Non-noble-metal bismuth nanoparticle-decorated bismuth vanadate nanoarray photoanode for efficient water splitting†
Ba-Ri
Wulan
,
Sha-Sha
Yi
,
Si-Jia
Li
,
Yan-Xin
Duan
,
Jun-Min
Yan
 *,
Xin-Bo
Zhang
*,
Xin-Bo
Zhang
 and
Qing
Jiang
and
Qing
Jiang

Key Laboratory of Automobile Materials (Jilin University), Ministry of Education, Department of Materials Science and Engineering, Jilin University, Changchun, 130022, China. E-mail: junminyan@jlu.edu.cn
First published on 4th July 2018
Abstract
Sunlight-driven photoelectrochemical (PEC) water splitting using earth-abundant semiconductor-based materials offers one promising strategy to produce attainable and sustainable carbon free energy. Herein, we demonstrate for the first time that a heterojunction nanostructure of a Bi/BiVO4 photoanode is fabricated by directly coupling semimetal Bi nanoparticles to BiVO4 nanoarrays, showing excellent water oxidation performance. The as-obtained photoanode exhibits a remarkable photocurrent density of 1.96 mA cm−2 at 1.23 V versus a reversible hydrogen electrode (vs. RHE) under AM 1.5G (100 mW cm−2) irradiation, which is approximately 2-fold higher than that of the pristine BiVO4. Based on the detailed analyses of J–V, i–t, M–S and EIS plots, the reason for the high photocurrent density of Bi/BiVO4 can be attributed to the improved charge separation efficiency, the enhanced hole injection efficiency and the suppressed back reaction of water oxidation.
1. Introduction
Harnessing solar energy for chemical energy conversion and storage has long been targeted as an attractive strategy to meet the rapid depletion of fossil fuels,1–3 especially for photoelectrochemical (PEC) water splitting because its product, hydrogen (H2), is a clean and easy-to-store fuel source that cannot produce greenhouse gas emissions on combustion.4 Semiconductor-based materials for high-efficiency PEC water oxidation reactions strongly depend on superior visible light utilization, efficient photogenerated electron–hole pair separation and rapid charge transfer character.5,6 In this regard, BiVO4 is attractive because of its narrow energy bandgap (2.4–2.5 eV), and suitable band edge positions for the oxygen evolution reaction (OER) from water splitting.7,8 However, its solar-to-energy conversion efficiency has been impeded by its poor carrier mobility and fast electron–hole recombination.9,10 Consequently, these disadvantages directly affect the bulk charge separation efficiency (Psep) and surface hole injection efficiency (Pinj) of a BiVO4 photoanode, which are two significant criteria for evaluating its PEC performance.11In related recent studies, in order to enhance the Psep and Pinj of BiVO4 photoanodes, various strategies focused on doping elements,12,13 depositing oxygen evolution catalysts (OECs),14–16 constructing heterojunctions,17,18 controlling the nanostructure,19 loading precious metal (e.g., Au, Ag and Pd) nanoparticles,20–22etc., have been conducted, which are aimed at enhancing the charge carrier concentration and regulating their separation and transfer efficiency. Among these strategies, it is not surprising that loading noble metals (NMs) onto the surface of BiVO4 is undoubtedly an efficient pathway, due to their low overpotential and relatively large work function.23,24 As the Fermi level of NMs is lower than that of BiVO4, the photogenerated electrons in the conduction band of BiVO4 can be rapidly transferred to the metals when they come in contact, reducing the electron–hole recombination and enhancing their separation.25 However, the high cost of NMs greatly restricts their extensive usage, and attention is now turning to the much more low-cost metals and how these metals influence the PEC water oxidation reaction of a BiVO4 photoanode. Semimetal bismuth (Bi) possesses a unique electronic structure, surface plasmon resonance (SPR) and small energy overlap between the conduction band (ECB) and valence band (EVB), and has attracted much attention in the area of PEC water oxidation and removal of NO.26–28 However, heterojunction nanostructures constructed by Bi and BiVO4 have rarely been reported, and we have reason to believe that the Bi/BiVO4 hybrid structure can achieve a high PEC performance for water oxidation.
Herein, as a proof-of-concept experiment, a Bi/BiVO4 photoanode is successfully synthesized using a facile reduction method, in which tannic acid (TA) and NaBH4 are used as the dispersant and reducing agent respectively to obtain small metallic Bi nanoparticles. The well-designed as-prepared Bi/BiVO4 photoanode displays an enhanced photocurrent density of 1.96 mA cm−2 at 1.23 V vs. RHE with respect to pristine BiVO4, which may result from the promoted electron–hole pair separation, the enhanced hole injection efficiency and the suppressed back reaction of PEC water oxidation.
2. Results and discussion
The synthesis process for Bi/BiVO4 is demonstrated in Fig. 1a. Typically, BiVO4 is primarily synthesized by the electrodeposition method.15 And then, Bi(NO3)3·5H2O is introduced into a DMSO solution with strong magnetic stirring. After that, a TA and NaBH4 mixed solution is slowly added into the above solution. After 10 min of magnetic stirring at room temperature, Bi/BiVO4 is obtained.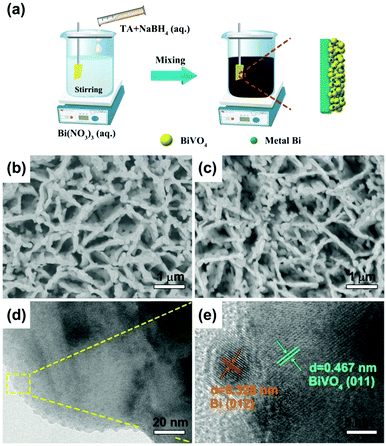 | ||
| Fig. 1 (a) Scheme illustration of the preparation process for Bi/BiVO4. Top-view FESEM images of (b) BiVO4 and (c) 0.1Bi/BiVO4. (d) TEM and (e) HRTEM images of 0.1Bi/BiVO4. | ||
Fig. 1b and c demonstrate the field emission scanning electron microscopy (FESEM) images of BiVO4 and 0.1Bi/BiVO4. It can be seen that the synthesized 0.1Bi/BiVO4 has a similar structure to pure BiVO4, while no discernable particles of Bi are observed on the surface of BiVO4, owning to its low content and small particle size. Transmission electron microscopy (TEM) and high resolution TEM (HRTEM) images give further insight into the structure of the composite sample, as shown in Fig. 1d and e. Small particles on the surface of BiVO4 can be obviously observed in the 0.1Bi/BiVO4 hybrid. The HRTEM image (Fig. 1e) of 0.1Bi/BiVO4 clearly reveals lattice fringe spacings of 0.467 and 0.328 nm, corresponding to the (011) and (012) planes of BiVO4 and Bi metal, respectively, proving that the metallic Bi particles are successfully loaded onto the BiVO4.
Furthermore, we perform X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) to investigate the structure, surface chemical composition and valence states of the specimens. In Fig. 2a, no XRD distinct peaks of Bi can be detected in the specimen of 0.1Bi/BiVO4, expect for the peaks of BiVO4 (JCPDS 14-0688)29 and SnO2 (JCPDS 46-1088)30 due to its low content or high dispersion. Fig. 2b shows the high-resolution XPS spectrum of Bi 4f; two pairs of spin–orbit doublets can be observed in 0.1Bi/BiVO4, suggesting the existence of Bi0 (Bi–Bi bonding energy peaks at 156.9 and 162.2 eV) and Bi3+ (Bi–O bonding energy peaks at 158.9 and 164.2 eV).31 The O 1s of 0.1Bi/BiVO4 in Fig. 2c can be separated into two peaks at binding energy values of 529.8 and 531.0 eV, assigned to lattice oxygen atoms (O2−) and the absorbed hydroxyl groups, respectively.32,33 Two bands located at 516.5 and 523.9 eV are attributed to the V5+ ions in BiVO4 as shown in Fig. 2d.31 Additionally, the XPS bonding energies of Bi 4f, V 2p and O 1s in 0.1Bi/BiVO4 all slightly shift to lower values (ca. 0.1–0.2 eV) compared with BiVO4. These shifts demonstrate that some electrons are transferred from Bi to BiVO4 in Bi/BiVO4. Such electrons have the potential to endow high performance for PEC water oxidation.
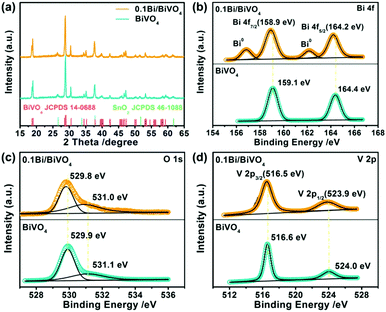 | ||
| Fig. 2 (a) XRD patterns of BiVO4 and 0.1Bi/BiVO4. (b–d) High-resolution XPS spectra of Bi 4f, O 1s and V 2p of BiVO4 and 0.1Bi/BiVO4, respectively. | ||
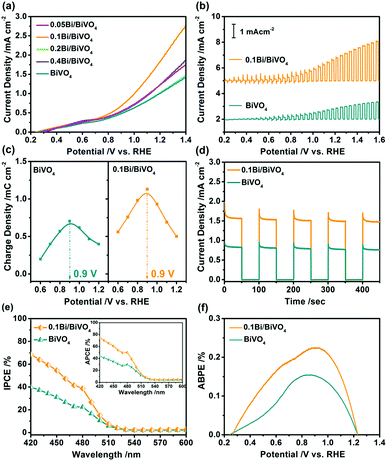
PEC water oxidation measurements were performed to study the performance of water splitting of the as-obtained photoelectrodes in a three-electrode system. The photocurrent–potential (J–V) curves of Bi/BiVO4 for water oxidation are shown in Fig. 3a, pure BiVO4 shows low photocurrent density in the whole potential because of its fast electron–hole recombination. Interestingly, after loading of Bi, the Bi/BiVO4 specimens show remarkably increased photocurrent density with respect to BiVO4. Particularly for 0.1Bi/BiVO4, it possesses the highest photocurrent density of 1.96 mA cm−2 at 1.23 V vs. RHE, which is nearly two times higher than that of the bulk BiVO4. Fig. 3b shows the transient J–V curves of 0.1Bi/BiVO4 and BiVO4, they all display a good response with light turn on/off cycles. In addition, the spike peaks are evidently detected at various voltages, which can be attributed to the accumulation of the photogenerated holes on the electrode/electrolyte interface.34,35 For further investigation, the accumulated positive charge density as a function of applied potential for the two samples is collected as shown in Fig. S1 (ESI†). As a result, the amount of hole storage of 0.1Bi/BiVO4 is remarkable increased compared with that of BiVO4 at the bias potential range from 0.6 to 1.2 V vs. RHE (Fig. 3c), indicating that the photogenerated holes from the bulk BiVO4 can be efficiently extracted by the metallic Bi on its surface and subsequently participate in water oxidation.36 The transient i–t curves are also performed to investigate the performance of the samples, and as illustrated in Fig. 3d, 0.1Bi/BiVO4 shows a high light response value, which is in agreement with the results in the J–V curves.
In order to further understand the performance of the samples, the incident photon-to-current efficiency (IPCE), absorbed photon-to-current efficiency (APCE) and applied bias photon-to-current efficiency (ABPE) are determined. Fig. 3e shows the IPCE spectra of the 0.1Bi/BiVO4 and BiVO4 electrodes at 1.23 V vs. RHE, indicating that the hybrid photoanode exhibits a remarkable improvement in IPCE value in the wavelength range from 420–600 nm and displays nearly two times enhancement at 420 nm (70%) related to that of the BiVO4 (40%). The inset image of Fig. 3e also shows the superior APCE performance of 0.1Bi/BiVO4 as compared with BiVO4, with its highest value of 80% achieved at 420 nm. In Fig. 3f, a maximum ABPE of 0.225% is achieved at 0.9 V vs. RHE for 0.1Bi/BiVO4, which is 1.48 times higher than that of BiVO4 (0.152%). Furthermore, the 0.1Bi/BiVO4 photoanode possesses fairly good stability (Fig. S2, ESI†).
To investigate the influence of metallic Bi on the electrode's optical properties, ultraviolet-visible diffuse reflectance spectra (UV-vis DRS) are performed. As shown in Fig. 4a, there is no obvious difference between the 0.1Bi/BiVO4 and BiVO4 photoanode in the light range of 300–500 nm, which might be attributed to the low loading of Bi on the 0.1Bi/BiVO4. Accordingly, the light harvesting efficiency (LHE) has similar results (Fig. S3a, ESI†). In addition, the band gaps (Eg) of the BiVO4 and 0.1Bi/BiVO4 are evaluated to be 2.53 and 2.49 eV, respectively (Fig. 4b), based on the Kubelka–Munk function.
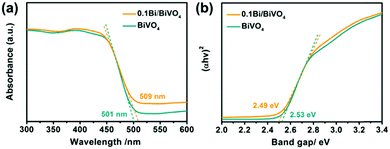 | ||
| Fig. 4 (a) UV-vis DRS and (b) plots of the (αhν)2vs. photon energy (hν) for BiVO4 and 0.1Bi/BiVO4, respectively. | ||
Fundamentally, the photocurrent (Jph) of a photoelectrode is determined by three main factors including LHE, Psep and Pinj.11,37–39 For calculations of Psep and Pinj, the Jabs (the photo absorption rate expressed as current density) of the two photoelectrodes is first obtained (Fig. S3b, ESI†). In our experiments, the influence of Bi on the Psep and Pinj of BiVO4 is studied with the sacrificial donor of Na2SO3 (Fig. S4, ESI†); as a result, 0.1Bi/BiVO4 shows evidently enhanced Psep and Pinj compared with the pure BiVO4 (Fig. 5a and b). Particularly for Pinj, 0.1Bi/BiVO4 shows the value of 41.3% at 1.23 V vs. RHE relative to the BiVO4 (22.4%), indicating that metallic Bi may serve as a cocatalyst to reduce the injection barrier of photogenerated holes, resulting in enhanced water oxidation efficiency on the surface of BiVO4.
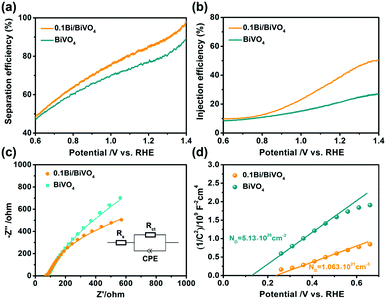 | ||
| Fig. 5 (a) Separation efficiency. (b) Injection efficiency. (c) Nyquist plots and (d) Mott–Schottky plots of BiVO4 and 0.1Bi/BiVO4 photoelectrodes, respectively. | ||
Fig. 5c shows the typical electrochemical impedance spectroscopy (EIS) of the as-obtained electrodes recorded at the open circuit potential under illumination, which is used to evaluate the charge transfer resistance based on the equivalent circuit model (inset of Fig. 5c). Rs shows the solution resistance, and Rct and CPE represent the charge transfer resistance at the photoelectrode/electrolyte interface and capacitance phase element, respectively.40 Compared with the BiVO4 (412.1 Ω), the fitted value of Rct for 0.1Bi/BiVO4 is 189.7 Ω, indicating that the transfer resistance for the photogenerated holes from the electrode to the electrolyte is effectively decreased. In other words, Bi-decorated BiVO4 possesses a much higher charge transfer efficiency with respect to pristine BiVO4, which remarkably hinders the recombination of photogenerated electrons and holes. Mott–Schottky (M–S) plots are also performed to get further insight into the role that Bi plays in the 0.1Bi/BiVO4. As shown in Fig. 5d, after Bi-decoration, the carrier density (ND) value of BiVO4 (5.13 × 1020 cm−3) is increased to be 1.06 × 1021 cm−3, indicating that Bi can enhance the electronic conductivity of BiVO4 and thus improve the water oxidation performance.41,42
In order to further investigate the reason for the improved PEC water oxidation, the dark currents of the BiVO4 and 0.1Bi/BiVO4 are measured in 0.5 M Na2SO4 with different gas bubbling.35,43 As shown in Fig. 6, the 0.1Bi/BiVO4 and BiVO4 show a similar reduction dark current density when bubbling N2 into the electrolyte. Interestingly, significantly enhanced current densities can be detected for both 0.1Bi/BiVO4 and BiVO4 when the N2 is replaced by O2, and BiVO4 shows a much larger reduction current density than that of 0.1Bi/BiVO4. These above results imply that the O2 reduction reaction on the surface of BiVO4 is much more suppressed after decorating with metallic Bi. When the O2 is produced by oxidizing H2O on the electrode under continuous illumination, the photogenerated electrons on the conduction band might reduce O2 in part. Thus, the O2 reduction reaction is the back reaction of water oxidation, causing the decreased photocurrent. As a consequence, the suppression of water reduction is another reason for high PEC water oxidation activity.
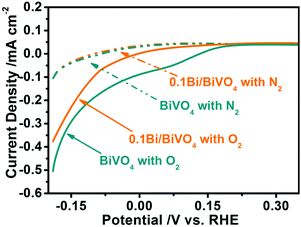 | ||
| Fig. 6 Reduction dark current of BiVO4 and 0.1Bi/BiVO4 in a 0.5 M Na2SO4 electrolyte with different gas bubbling (N2 or O2). | ||
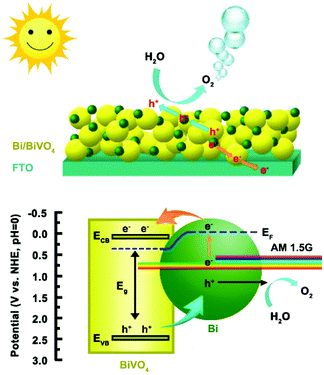
The energy band position of the 0.1Bi/BiVO4 photoanode is also determined to further investigate the reason for the improved performance. The flat band potential (Efb) of BiVO4 is estimated to be about 0.13 V vs. RHE (Fig. 5d). It is well known that, for a n-type semiconductor, the ECB is more negative by about 0.1 V than Efb.1,44 Thus, the ECB of BiVO4 is calculated to be 0.03 V vs. RHE. The EVB is calculated to be 2.56 V vs. RHE according to the equation of EVB = ECB + Eg.45 Based on the above analysis, the possible mechanism for the enhanced PEC water oxidation on 0.1Bi/BiVO4 is proposed, as shown in Scheme 1. It is apparent that the Bi loading on the surface of BiVO4 provides an appropriate structure for charge separation and transportation. Given that the Fermi level of BiVO4 (ca. 0.5 V vs. RHE) is lower than that of the metallic Bi (ca. −0.22 V vs. RHE),46 then the electrons will redistribute across the interface until the Fermi level reaches an equilibrium state. Under AM 1.5G irradiation, both Bi and BiVO4 can be excited to generate electrons and holes, wherein Bi shows the surface plasma resonance (SPR) effect.47,48 Then hot electrons from Bi can quickly inject into the CB of BiVO4, which can be evidenced by XPS results, and in the meantime the VB-holes of BiVO4 can transfer to the Bi metal, resulting in efficient charge separation and transportation. The electrons collected on BiVO4 then flow to the FTO substrate, and ultimately to the Pt counter electrode for water reduction. Also the holes accumulated on Bi can participate for the water oxidation reaction. In addition, the introduction of Bi on BiVO4 can also greatly suppress the water oxidation back reaction, which is beneficial for improving the PEC performance. Therefore, the efficient charge separation and transportation, the enhanced hole injection efficiency and the suppressed back reaction all contribute to the excellent performance for water oxidation on the Bi/BiVO4 hybrid.
3. Conclusions
In summary, heterojunction nanostructures constructed by Bi decorated BiVO4 have been successfully synthesized using a facile method for efficient PEC water oxidation. The 0.1Bi/BiVO4 photoelectrode exhibits a good photocurrent density and surface charge injection efficiency, due to the accelerated charge separation at the electrode/electrolyte interface as well as the suppressed water back reaction. Additionally, the present reduction method for the deposition of a co-catalyst may bring new insight for the fabrication of novel photoanodes towards solar energy conversion.4. Experimental section
4.1. Materials
Bismuth nitrate pentahydrate (Bi(NO3)3·5H2O, Xilong Scientific, ≥99%), potassium iodide (KI, Beijing Chemical Works, ≥98.5%), p-benzoquinone (C6H4O2, Sinopharm Chemical Reagent Co., Ltd, ≥98%), dimethyl sulfoxide (DMSO, Sinopharm Chemical Reagent Co., Ltd, ≥99%), vanadyl acetylacetonate (VO(acac)2, Sinopharm Chemical Reagent Co., Ltd, ≥99%), hydrogen nitrate (HNO3, Beijing Chemical Works, 65–68%), sodium hydroxide (NaOH, Sinopharm Chemical Reagent Co., Ltd, 96%), ethanol (C2H5OH, Beijing Chemical Works, ≥99.7%), sodium borohydride (NaBH4, Sinopharm Chemical Reagent Co., Ltd, 96%), and tannic acid (C76H52O46, Sinopharm Chemical Reagent Co., Ltd, 99.9%) were used. Fluorine-doped tin oxide (F: SnO2, FTO, <15 ohm sq−1) glass was purchased from Zhuhai Kaivo Optoelectronic Technology Co., Ltd. Before use, the FTO was cleaned by sonication in acetone, ethanol and water, respectively, for 10 min and dried at room temperature. Ultrapure water with the specific resistance of 18.2 MΩ cm was obtained by reverse osmosis followed by ion-exchange and filtration. All of the raw materials are analytical reagents and used as purchased without further purification.4.2. Preparation of the BiVO4 photoelectrode.
The BiVO4 photoelectrode was prepared by a previously reported electrodeposition method.15 Typically, the BiOI nanosheets were firstly synthesized via an electrochemical deposition in a typical three-electrode cell with an FTO working electrode (WE), a Ag/AgCl (sat. KCl) reference electrode (RE) and a platinum wire counter electrode (CE). 20 mmol of KI was dissolved in 50 mL of H2O and the pH was first adjusted to about 1.7 by adding 100 μL of HNO3. Then, 0.97 g of Bi(NO3)3·5H2O was added to the above solution with stirring for 10 min. The resultant solution was mixed with 20 mL of ethanol containing 0.23 M p-benzoquinone with magnetic stirring for 10 min. The electrodeposition was performed potentiostatically at −0.1 V vs. Ag/AgCl for 300 s, which formed the BiOI film on the surface of FTO. Then, 100 μL of DMSO solution containing 0.2 M vanadylacetylacetonage (Vo(acac)2) was dropped onto the as-obtained BiOI film and heated in a muffle furnace at 450 °C for 2 h with a heating rate of 2 °C min−1. After cooling down, the electrode was soaked in NaOH solution (1 M) for 30 min to remove excess V2O5. Finally, the resulting BiVO4 photoelectrode was rinsed completely with distilled water and dried at room temperature.4.3. Preparation of the Bi/BiVO4 photoelectrode.
A facile reduction method was used to deposit Bi nanoparticles on BiVO4. Briefly, BiVO4 grown on the FTO substrate was vertically soaked in 50 mL of DMSO solution containing 0.1 mmol of Bi(NO3)3·5H2O under stirring. And finally, a mixture of TA (50 mg) and NaBH4 (10 mg) was dissolved into the above mixed solution with magnetic stirring for 10 minutes, the resultant Bi/BiVO4 was dried before being washed with water and ethanol several times, which was denoted as 0.1Bi/BiVO4. For comparison, Bi/BiVO4 materials with different loading contents of Bi (0.05, 0.2 and 0.4 mmol) were also obtained by the method mentioned above, and were denoted here as 0.05Bi/BiVO4, 0.2Bi/BiVO4 and 0.4Bi/BiVO4, respectively.4.4. Characterization
Wide angle X-ray diffraction (XRD) measurements were performed on a Rigaku D/max-2500pc X-ray diffractometer with Cu Kα irradiation (λ = 1.5406 Å) with a scan rate of 4° min−1. A JEOL JSM-6700F field emission scanning electron microscope (FESEM) with an acceleration voltage of 8 kV was applied to analyze the microstructure. Transmission electron microscope (TEM) analyses were performed using a JEOL JEM-2010 microscope (accelerating voltage = 200 kV). X-ray photoelectron spectroscopy (XPS) analyses were conducted using an ESCALAB MK II (Vacuum Generators) spectrometer using Al Kα radiation (300 W). All binding energies were referenced to the C 1s peak at 284.6 eV of the surface adventitious carbon. Ultraviolet-visible diffuse reflectance spectra (UV-vis DRS) were recorded on a Beijing Purkinje General TU-1900 ultraviolet-visible spectrophotometer, in which BaSO4 was used as the background.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Ba-Ri Wulan and Sha-Sha Yi contributed equally to this work. This work is supported in part by the National Natural Science Foundation of China (51522101, 51471075, and 51631004); the Program for JLU Science and Technology Innovative Research Team (2017TD-09); and the Fundamental Research Funds for the Central Universities.References
- X. Li, J. Yu, J. Low, Y. Fang, J. Xiao and X. Chen, J. Mater. Chem. A, 2015, 3, 2485 RSC.
- R. Liu, Z. Zheng, J. Spurgeon and X. Yang, Energy Environ. Sci., 2014, 7, 2504 RSC.
- Z. Li, W. Luo, M. Zhang, J. Feng and Z. Zou, Energy Environ. Sci., 2013, 6, 347 RSC.
- M. G. Walter, E. L. Warren, J. R. McKone, S. W. Boettcher, Q. Mi, E. A. Santori and N. S. Lewis, Chem. Rev., 2010, 110, 6446 CrossRef PubMed.
- S. Shen, S. A. Lindley, X. Chen and J. Z. Zhang, Energy Environ. Sci., 2016, 9, 2744 RSC.
- Q. Lu, Y. Yu, Q. Ma, B. Chen and H. Zhang, Adv. Mater., 2016, 28, 1917 CrossRef PubMed.
- E. A. Mohamed, Z. N. Zahran and Y. Naruta, J. Mater. Chem. A, 2017, 5, 6825 RSC.
- D. K. Zhong, S. Choi and D. R. Gamelin, J. Am. Chem. Soc., 2011, 133, 18370 CrossRef PubMed.
- Y. Park, K. J. McDonald and K. S. Choi, Chem. Soc. Rev., 2013, 42, 2321 RSC.
- S. K. Pilli, T. E. Furtak, L. D. Brown, T. G. Deutsch, J. A. Turner and A. M. Herring, Energy Environ. Sci., 2011, 4, 5028 RSC.
- Q. Bu, S. Li, S. Cao, Q. Zhao, Y. Chen, D. Wang and T. Xie, Dalton Trans., 2017, 46, 10549 RSC.
- L. Fang, F. Nan, Y. Yang and D. Cao, Appl. Phys. Lett., 2016, 108, 093902 CrossRef.
- B. Zhang, H. Zhang, Z. Wang, X. Zhang, X. Qin, Y. Dai, Y. Liu, P. Wang, Y. Li and B. Huang, Appl. Catal., B, 2017, 211, 258 CrossRef.
- T. Wang, H. T. Hung, Y. R. Cheng, M. C. Huang, Y. K. Hsieh and C. F. Wang, RSC Adv., 2016, 6, 28236 RSC.
- T. W. Kim and K. S. Choi, Science, 2014, 343, 990 CrossRef PubMed.
- M. Zhong, T. Hisatomi, Y. Kuang, J. Zhao, M. Liu, A. Iwase, Q. Jia, H. Nishiyama, T. Minegishi, M. Nakabayashi, N. Shibata, R. Niishiro, C. Katayama, H. Shibano, M. Katayama, A. Kudo, T. Yamada and K. Domen, J. Am. Chem. Soc., 2015, 137, 5053 CrossRef PubMed.
- S. Y. Chae, C. S. Lee, H. Jung, O. S. Joo, B. K. Min, J. H. Kim and Y. J. Hwang, ACS Appl. Mater. Interfaces, 2017, 9, 19780 CrossRef PubMed.
- H. He, Y. Zhou, G. Ke, X. Zhong, M. Yang, L. Bian, K. Lv and F. Dong, Electrochim. Acta, 2017, 257, 181 CrossRef.
- M. G. Lee, D. H. Kim, W. Sohn, C. W. Moon, H. Park, S. Lee and H. W. Jang, Nano Energy, 2016, 28, 250 CrossRef.
- C. N. Van, W. S. Chang, J. W. Chen, K. A. Tsai, W. Y. Tzeng, Y. C. Lin, H. H. Kuo, H. J. Liu, K. D. Chang, W. C. Chou, C. L. Wu, Y. C. Chen, C. W. Luo, Y. J. Hsu and Y. H. Chu, Nano Energy, 2015, 15, 625 CrossRef.
- J. Li, J. Zhou, H. Hao and Z. Zhu, Mater. Lett., 2016, 170, 163–166 CrossRef.
- W. Yang, Y. Xiong, L. Zou, Z. Zou, D. Li, Q. Mi, Y. Wang and H. Yang, Nanoscale Res. Lett., 2016, 11, 283 CrossRef PubMed.
- L. Wu, F. Li, Y. Xu, J. W. Zhang, D. Zhang, G. Li and H. Li, Appl. Catal., B, 2015, 164, 217 CrossRef.
- D. Y. Leung, X. Fu, C. Wang, M. Ni, M. K. Leung, X. Wang and X. Fu, ChemSusChem, 2010, 3, 681 CrossRef PubMed.
- K. Chang, X. Hai and J. Ye, Adv. Energy Mater., 2016, 6, 1502555 CrossRef.
- F. Dong, Q. Li, Y. Sun and W. K. Ho, ACS Catal., 2014, 4, 4341 CrossRef.
- Z. Wang, S. Yan, Y. Sun, T. Xiong, F. Dong and W. Zhang, Appl. Catal., B, 2017, 214, 148 CrossRef.
- Q. Wang, J. He, Y. Shi, S. Zhang, T. Niu, H. She and Y. Bi, Chem. Eng. J., 2017, 326, 411 CrossRef.
- L. Xia, J. Bai, J. Li, Q. Zeng, L. Li and B. Zhou, Appl. Catal., B, 2017, 204, 127 CrossRef.
- L. Xi, P. S. Bassi, S. Y. Chiam, W. F. Mak, P. D. Tran, J. Barber, J. S. Chye Loo and L. H. Wong, Nanoscale, 2012, 4, 4430 RSC.
- J. Sun, X. Li, Q. Zhao, M. O. Tadé and S. Liu, Appl. Catal., B, 2017, 219, 259 CrossRef.
- C. Y. Wang, Y. J. Zhang, W. K. Wang, D. N. Pei, G. X. Huang, J. J. Chen, X. Zhang and H. Q. Yu, Appl. Catal., B, 2018, 221, 320 CrossRef.
- P. Li, X. Chen, H. He, X. Zhou, Y. Zhou and Z. Zou, Adv. Mater., 2018, 30, 1703119 CrossRef PubMed.
- S. Li, Q. Zhao, D. Meng, D. Wang and T. Xie, J. Mater. Chem. A, 2016, 4, 16661 RSC.
- Z. Fan, Z. Xu, S. Yan and Z. Zou, J. Mater. Chem. A, 2017, 5, 8402 RSC.
- Y. Yang, G. Liu, J. T. Irvine and H. M. Cheng, Adv. Mater., 2016, 28, 5850 CrossRef PubMed.
- Z. Wang, J. Han, Z. Li, M. Li, H. Wang, X. Zong and C. Li, Adv. Energy Mater., 2016, 6, 1600864 CrossRef.
- Z. Wang, G. Liu, C. Ding, Z. Chen, F. Zhang, J. Shi and C. Li, J. Phys. Chem. C, 2015, 119, 19607 CrossRef.
- G. Liu, S. Ye, P. Yan, F. Xiong, P. Fu, Z. Wang, Z. Chen, J. Shi and C. Li, Energy Environ. Sci., 2016, 9, 1327 RSC.
- K. P. Parmar, H. J. Kang, A. Bist, P. Dua, J. S. Jang and J. S. Lee, ChemSusChem, 2012, 5, 1926 CrossRef PubMed.
- S. P. Berglund, A. J. Rettie, S. Hoang and C. B. Mullins, Phys. Chem. Chem. Phys., 2012, 14, 7065 RSC.
- Y. Ma, S. R. Pendlebury, A. Reynal, F. Le Formal and J. R. Durrant, Chem. Sci., 2014, 5, 2964 RSC.
- D. Cao, W. Luo, J. Feng, X. Zhao, Z. Li and Z. Zou, Energy Environ. Sci., 2014, 7, 752 RSC.
- S. S. Yi, J. M. Yan, B. R. Wulan, S. J. Li, K. H. Liu and Q. Jiang, Appl. Catal., B, 2017, 200, 477 CrossRef.
- S. S. Yi, J. M. Yan, B. R. Wulan and Q. Jiang, J. Mater. Chem. A, 2017, 5, 15862 RSC.
- Z. Jiao, Y. Zhang, S. Ouyang, H. Yu, G. Lu, J. Ye and Y. Bi, ACS Appl. Mater. Interfaces, 2014, 6, 19488 CrossRef PubMed.
- P. Zhang, T. Song, T. Wang and H. Zeng, Appl. Catal., B, 2018, 225, 172 CrossRef.
- M. G. Lee, C. W. Moon, H. Park, W. Sohn, S. B. Kang, S. Lee, K. J. Choi and H. W. Jang, Small, 2017, 13, 1701644 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8qm00239h |
| This journal is © the Partner Organisations 2018 |