Open Access Article
Open Access ArticleCatalytic stereoselective synthesis of all-carbon tetra-substituted alkenes via Z-selective alkyne difunctionalization†
Prashant S.
Shinde‡
a,
Valmik S.
Shinde‡
ab and
Magnus
Rueping
 *a
*a
aKAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Saudi Arabia. E-mail: magnus.rueping@kaust.edu.sa
bMedicinal and Process Chemistry Division, CSIR-Central Drug Research Institute, Lucknow 226031, Uttar Pradesh, India
First published on 13th March 2025
Abstract
We report a Ni-catalyzed cascade reaction leading to the arylation of an alkyne-induced acyl migration and the formation of all-carbon tetra-substituted alkenes in good yields with exclusive Z-selectivity. This transformation involves the generation of a nucleophilic vinyl-Ni species through regioselective syn-aryl nickelation of the alkynes, followed by an intramolecular acyl migration. The steric and electronic properties of the phosphine ligands are crucial for achieving high regio- and stereocontrol in this migratory carbo-acylation process. The synthetic utility of the resulting Z-tetra-substituted alkenes is also demonstrated.
All-carbon tetrasubstituted olefins bearing four different carbon-based groups are ubiquitous motifs present in numerous natural products and have various applications from medicinal to materials chemistry (Fig. 1).1–9 Due to their broad applications, significant research efforts are focused on developing general protocols for the challenging stereoselective synthesis, particularly for acyclic structures.8,10–16 While strategies exist for synthesizing stereo defined E-alkenes, their thermodynamically less stable Z-isomers remain considerably more challenging. Achieving Z-alkenes with four distinct carbon-based substituents and an adjacent reactive functionality remains a formidable challenge.
Classical methods for forming carbon–carbon double bonds include carbonyl olefinations such as the Wittig reaction and its variants (e.g., Julia, Peterson, McMurry, and Horner–Wadsworth–Emmons reactions), as well as metathesis reactions, elimination processes, and additions to triple bonds (Fig. 2a).17–20 These methods are effective for producing di- or tri-substituted alkenes; however, they typically yield mixtures of stereoisomers when applied to tetrasubstituted alkenes.11
 | ||
| Fig. 2 (a) Synthetic strategies for multi-substituted alkenes; (b) nickel-catalyzed stereoselective synthesis of all-carbon tetra-substituted alkenes via Z-selective alkyne difunctionalization | ||
Additionally, their efficiency diminishes when faced with the high steric demands of tetra-substituted alkenes, making the selective synthesis of these structures a key area of research, particularly over the past years. An alternative approach involves the stereoselective insertion of two carbon-based groups across a C–C triple bond, either in a stepwise manner or through multicomponent strategies, offering a promising route for the synthesis of complex alkenyl products.21–28 However, a major limitation in these transformations is the challenge of achieving regioselectivity, especially with alkynes that have substituents of similar steric or electronic properties. Therefore, developing a general method that enables the synthesis of highly substituted alkene with precise regio- and stereocontrol is crucial for expanding their synthetic utility. A commonly employed method for synthesizing tetrasubstituted alkenes, particularly those with four distinct functional groups, involves the carbometalation of internal alkynes to generate trisubstituted alkenyl metal nucleophiles. These intermediates then react with different electrophiles, often through transition metal-catalyzed cross-coupling processes.21,25–42 Intramolecular capture, particularly in the form of arylative cyclization, occurs readily, facilitating the formation of cyclic scaffolds with good efficiency.43–47 Prompted by these reports and by our continuing research interests in nickel-catalyzed transformations,48–52 we envisioned that by using alkyne-tethered phenolic ester substrate, regioselective syn-aryl nickelation of an alkyne would generate nucleophilic vinyl Ni[II] species that may undergo nucleophilic addition to the carbonyl carbon of tethered ester group (Fig. 2b).47,53,54 We anticipated that the careful choice of bulkier ligands55–66 could assist in the C–O bond cleavage of the intermediate which would result in subsequent intramolecular acyl group migration67 and formation of tetrasubstituted alkene products in a stereoselective manner (Fig. 2b). Herein, we describe the successful development of a nickel-catalyzed tandem alkyne hydroarylation acylation strategy, which proceeds with complete Z-selectivity and high regioselectivity to produce a variety of tetra-substituted alkene products in good-to-excellent yields. The key to the success of this method is the use of Ni-catalysts with bulky monodentate phosphine ligands.
We began our study by reacting 2-hexynyl phenol ester 1a, synthesized in two steps from 2-iodophenol, with phenyl boronic acid 2a using various nickel catalysts in acetonitrile at 90 °C (Table 1). Notably, when using Ni(acac)2·4H2O in combination with the bidentate phosphine ligand 1,2-bis(diphenylphosphino)ethane (L1), the desired alkene product 3a was obtained with high stereoselectivity, achieving a 57% yield (Table 1, entry 1). Analysis of the purified reaction mixture by 1H NMR spectroscopy confirmed the formation of the expected tetrasubstituted alkene 3a with excellent Z-selectivity. The choice of the ligand had a significant effect on the reactivity and selectivity of the transformation.68–71 Systematic studies of various bidentate and monodentate ligands revealed that the use of bulkier monodentate ligands afforded better yield and selectivity (Table 1, entries 1–7). Among the ligands tested, triisopropylphosphine ligand (L7) demonstrated the highest reactivity and was selected for further investigation due to its cleaner reaction profile and excellent Z-selectivity (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Table 1, entry 7). We also evaluated various commercially available Ni-salts in combination with ligand L7, which yielded comparable reactivity (entries 8–11). However, using Ni(0) in place of Ni(II) led to slightly reduced reactivity and selectivity (entry 11). Acetonitrile (MeCN) emerged as the optimal solvent among those tested (entries 12–14). Control experiments confirmed that both the Ni complex and ligand were crucial for the reaction's success. Reducing the catalyst loading to 5 mol% had a detrimental effect on the reaction, resulting in lower yields and longer reaction times. The use of bases, typically used for coupling reactions, resulted in no product formation as the substrate is prone to ester hydrolysis (entry 15). Changing the substrate to the corresponding acetylated phenol resulted in the formation of the corresponding benzofurane product (vide infra).
1) (Table 1, entry 7). We also evaluated various commercially available Ni-salts in combination with ligand L7, which yielded comparable reactivity (entries 8–11). However, using Ni(0) in place of Ni(II) led to slightly reduced reactivity and selectivity (entry 11). Acetonitrile (MeCN) emerged as the optimal solvent among those tested (entries 12–14). Control experiments confirmed that both the Ni complex and ligand were crucial for the reaction's success. Reducing the catalyst loading to 5 mol% had a detrimental effect on the reaction, resulting in lower yields and longer reaction times. The use of bases, typically used for coupling reactions, resulted in no product formation as the substrate is prone to ester hydrolysis (entry 15). Changing the substrate to the corresponding acetylated phenol resulted in the formation of the corresponding benzofurane product (vide infra).
| Entry | Ni-catalyst | Solvent | L | Yieldb,c (%) |
Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E E |
|---|---|---|---|---|---|
| a All reaction were carried out on a 0.2 mmol scale. b Yield determined by GC using dodecane as an internal standard. c Isolated yield. | |||||
| 1 | Ni(acac)2·4H2O | MeCN | L1 | 57 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | Ni(acac)2·4H2O | MeCN | L2 | 52 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | Ni(acac)2·4H2O | MeCN | L3 | 39 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 4 | Ni(acac)2·4H2O | MeCN | L4 | 52 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 5 | Ni(acac)2·4H2O | MeCN | L5 | 80 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | Ni(acac)2·4H2O | MeCN | L6 | 87 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | Ni(acac) 2 ·4H 2 O | MeCN | L7 | 90c |
99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1
|
| 8 | Ni(OAc)2·4H2O | MeCN | L7 | 84 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | Ni(ClO4)2·6H2O | MeCN | L7 | 57 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 10 | NiBr2·3H2O | MeCN | L7 | 62 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 11 | Ni(COD)2 | MeCN | L7 | 86 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 12 | Ni(acac)2·4H2O | Dioxane | L7 | 66 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 13 | Ni(acac)2·4H2O | THF | L7 | 62 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 14 | Ni(acac)2·4H2O | MeCN : 2-Me THF | L7 | 78 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 15 | Ni(acac)2·4H2O, K2CO3, CsCO3 | MeCN | L7 | 0 | NA |
With the optimized conditions established, we next explored the scope of the aryl acylation reaction of alkynes. We began by examining the suitability of various aryl boronic acids (2) as coupling partners (Fig. 3). Generally, the steric and electronic properties of the phenyl ring in para- and meta-substituted aryl boronic acids did not significantly influence the reaction yield. Both electron-rich and electron-deficient aryl boronic acids, featuring substituents such as methyl, t-butyl, halogen, trifluoromethyl, o-phenoxy, and cyano-groups, successfully reacted with o-hexynyl phenol ester 1a, producing the corresponding rearranged products 3 in good yields (3a–3l). In most cases, boronic acids bearing electron-donating groups (e.g., 3j) resulted in slightly higher yields compared to those with electron-withdrawing groups (e.g., 3k). Additionally, hetero-aromatic boronic acids proved compatible with this reaction, delivering a lower yield of the desired product (3l). We then explored the generality of this Ni-catalyzed aryl acylation reaction with 2-alkynyl phenol esters 1, featuring various ester groups in (Fig. 4). Variations in the ester group on the phenol did not significantly impact the reaction efficiency. The reaction conditions were well-tolerated with a range of ester groups, including strained cyclopropyl (3n), 4-methyl (3m), naphthyl (3o), p-chloro (3p), o-bromo (3q), pentafluoro (3r), electron-donating groups such as methoxy (3s) and N,N-dimethylamine (3t), as well as electron-withdrawing groups like nitro (3v). Additionally, heteroatomic thiophene-containing esters (3w) were also compatible. Notably, the reaction was not restricted to simple esters; phosphoryl esters also yielded the desired product, albeit as a Z/E mixture (77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23) (3x). Next, we investigated the reaction scope with variations in the alkyne side chain (R2). When 2-alkynyl phenol ester 1 containing a shorter alkyl chain substituent on the alkynyl moiety was employed, the reaction afforded a single isomer, yielding 76% of the corresponding tetrasubstituted alkene (3y) as a white solid. The structure of (3y) was unambiguously confirmed through X-ray crystallographic analysis (CCDC: 2110836).
23) (3x). Next, we investigated the reaction scope with variations in the alkyne side chain (R2). When 2-alkynyl phenol ester 1 containing a shorter alkyl chain substituent on the alkynyl moiety was employed, the reaction afforded a single isomer, yielding 76% of the corresponding tetrasubstituted alkene (3y) as a white solid. The structure of (3y) was unambiguously confirmed through X-ray crystallographic analysis (CCDC: 2110836).
Reactions of 1, bearing longer alkyl chain, heteroatomic, and phenyl group substituents on the alkyne moiety, also resulted in the formation of the corresponding tetra-substituted alkenes (3z–3ac) in high yields. We also examined substrates with various substituents on the phenol ring, including phenyl, methyl ester, fluoro, and chloro groups. In all cases, the expected products were obtained in good to excellent yields (3ad–3ag).
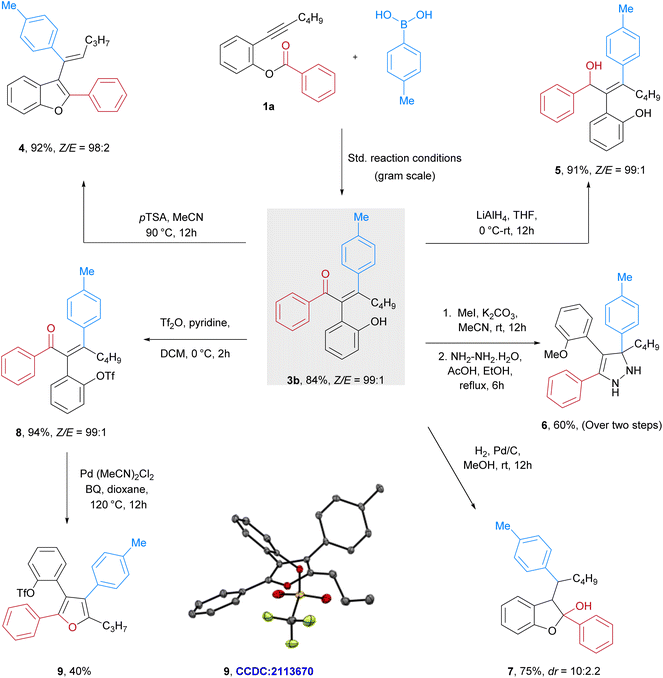
Notably, expanding the scope of the syn-arylative rearrangement to include an amide moiety in place of the ester also proved successful under standard conditions, yielding the corresponding alkene derivative (3ah) in 52%. Furthermore, the scalability of our protocol was exemplified by the aryl-acylation of 2-hexynyl phenol ester 1a on a 1.2-gram scale affording 84% of Z-alkene 3b. We next showcased the synthetic utility of the products through a series of post-functionalization reactions (Fig. 5). PTSA-catalyzed dehydrative cyclization of (3b) yielded the corresponding 2,3-difunctionalized benzofuran47,72,734 in good yield. Treatment of 3b with LiAlH4 led to the selective reduction of the ketone moiety, providing the reduced product in excellent yield. We then aimed to transform 3b into the highly functionalized pyrazole structure 6 through a selective five-membered ring cyclization using hydrazine hydrate. Additionally, Pd/C-catalyzed hydrogenation of 3b produced substituted benzofuran-2-ol 7 in 75% yield. Additionally, we leveraged the phenol group on 3b as a functional handle, converting it into the corresponding triflate 8 in 94% yield. This triflate was subsequently subjected to a Pd-catalyzed oxidative coupling reaction. Interestingly, an unexpected transformation occurred and yielded a tetrasubstituted furan 9. The structure of the furan 9 was confirmed by X-ray crystallographic analysis (CCDC: 2113670). On the basis of the literature reports47,53,54,57,74,75 and the experimental results we propose a catalytic cycle (Fig. 6). Initially, the nickel complex undergoes a transmetalation with boronic acids 2a to form the aryl-Ni intermediate A, which regioselectively adds in syn fashion across the alkyne in 1a to form the alkenyl-Ni species B. The organo-nickel intermediate B subsequently adds to the carbonyl carbon of the ester moiety that results in cyclic intermediate C. Finally, the C–O bond cleavage with ring opening leads to the formation of alkene product 3a with acyl group migration along with the regeneration of the Ni complex.
In summary, we have developed an unconventional Ni-catalyzed approach for the synthesis of tetrasubstituted alkenes from alkynes and boronic acids. This method enables a one-step difunctionalization of internal alkynes through the simultaneous addition of both aryl and acyl groups across triple bonds, providing streamlined access to tetrasubstituted alkenes with high regio- and stereocontrol; challenging to achieve with conventional methods. The process exhibits excellent functional group compatibility and broad synthetic applicability, even in complex molecular settings. Its practicality is further demonstrated by gram-scale synthesis and diverse post-functionalization of complex molecules. This straightforward protocol opens new avenues in multi-substitution chemistry for acyclic, all-carbon tetrasubstituted Z-olefinic products.
Data availability
Experiment procedures, characterization of the new compounds are available in the ESI.†Author contributions
P. S. S., V. S. S., and M. R. conceived and designed the experiments. P. S. S., V. S. S. conducted the experiments, analyzed the data and wrote the manuscript, while M. R. supervised the project and the manuscript.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work was financially supported by the King Abdullah University of Science and Technology (KAUST), Saudi Arabia, Office of Sponsored Research (URF/1/4405).Notes and references
- M. W. DeGregorio and V. J. Wiebe, Tamoxifen and breast cancer, Yale University Press, New Haven, CT, 2nd edn, 1999 Search PubMed.
- A. Levenson and V. Jordan, Selective oestrogen receptor modulation: molecular pharmacology for the millennium, Eur. J. Cancer, 1999, 35, 1974–1985 CrossRef CAS PubMed.
- R. McCague, G. Leclercq, N. Legros, J. Goodman, G. M. Blackburn, M. Jarman and A. B. Foster, Derivatives of tamoxifen. Dependence of antiestrogenicity on the 4-substituent, J. Med. Chem., 1989, 32, 2527–2533 Search PubMed.
- C. E. Connor, J. D. Norris, G. Broadwater, T. M. Willson, M. M. Gottardis, M. W. Dewhirst and D. P. McDonnell, Circumventing tamoxifen resistance in breast cancers using antiestrogens that induce unique conformational changes in the estrogen receptor, Cancer Res., 2001, 61, 2917–2922 Search PubMed.
- A. Lai, M. Kahraman, S. Govek, J. Nagasawa, C. Bonnefous, J. Julien, K. Douglas, J. Sensintaffar, N. Lu and K.-j. Lee, Identification of GDC-0810 (ARN-810), an orally bioavailable selective estrogen receptor degrader (SERD) that demonstrates robust activity in tamoxifen-resistant breast cancer xenografts, J. Med. Chem., 2015, 58, 4888–4904 CrossRef CAS PubMed.
- B. L. Feringa, R. A. Van Delden, N. Koumura and E. M. Geertsema, Chiroptical molecular switches, Chem. Rev., 2000, 100, 1789–1816 CrossRef CAS PubMed.
- A. Schreivogel, J. Maurer, R. Winter, A. Baro and S. Laschat, Synthesis and Electrochemical Properties of Tetrasubstituted Tetraphenylethenes, Eur. J. Org Chem., 2006, 2006, 3395–3404 CrossRef.
- N. Mukherjee, S. Planer and K. Grela, Formation of tetrasubstituted C–C double bonds via olefin metathesis: challenges, catalysts, and applications in natural product synthesis, Org. Chem. Front., 2018, 5, 494–516 RSC.
- D. D. La, S. V. Bhosale, L. A. Jones and S. V. Bhosale, Tetraphenylethylene-based AIE-active probes for sensing applications, ACS Appl. Mater. Interfaces, 2017, 10, 12189–12216 CrossRef PubMed.
- O. Reiser, Palladium-Catalyzed Coupling Reactions for the Stereoselective Synthesis of Tri-and Tetrasubstituted Alkenes, Angew. Chem., Int. Ed., 2006, 45, 2838–2840 CrossRef CAS PubMed.
- A. B. Flynn and W. W. Ogilvie, Stereocontrolled synthesis of tetrasubstituted olefins, Chem. Rev., 2007, 107, 4698–4745 CrossRef CAS PubMed.
- K. Murakami and H. Yorimitsu, Recent advances in transition-metal-catalyzed intermolecular carbomagnesiation and carbozincation, Beilstein J. Org. Chem., 2013, 9, 278–302 CrossRef CAS PubMed.
- V. P. Boyarskiy, D. S. Ryabukhin, N. A. Bokach and A. V. Vasilyev, Alkenylation of arenes and heteroarenes with alkynes, Chem. Rev., 2016, 116, 5894–5986 CrossRef CAS PubMed.
- P. Polák, H. Váňová, D. Dvořák and T. Tobrman, Recent progress in transition metal-catalyzed stereoselective synthesis of acyclic all-carbon tetrasubstituted alkenes, Tetrahedron Lett., 2016, 57, 3684–3693 CrossRef.
- D. Müller and I. Marek, Copper mediated carbometalation reactions, Chem. Soc. Rev., 2016, 45, 4552–4566 RSC.
- B. M. Trost and J. S. Tracy, Vanadium-catalyzed synthesis of geometrically defined acyclic tri-and tetrasubstituted olefins from propargyl alcohols, ACS Catal., 2019, 9, 1584–1594 CrossRef CAS.
- B. E. Maryanoff and A. B. Reitz, The Wittig olefination reaction and modifications involving phosphoryl-stabilized carbanions. Stereochemistry, mechanism, and selected synthetic aspects, Chem. Rev., 1989, 89, 863–927 CrossRef CAS.
- Y. Yang, S.-F. Zhu, C.-Y. Zhou and Q.-L. Zhou, Nickel-catalyzed enantioselective alkylative coupling of alkynes and aldehydes: synthesis of chiral allylic alcohols with tetrasubstituted olefins, J. Am. Chem. Soc., 2008, 130, 14052–14053 CrossRef CAS PubMed.
- D. Zell, C. Kingston, J. Jermaks, S. R. Smith, N. Seeger, J. Wassmer, L. E. Sirois, C. Han, H. Zhang and M. S. Sigman, Stereoconvergent and-divergent synthesis of tetrasubstituted alkenes by nickel-catalyzed cross-couplings, J. Am. Chem. Soc., 2021, 143, 19078–19090 CrossRef CAS PubMed.
- Y. Weng, Y. Zhang, A. Turlik, X. Wu, H. Li, F. Fei, Y. Yao, C. Wang, Z. Guo and J. Qu, Nickel-catalysed regio-and stereoselective acylzincation of unsaturated hydrocarbons with organozincs and CO, Nat. Synth., 2023, 2, 261–274 CrossRef CAS.
- W. You, Y. Li and M. K. Brown, Stereoselective synthesis of all-carbon tetrasubstituted alkenes from in situ generated ketenes and organometallic reagents, Org. Lett., 2013, 15, 1610–1613 CrossRef CAS PubMed.
- B. X. Li, D. N. Le, K. A. Mack, A. McClory, N.-K. Lim, T. Cravillion, S. Savage, C. Han, D. B. Collum and H. Zhang, Highly stereoselective synthesis of tetrasubstituted acyclic all-carbon olefins via enol tosylation and Suzuki–Miyaura coupling, J. Am. Chem. Soc., 2017, 139, 10777–10783 Search PubMed.
- Z. Lin, W. Hu, L. Zhang and C. Wang, Nickel-catalyzed asymmetric cross-electrophile trans-aryl-benzylation of α-naphthyl propargylic alcohols, ACS Catal., 2023, 13, 6795–6803 CrossRef CAS.
- M. Bera, S. D. Tambe, H. S. Hwang, S. Kim and E. J. Cho, Base-free NiH-catalyzed regio-and stereo-selective hydroacylation of allenes: A new route to synthesis of tetra-substituted olefins, Chem Catal., 2023, 3, 100606 CrossRef CAS.
- U. Wille, Radical cascades initiated by intermolecular radical addition to alkynes and related triple bond systems, Chem. Rev., 2013, 113, 813–853 CrossRef CAS PubMed.
- T. Besset, T. Poisson and X. Pannecoucke, Direct vicinal difunctionalization of alkynes: an efficient approach towards the synthesis of highly functionalized fluorinated alkenes, Eur. J. Org Chem., 2015, 2015, 2765–2789 Search PubMed.
- T. Koike and M. Akita, A versatile strategy for difunctionalization of carbon–carbon multiple bonds by photoredox catalysis, Org. Chem. Front., 2016, 3, 1345–1349 RSC.
- M. C. Haibach, S. Shekhar, T. S. Ahmed and A. R. Ickes, Recent advances in nonprecious metal catalysis, Org. Process Res. Dev., 2023, 27, 423–447 CrossRef CAS.
- N. Iqbal, J. Jung, S. Park and E. J. Cho, Controlled trifluoromethylation reactions of alkynes through visible-light photoredox catalysis, Angew. Chem., Int. Ed., 2014, 126, 549–552 CrossRef.
- T. Xu, C. W. Cheung and X. Hu, Iron-Catalyzed 1,2-Addition of Perfluoroalkyl Iodides to Alkynes and Alkenes, Angew. Chem., Int. Ed., 2014, 126, 4910–4914 CrossRef PubMed.
- L. Huang, M. Rudolph, F. Rominger and A. S. K. Hashmi, Photosensitizer-free visible-light-mediated gold-catalyzed 1,2-difunctionalization of alkynes, Angew. Chem., Int. Ed., 2016, 55, 4808–4813 CrossRef CAS PubMed.
- M. H. Babu, G. R. Kumar, R. Kant and M. S. Reddy, Ni-Catalyzed regio-and stereoselective addition of arylboronic acids to terminal alkynes with a directing group tether, Chem. Commun., 2017, 53, 3894–3897 RSC.
- Z. Li, E. Merino and C. Nevado, Stereoselective Carboperfluoroalkylation of Internal Alkynes: Mechanistic Insights, Top. Catal., 2017, 60, 545–553 CrossRef CAS.
- H. Yue, C. Zhu, R. Kancherla, F. Liu and M. Rueping, Regioselective Hydroalkylation and Arylalkylation of Alkynes by Photoredox/Nickel Dual Catalysis: Application and Mechanism, Angew. Chem., Int. Ed., 2020, 59, 5738–5746 CrossRef CAS PubMed.
- H. Yao, W. Hu and W. Zhang, Difunctionalization of alkenes and alkynes via intermolecular radical and nucleophilic additions, Molecules, 2020, 26, 105 CrossRef PubMed.
- Y. Jiang, J. Pan, T. Yang, Y. Zhao and M. J. Koh, Nickel-catalyzed site-and stereoselective reductive alkylalkynylation of alkynes, Chem, 2021, 7, 993–1005 CAS.
- N. Xiao, Y.-Z. Zhan, H. Meng and W. Shu, Access to Z-selective 1,3-enynes via Ni-catalyzed intermolecular cross-alkylalkynylation of terminal alkynes, Org. Lett., 2021, 23, 5186–5191 CrossRef CAS PubMed.
- Y.-Z. Zhan, N. Xiao and W. Shu, Ni-catalyzed regio-and stereo-defined intermolecular cross-electrophile dialkylation of alkynes without directing group, Nat. Commun., 2021, 12, 928 CrossRef CAS PubMed.
- S. Maiti and J. H. Rhlee, Reductive Ni-catalysis for stereoselective carboarylation of terminal aryl alkynes, Chem. Commun., 2021, 57, 11346–11349 RSC.
- Y.-Z. Zhan, H. Meng and W. Shu, Rapid access to t-butylalkylated olefins enabled by Ni-catalyzed intermolecular regio-and trans-selective cross-electrophile t-butylalkylation of alkynes, Chem. Sci., 2022, 13, 4930–4935 RSC.
- Y. Dai, F. Wang, S. Zhu and L. Chu, Selective Ni-catalyzed cross-electrophile coupling of alkynes, fluoroalkyl halides, and vinyl halides, Chin. Chem. Lett., 2022, 33, 4074–4078 CrossRef CAS.
- H. Li, F. Wang, S. Zhu and L. Chu, Selective Fluoromethyl Couplings of Alkynes via Nickel Catalysis, Angew. Chem., Int. Ed., 2022, 134, e202116725 CrossRef.
- L. Zhou, M. Zhang, W. Li and J. Zhang, Furan-Based o-Quinodimethanes by Gold-Catalyzed Dehydrogenative Heterocyclization of 2-(1-Alkynyl)-2-alken-1-ones: A Modular Entry to 2,3-Furan-Fused Carbocycles, Angew. Chem., Int. Ed., 2014, 53, 6542–6545 CrossRef CAS PubMed.
- J. Sun, J.-K. Qiu, Y.-N. Wu, W.-J. Hao, C. Guo, G. Li, S.-J. Tu and B. Jiang, Silver-Mediated Radical C (sp3)–H Biphosphinylation and Nitration of β-Alkynyl Ketones for Accessing Functional Isochromenes, Org. Lett., 2017, 19, 754–757 CrossRef CAS PubMed.
- J. Wang, X. Cao, S. Lv, C. Zhang, S. Xu, M. Shi and J. Zhang, Synthesis and structures of gold and copper carbene intermediates in catalytic amination of alkynes, Nat. Commun., 2017, 8, 14625 CrossRef PubMed.
- S. Ge, W. Cao, T. Kang, B. Hu, H. Zhang, Z. Su, X. Liu and X. Feng, Bimetallic Catalytic Asymmetric Tandem Reaction of β-Alkynyl Ketones to Synthesize 6,6-Spiroketals, Angew. Chem., Int. Ed., 2019, 58, 4017–4021 CrossRef CAS PubMed.
- N. Iqbal, N. Iqbal, D. Maiti and E. J. Cho, Access to Multifunctionalized Benzofurans by Aryl Nickelation of Alkynes: Efficient Synthesis of the Anti-Arrhythmic Drug Amiodarone, Angew. Chem., Int. Ed., 2019, 58, 15808–15812 CrossRef CAS PubMed.
- L. Guo and M. Rueping, Decarbonylative cross-couplings: nickel catalyzed functional group interconversion strategies for the construction of complex organic molecules, Acc. Chem. Res., 2018, 51, 1185–1195 CrossRef CAS PubMed.
- A. Chatupheeraphat, H.-H. Liao, W. Srimontree, L. Guo, Y. Minenkov, A. Poater, L. Cavallo and M. Rueping, Ligand-controlled chemoselective C (acyl)–O bond vs. C (aryl)–C bond activation of aromatic esters in nickel catalyzed C (sp2)–C (sp3) cross-couplings, J. Am. Chem. Soc., 2018, 140, 3724–3735 CrossRef CAS PubMed.
- S.-C. Lee, L. Guo and M. Rueping, Nickel-catalyzed exo-selective hydroacylation/Suzuki cross-coupling reaction, Chem. Commun., 2019, 55, 14984–14987 RSC.
- C. Zhu, H. Yue and M. Rueping, Nickel catalyzed multicomponent stereodivergent synthesis of olefins enabled by electrochemistry, photocatalysis and photo-electrochemistry, Nat. Commun., 2022, 13, 3240 CrossRef CAS PubMed.
- T. Long, C. Zhu, L. Li, L. Shao, S. Zhu, M. Rueping and L. Chu, Ligand-controlled stereodivergent alkenylation of alkynes to access functionalized trans-and cis-1,3-dienes, Nat. Commun., 2023, 14, 55 CrossRef CAS PubMed.
- J. Corpas, P. Mauleon, R. G. Arrayás and J. C. Carretero, Transition-metal-catalyzed functionalization of alkynes with organoboron reagents: new trends, mechanistic insights, and applications, ACS Catal., 2021, 11, 7513–7551 CrossRef CAS.
- S. M. Gillbard and H. W. Lam, Nickel-Catalyzed Arylative Cyclizations of Alkyne-and Allene-Tethered Electrophiles using Arylboron Reagents, Chem.–Eur. J., 2022, 28, e202104230 CrossRef CAS PubMed.
- S. Z. Tasker, E. A. Standley and T. F. Jamison, Recent advances in homogeneous nickel catalysis, Nature, 2014, 509, 299–309 CrossRef CAS PubMed.
- V. P. Ananikov, Nickel: The “Spirited Horse” of Transition Metal Catalysis, ACS Catal., 2015, 5, 1964–1971 CrossRef CAS.
- C. Clarke, C. A. Incerti-Pradillos and H. W. Lam, Enantioselective nickel-catalyzed anti-carbometallative cyclizations of alkynyl electrophiles enabled by reversible alkenylnickel E/Z isomerization, J. Am. Chem. Soc., 2016, 138, 8068–8071 CrossRef CAS PubMed.
- X. Zhang, X. Xie and Y. Liu, Nickel-catalyzed cyclization of alkyne-nitriles with organoboronic acids involving anti-carbometalation of alkynes, Chem. Sci., 2016, 7, 5815–5820 RSC.
- Z. Li, A. García-Domínguez and C. Nevado, Nickel-catalyzed stereoselective dicarbofunctionalization of alkynes, Angew. Chem., Int. Ed., 2016, 55, 6938–6941 CrossRef CAS PubMed.
- C. Yap, G. M. J. Lenagh-Snow, S. N. Karad, W. Lewis, L. J. Diorazio and H. W. Lam, Enantioselective Nickel-Catalyzed Intramolecular Allylic Alkenylations Enabled by Reversible Alkenylnickel E/Z Isomerization, Angew. Chem., Int. Ed., 2017, 56, 8216–8220 CrossRef CAS PubMed.
- S. M. Gillbard, C.-H. Chung, S. N. Karad, H. Panchal, W. Lewis and H. W. Lam, Synthesis of multisubstituted pyrroles by nickel-catalyzed arylative cyclizations of N-tosyl alkynamides, Chem. Commun., 2018, 54, 11769–11772 RSC.
- S. N. Karad, H. Panchal, C. Clarke, W. Lewis and H. W. Lam, Enantioselective Synthesis of Chiral Cyclopent-2-enones by Nickel-Catalyzed Desymmetrization of Malonate Esters, Angew. Chem., Int. Ed., 2018, 57, 9122–9125 CrossRef CAS PubMed.
- X. Zhang, X. Xie and Y. Liu, Nickel-catalyzed highly regioselective hydrocyanation of terminal alkynes with Zn (CN)2 using water as the hydrogen source, J. Am. Chem. Soc., 2018, 140, 7385–7389 CrossRef CAS PubMed.
- C. Zhu, H. Yue, B. Maity, I. Atodiresei, L. Cavallo and M. Rueping, A multicomponent synthesis of stereodefined olefins via nickel catalysis and single electron/triplet energy transfer, Nat. Catal., 2019, 2, 678–687 CrossRef CAS.
- C. Zhu, H. Yue, L. Chu and M. Rueping, Recent advances in photoredox and nickel dual-catalyzed cascade reactions: pushing the boundaries of complexity, Chem. Sci., 2020, 11, 4051–4064 RSC.
- C. Zhu, H. Yue, J. Jia and M. Rueping, Nickel-Catalyzed C-Heteroatom Cross-Coupling Reactions under Mild Conditions via Facilitated Reductive Elimination, Angew. Chem., Int. Ed., 2021, 60, 17810–17831 CrossRef CAS PubMed.
- T. Miura, M. Shimada and M. Murakami, Acyl 1,3-migration in rhodium-catalyzed reactions of acetylenic β-ketoesters with aryl boronic acids: application to two-carbon-atom ring expansions, Angew. Chem., Int. Ed., 2005, 44, 7598–7600 CrossRef CAS PubMed.
- E. P. Jackson, H. A. Malik, G. J. Sormunen, R. D. Baxter, P. Liu, H. Wang, A.-R. Shareef and J. Montgomery, Mechanistic basis for regioselection and regiodivergence in nickel-catalyzed reductive couplings, Acc. Chem. Res., 2015, 48, 1736–1745 CrossRef CAS PubMed.
- E. D. Entz, J. E. Russell, L. V. Hooker and S. R. Neufeldt, Small Phosphine Ligands Enable Selective Oxidative Addition of Ar–O over Ar–Cl Bonds at Nickel(0), J. Am. Chem. Soc., 2020, 142, 15454–15463 CrossRef CAS PubMed.
- M. H. Tse, P. Y. Choy and F. Y. Kwong, Facile assembly of modular-type phosphines for tackling modern arylation processes, Acc. Chem. Res., 2022, 55, 3688–3705 CrossRef CAS PubMed.
- T. Gensch, S. R. Smith, T. J. Colacot, Y. N. Timsina, G. Xu, B. W. Glasspoole and M. S. Sigman, Design and application of a screening set for monophosphine ligands in cross-coupling, ACS Catal., 2022, 12, 7773–7780 CrossRef CAS.
- C. Martinez, R. Alvarez and J. M. Aurrecoechea, Palladium-catalyzed sequential oxidative cyclization/coupling of 2-alkynylphenols and alkenes: a direct entry into 3-alkenylbenzofurans, Org. Lett., 2009, 11, 1083–1086 CrossRef CAS PubMed.
- R. Álvarez, C. Martínez, Y. Madich, J. G. Denis, J. M. Aurrecoechea and Á. R. de Lera, A General Synthesis of Alkenyl-Substituted Benzofurans, Indoles, and Isoquinolones by Cascade Palladium-Catalyzed Heterocyclization/Oxidative Heck Coupling, Chem.–Eur. J., 2010, 16, 12746–12753 CrossRef PubMed.
- N. Iqbal, D. S. Lee, H. Jung and E. J. Cho, Synergistic effects of boron and oxygen interaction enabling nickel-catalyzed exogenous base-free stereoselective arylvinylation of alkynes through vinyl transposition, ACS Catal., 2021, 11, 5017–5025 CrossRef CAS.
- J. Bae, W. Lee, H. S. Hwang, S. Kim, J. Kang, N. Iqbal and E. J. Cho, Nonclassical Arylative Meyer–Schuster Rearrangement through Ni-Catalyzed Inner-Sphere Acyloxy Migration, ACS Catal., 2023, 13, 10756–10764 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sc00297d |
| ‡ PSS and VSS contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |