van Heiningen and E. Sklavounos, Api Intellectual Property Holdings, LLC, US2014004582A1, 2014.
- P. D. Kouris, X. Huang, X. Ouyang, D. J. G. P. van Osch, G. J. W. Cremers, M. D. Boot and E. J. M. Hensen, Catalysts, 2021, 11, 750 CrossRef CAS.
- B. V. Vertoro, EP3798286A1, 2021.
- Vertoro, https://vertoro.com/technology/, (accessed October 11, 2022).
- American Science and Technology Corporation, US9365525B2, 2016.
- E. Ramírez, R. Bringué, C. Fité, M. Iborra, J. Tejero and F. Cunill, Appl. Catal., A, 2021, 612, 117988 CrossRef.
- P. Ligero, A. Vega and M. Bao, Ind. Crops Prod., 2005, 21, 235–240 CAS.
- Acetocell, GMBH and CO. KG, EP0503304A1, 1992.
- Nimz and H. Horst, DE3445132A1, 1986.
- J. J. Villaverde, P. Ligero and A. Vega, Ind. Crops Prod., 2015, 77, 275–281 CrossRef CAS.
- Gebruder Kammerer Projekt Agentus GMBH, US6139683A, 2000.
- A. Ferrer, A. Vega, A. Rodríguez, P. Ligero and L. Jiménez, Bioresour. Technol., 2011, 102, 9755–9762 CrossRef CAS PubMed.
- Chempolis OY, US6156156A, 2000.
- L. Kupiainen, J. Ahola and J. Tanskanen, Bioresour. Technol., 2012, 116, 29–35 CrossRef CAS PubMed.
- J. Anttila, J. Tanskanen, P. Rousu, P. Rousu and K. Hytoenen, US2010240112A1, 2010.
- F. Abdelkafi, H. Ammar, B. Rousseau, M. Tessier, R. El Gharbi and A. Fradet, Biomacromolecules, 2011, 12, 3895–3902 CrossRef CAS PubMed.
- A. D. Mountraki, B. Benjelloun-Mlayah and A. C. Kokossis, Front. Chem. Eng., 2020, 2, 568196 CrossRef.
- Compagnie Industrielle de la Matiere Vegetale CIMV, US2010285553A1, 2010.
- Valtion teknillinen tutkimuslaitos, H. Mikkonen, S. Peltonen, A. Kallioinen, A. Suurnaekki, V. Kunnari and T. Malm, WO2009066007A2, 2009.
- H. Kangas, T. Tamminen, T. Liitia, T. K. Hakala, W. Vorwerg and K. Poppius-Levlin, Cellul. Chem. Technol., 2014, 48, 765–771 CAS.
- Ecole Polytechnique Federale de Lausanne, WO2017178513A1, 2017.
- S. Bertella and J. S. Luterbacher, Green Chem., 2021, 23, 3459–3467 RSC.
- L. Shuai, M. T. Amiri, Y. M. Questell-Santiago, F. Héroguel, Y. Li, H. Kim, R. Meilan, C. Chapple, J. Ralph and J. S. Luterbacher, Science, 2016, 354, 329–333 CrossRef CAS PubMed.
- J. Bergrath, J. Rumpf, R. Burger, X. T. Do, M. Wirtz and M. Schulze, Macromol. Mater. Eng., 2023, 308, 2300093 CrossRef.
- S. Mastrolitti, E. Borsella, A. Giuliano, M. T. Petrone, I. De Bari, R. Gosselink, G. van Erven, E. Annevelink, K. S. Triantafyllidis and H. Stichnothe, Sustainable lignin valorization, https://www.ieabioenergy.com/blog/publications/sustainable-lignin-valorization/, (accessed October 11, 2022).
- Glycell, https://leafresources.com.au/uncategorized/manufacturing/rebuilding-and-upgrading/, (accessed October 11, 2022).
- American Science and Technology corporation, RFI Category 2 – LIGNIN, extension://elhekieabhbkpmcefcoobjddigjcaadp/https://www.energy.gov/sites/prod/files/2016/10/f33/american_science_and_technology_de_foa_0001615_lignin_0.pdf, (accessed October 11, 2022).
- Fraction project, https://fraction-project.eu/, (accessed October 11, 2022).
- Bloom Biorenewables, https://www.bloombiorenewables.com/, (accessed October 11, 2022).
- Sekab website, Sekab and Vertoro to build large-scale demonstration plant to produce a new platform for sustainable fuels, chemicals, and materials, https://www.sekab.com/en/press-releases/sekab-and-vertoro-to-build-large-scale-demonstration-plant-to-produce-a-new-platform-for-sustainable-fuels-chemicals-and-materials/, (accessed October 11, 2022).
- H. Wikberg, in Development steps in commercialization of organosolv lignin, The FinnCERES Scientific Seminar 2022 Back to Fundamentals - Fibre, Water & Interactions, Espoo, Finland, 2022 Search PubMed.
- R. Nitzsche, M. Budzinski and A. Gröngröft, Bioresour. Technol., 2016, 200, 928–939 CrossRef CAS PubMed.
- M. Budzinski and R. Nitzsche, Bioresour. Technol., 2016, 216, 613–621 CrossRef CAS PubMed.
- X. Zhao, S. Li, R. Wu and D. Liu, Biofuels, Bioprod. Biorefin., 2017, 11, 567–590 CrossRef CAS.
- J. Moncada, I. Vural Gursel, W. J. J. Huijgen, J. W. Dijkstra and A. Ramírez, J. Cleaner Prod., 2018, 170, 610–624 CrossRef CAS.
- A. Rodrigues Gurgel da Silva, M. Errico and B.-G. Rong, Clean Technol. Environ. Policy, 2018, 20, 1401–1412 CrossRef.
- C. I. Santos, C. C. Silva, S. I. Mussatto, P. Osseweijer, L. A. M. van der Wielen and J. A. Posada, Renewable Energy, 2018, 129, 733–747 CrossRef CAS.
- S. Bello, C. Ríos, G. Feijoo and M. T. Moreira, Biofuels, Bioprod. Biorefin., 2018, 12, 1047–1064 CrossRef CAS.
- M. K. Patel, A. Bechu, J. D. Villegas, M. Bergez-Lacoste, K. Yeung, R. Murphy, J. Woods, O. N. Mwabonje, Y. Ni, A. D. Patel, J. Gallagher and D. Bryant, Biofuels, Bioprod. Biorefin., 2018, 12, 426–441 CrossRef CAS.
- M. Nieder-Heitmann, K. Haigh, J. Louw and J. F. Görgens, Biofuels, Bioprod. Biorefin., 2020, 14, 55–77 CrossRef CAS.
- S. Mesfun, L. Matsakas, U. Rova and P. Christakopoulos, Energies, 2019, 12, 4206 CrossRef CAS.
- A. Rodrigues Gurgel da Silva, A. Giuliano, M. Errico, B.-G. Rong and D. Barletta, Clean Technol. Environ. Policy, 2019, 21, 637–654 CrossRef.
- A. Herry, Large Scale Lignin Organosolv Extraction, University of Groningen, 2020 Search PubMed.
- S. Soltanian, M. Aghbashlo, F. Almasi, H. Hosseinzadeh-Bandbafha, A.-S. Nizami, Y. S. Ok, S. S. Lam and M. Tabatabaei, Energy Convers. Manage., 2020, 212, 112792 CrossRef CAS.
- N. Ryan and P. Yaseneva, Philos. Trans. R. Soc., A, 2021, 379, 20200335 CrossRef CAS PubMed.
- L. B. Dornelles, R. M. Filho and A. P. Mariano, Ind. Crops Prod., 2021, 173, 114097 CrossRef CAS.
- R. A. Ospina-Varón, F. E. López-Suárez and V. Aristizábal-Marulanda, Biomass Convers. Biorefin., 2022, 12, 4245–4256 CrossRef.
- M. Ouhimmou, M. Rönnqvist and L.-A. Lapointe, Omega, 2021, 99, 102173 CrossRef.
- L. Zeilerbauer, J. Lindorfer, R. Süss and B. Kamm, Biofuels, Bioprod. Biorefin., 2022, 16, 370–388 CrossRef CAS.
- K. Heiko, S. Julian, R. Guido, G. Sven and R. Nils, Integrated sustainability assessment of an innovative lignocellulose biorefinery concept based on the acetone organosolv process, UNRAVEL project GA No. 792004,EU Horizon 2020, https://www.ifeu.de/en/project/unravel/.
- D. W. K. Chin, S. Lim, Y. L. Pang and M. K. Lam, Biofuels, Bioprod. Biorefin., 2020, 14, 808–829 CrossRef.
- L. G. Nair, K. Agrawal and P. Verma, Bioresour. Bioprocess., 2023, 10, 50 CrossRef.
- P. P. Thoresen, L. Matsakas, U. Rova and P. Christakopoulos, Bioresour. Technol., 2020, 306, 123189 CrossRef CAS PubMed.
- A. De Santi, M. V. Galkin, C. W. Lahive, P. J. Deuss and K. Barta, ChemSusChem, 2020, 13, 4468–4477 CrossRef CAS.
- Á. Valdespino-Rodríguez, D. J. Pérez-Martín, M. F. González-Barajas, H. Villicaña-Ambriz, D. Campos-Castellanos, F. M. Escalante, A. Sánchez and E. Aguilar-Garnica, J. Chem. Technol. Biotechnol., 2020, 95, 594–603 CrossRef.
- M. E. Vuillemin, M. C. Quesada-Salas, C. Hadad, J. Jasniewski, E. Husson and C. Sarazin, RSC Sustainability, 2023, 1, 853–865 RSC.
- G. T. Wurzler, V. T. da Silva, D. de Almeida Azevedo, A. S. Ana da Silva and F. B. Noronha, Fuel Process. Technol., 2022, 230, 107208 CrossRef CAS.
- D. Pradhan, S. Jaiswal and A. K. Jaiswal, Biofuels, Bioprod. Biorefin., 2022, 16, 1849–1868 CrossRef CAS.
- D. Sidiras, D. Politi, G. Giakoumakis and I. Salapa, Bioresour. Technol., 2022, 343, 126158 CrossRef CAS PubMed.
- M. Karlsson, V. L. Vegunta, R. Deshpande and M. Lawoko, Green Chem., 2022, 24, 1211–1223 RSC.
- S. Serna-Loaiza, J. Adamcyk, S. Beisl, M. Miltner and A. Friedl, Waste Biomass Valorization, 2022, 13, 4771–4784 CrossRef CAS.
- N. Asadi, M. Karimi Alavijeh and H. Zilouei, Int. J. Hydrogen Energy, 2018, 43, 8718–8728 CrossRef CAS.
- O. Gordobil, R. Herrera, M. Yahyaoui, S. İlk, M. Kaya and J. Labidi, RSC Adv., 2018, 8, 24525–24533 RSC.
- C. Scarica, R. Suriano, M. Levi, S. Turri and G. Griffini, ACS Sustainable Chem. Eng., 2018, 6, 3392–3401 CrossRef CAS.
- D. B. Tiz, F. A. Vicente, A. Kroflič and B. Likozar, ACS Sustainable Chem. Eng., 2023, 11, 13836–13867 CrossRef CAS.
- R. Nitzsche, H. Etzold, M. Verges, A. Gröngröft and M. Kraume, Membranes, 2022, 12, 82 CrossRef CAS PubMed.
- R. M. Abdilla-Santes, W. Guo, P. C. A. Bruijnincx, J. Yue, P. J. Deuss and H. J. Heeres, ChemSusChem, 2019, 12, 4304–4312 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |