CoO microspheres and metallic Co evolved from hexagonal α-Co(OH)2 plates in a hydrothermal process for lithium storage and magnetic applications†
KeYuan
Ma
a,
F.
Liu
a,
Y. F.
Yuan
b,
X. Q.
Liu
c,
Jun
Wang
d,
J.
Xie
 a and
J. P.
Cheng
a and
J. P.
Cheng
 *a
*a
aState Key Laboratory of Silicon Materials, School of Materials Science & Engineering, Key Laboratory of Advanced Materials and Applications for Batteries of Zhejiang Province, Zhejiang University, Hangzhou 310027, China. E-mail: chengjp@zju.edu.cn; Fax: +86-571-87951411; Tel: +86-571-87951411
bCollege of Machinery and Automation, Zhejiang Sci-Tech University, Hangzhou 310018, China
cLaboratory of Dielectric Materials, School of Materials Science & Engineering, Zhejiang University, Hangzhou 310027, China
dSchool of Physical Science and Technology, Lanzhou University, Lanzhou 730000, China
First published on 1st December 2017
Abstract
CoO microspheres and metallic Co could be successfully synthesized by simply reacting cobalt acetate with a mixture solvent of ethylene glycol and deionized water in a hydrothermal process for different times. As the reaction proceeded, α-Co(OH)2, CoO and metallic Co were produced. To understand the phase evolution processes from α-Co(OH)2 to CoO and then metallic Co, a range of time-dependent experiments were carried out, and the intermediate products obtained at different reaction times were investigated in detail. The investigation revealed that CoO microspheres were actually evolved from α-Co(OH)2 as a precursor. Just elongating the reaction time, CoO microspheres could be further reduced to metallic Co. With a pure ethylene glycol medium for the same reaction, only α-Co(OH)2 could be generated, indicating an important role of water. When the obtained CoO microspheres were used as anode materials for lithium-ion batteries, they delivered a specific capacity of 803 mA h g−1 at 0.1 A g−1 with a retention of 453 mA h g−1 after 70 cycles. Meanwhile, the magnetic properties of the obtained CoO microspheres and metallic Co were investigated, with the CoO microspheres showing an antiferromagnetic behavior and the metallic Co exhibiting ferromagnetic characteristics. This study suggested a novel method for synthesizing CoO with a uniform microsphere morphology and bulk metallic Co easily.
1. Introduction
Metallic cobalt and Co-based compounds are attractive functional materials for investigation owing to their wide applications in diverse fields.1–8 For instance, metallic Co with various nanostructures has been widely investigated for magnetic and catalytic applications.1–4 Co(OH)2 with a unique layered structure is extensively used as an electrode material for energy storage.6–9 Cobalt oxides have been also widely studied for their widespread applications in sensors, catalysts, anode materials for batteries and so on.10–14 Therefore, the controllable preparation of cobalt and cobalt compounds has become more and more attractive and important for both fundamental studies and practical applications. Up to now, a lot of methods have been put forward to prepare these materials for various purposes.15–19 Notably, the preparation of single-crystalline CoO with a high purity is not easy, because it is in a metastable phase state and can be easily oxidized into Co3O4 or be reduced into metallic cobalt.19Many methods including thermal decomposition, solvothermal methods, sol–gel processes and hydrothermal methods have been developed to prepare CoO with various morphologies.19–25 Among these methods, the most widely used method is heating Co-based intermediate compounds (such as Co(OH)2, CoCO3 and CoC2O4) in an inert atmosphere.14,26 For example, Zhou prepared CoO nanosheets by heating Co2(OH)2CO3 under an Ar atmosphere.26 Sun et al. synthesized mesoporous CoO nanodisks by heating Co(OH)2 in a N2 atmosphere at 450 °C.14 The obtained CoO products in these processes were of high purity. However, it should be noted that the post-heat treatment process in inert conditions usually suffers from complex procedures. Thus, it remains significant to develop a simple one-step synthetic approach to synthesize high-purity CoO. In recent years, transition metal oxides with micrometer-sized sphere structures have attracted much attention due to their widespread applications in catalysis, energy storage and so on.27–29 However, there have been few reports on the preparation of CoO microspheres with a high purity by chemical solution method until now.
In this work, we report the synthesis of uniform CoO microspheres by a facile one-step hydrothermal process. CoO microspheres with a diameter ranging from 0.1 to 0.5 μm were successfully prepared by reacting cobalt acetate with a mixture solvent of ethylene glycol and deionized water at 200 °C for an intermediate period. Interestingly, we found that these CoO microspheres were gradually evolved from α-Co(OH)2 plates as a precursor, and that by extending the reaction time the CoO microspheres could be further reduced to metallic Co. These transformation processes were investigated in detail via a series of time-dependent experiments. We believe that this process signifies a novel important method to prepare uniform CoO microspheres and metallic Co by solution method under low temperature. Besides, the lithium storage properties of the as-prepared CoO microspheres were studied, and the magnetic properties of the CoO microspheres and metallic Co were also investigated.
2. Experimental section
2.1. Preparation of Co(OH)2 hexagonal nanoplates in pure ethylene glycol
Co(OH)2 hexagonal nanoplates were simply synthesized via a hydrothermal process. Specifically, 1 g cobalt acetate (Co(CH3COO)2·4H2O) was dissolved into 60 mL pure ethylene glycol (EG, (CH2OH)2) under magnetic stirring to form a clear solution. Then, the solution was transferred into a 90 mL Teflon-lined stainless-steel autoclave and closely sealed. The autoclave was heated and kept at 200 °C for 8 hours reaction. After cooling down to room temperature, the products was collected by centrifugation, washed with deionized water and alcohol repeatedly, and dried at 80 °C. Even when the reaction time was extended to 24 hours, we only obtained the product with the same phase and morphology.2.2. Preparation of CoO microspheres and metallic Co in a mixture of ethylene glycol and water
CoO microspheres and metallic Co were prepared by the same route as that of Co(OH)2 hexagonal nanoplates, but in a different solvent. In a typical process, 1 g cobalt acetate was dissolved into a mixture solution of 55 mL EG and 5 mL deionized water under magnetic stirring to form a clear solution. Subsequently, the solution was transferred into a 90 mL Teflon-lined stainless-steel autoclave. The autoclave was sealed, heated and kept at 200 °C for 8 hours. After cooling down to room temperature, the CoO product was collected, washed with deionized water and alcohol repeatedly, and dried at 80 °C. To obtain Co metal, the reaction time was just elongated to 18 hours, and the bulk Co product was easily collected by removing the solution.To investigate the reaction process, a range of time-dependent experiments were carried out under the same conditions, but at different times. After the autoclave was reacted at 200 °C for a specific time (1, 3, 6, 8, 12, and 18 hours), it was quickly cooled down to room temperature by being immersed in a mixture of water and ice. The obtained intermediate products were collected for further analysis.
2.3. Material characterization
The crystal structure and phase of the cobalt compounds were characterized using a powder X-ray diffractometer (Shimadzu, XRD-6000) using Cu-Kα irradiation (λ = 1.5406 Å). A scanning electron microscope (SEM, Hitachi S-4800) and a transmission electron microscope (Philips CM200, HV = 160 kV) were used to characterize their morphology and structure. The elemental analysis of the samples was measured using an electron dispersive X-ray spectrometer (EDS) coupled to the SEM and X-ray photoelectron spectroscopy (XPS, Shimadzu, AXIS Supra). Fourier transformed infrared (FTIR) spectra were acquired using a Bruker spectrometer (TENSOR27). Thermogravimetric (TG) analysis of Co(OH)2 was carried out using an SDT TA Q600 instrument in a temperature range from room temperature to 800 °C at a heating rate of 10 °C min−1 in air. The specific surface area and pore diameter distribution of the CoO microspheres were measured by a Micromeritics ASAP 2020. The magnetic properties of CoO and Co were investigated by a superconducting quantum interference device magnetometer (VSM-VersaLab).2.4. Electrochemical tests
The electrochemical tests were performed using two-electrode coin-type cells. Anode electrodes were prepared using the following processes: CoO microspheres, acetylene black and PVDF binder were mixed in a weight ratio of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 in N-methyl-2-pyrrolidinone to form an electrode slurry. Subsequently, the slurry was cast onto copper foil as a current collector, and dried at 80 °C in a vacuum oven to yield the working electrode. After drying, the electrode was pressed using a roller to enhance the packing and contact of the electrode materials. The mass loading of an electrode was about 2.0 mg cm−2. The electrodes were punched in the form of disks and then vacuum-dried at 120 °C for 12 h. Then, half cells (CR 2032 coin-type cell) were assembled with Li foil as both the counter and reference electrode, 1 M LiPF6 dissolved in ethylene carbonate (EC)/dimethyl carbonate (DMC)/ethyl methyl carbonate (EMC) (1
20 in N-methyl-2-pyrrolidinone to form an electrode slurry. Subsequently, the slurry was cast onto copper foil as a current collector, and dried at 80 °C in a vacuum oven to yield the working electrode. After drying, the electrode was pressed using a roller to enhance the packing and contact of the electrode materials. The mass loading of an electrode was about 2.0 mg cm−2. The electrodes were punched in the form of disks and then vacuum-dried at 120 °C for 12 h. Then, half cells (CR 2032 coin-type cell) were assembled with Li foil as both the counter and reference electrode, 1 M LiPF6 dissolved in ethylene carbonate (EC)/dimethyl carbonate (DMC)/ethyl methyl carbonate (EMC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume) as the electrolyte, and Celgard 2300 polypropylene as the separator in a high purity argon-filled glovebox. Cyclic voltammetry tests were performed using a ParStat 2273 advanced electrochemical workstation. Galvanostatic charge/discharge measurements were performed by a Neware battery test system at ambient temperature in the potential range of 0.01–3 V (vs. Li+/Li) and the cycle stability was evaluated at the current density of 0.1 A g−1.
1 in volume) as the electrolyte, and Celgard 2300 polypropylene as the separator in a high purity argon-filled glovebox. Cyclic voltammetry tests were performed using a ParStat 2273 advanced electrochemical workstation. Galvanostatic charge/discharge measurements were performed by a Neware battery test system at ambient temperature in the potential range of 0.01–3 V (vs. Li+/Li) and the cycle stability was evaluated at the current density of 0.1 A g−1.
3. Results and discussion
3.1. Structures and morphologies of the as-prepared cobalt compounds
In the fabrication processes, cobalt acetate was used as a reactant to react in different solutions and reaction times to obtain the various products, and Fig. 1(a) presents the photographs of the prepared samples. XRD patterns are employed to investigate the phase of the products, as shown in Fig. 1(b) and (c). The XRD pattern of the collected Co(OH)2 products with a light pink color shown in Fig. 1(a) exhibits a typical layered hydrotalcite-like structure, and it corresponds to the pattern of standard α-Co(OH)2 (PDF No. 51-1731). The small angle deviation compared with the standard pattern is originated from different interlayer intercalations. The CoO powder with an ocher color displayed an XRD profile that is in good agreement with the standard face centered cubic (fcc) CoO (PDF No. 78-0431), and the sharp diffraction peaks indicate that the obtained product is of high crystallinity and phase purity. The five major diffraction peaks located at around 36.4°, 42.2°, 61.3°, 73.4° and 77.2° correspond to the (111), (200), (220), (311) and (222) planes of fcc CoO crystals, respectively.3,30 The metallic Co product shows strong diffraction peaks at about 44.3°, 51.6°, and 76.1°, corresponding to the (111), (200) and (220) planes of fcc Co (PDF No. 15-0806), respectively.3 However, it should be noted that in addition to the peaks from fcc Co, a small peak at about 47.5° can be also clearly discerned, and it can be well indexed to metallic Co with a hexagonal closed packed (hcp) crystal structure (PDF No. 05-0727). As a result, the obtained metallic Co sample has both fcc and hcp phase structures. From Fig. 1(a), it can be clearly seen that the as-obtained Co product is ferromagnetic as it can be tightly attracted to a magnet. These results suggest that Co(OH)2, CoO and metallic Co can be controllably obtained via a simple hydrothermal method by easily changing the solvent and reaction time. | ||
| Fig. 1 (a) Photographs of the as prepared samples, (b) XRD pattern of Co(OH)2, (c) XRD patterns of CoO and Co, and (d) FTIR spectra for Co(OH)2, CoO and Co. | ||
The obtained cobalt products were further investigated by FTIR. As shown in Fig. 1(d), the Co(OH)2 sample shows a broad peak at around 3440 cm−1, which is assigned to the stretching vibration of –OH from absorbed water and Co–OH groups.31 The strong absorption band at 650 cm−1 is ascribed to the bending vibrations of Co–OH groups in the brucite-like sheets of Co(OH)2.31,32 The characteristic absorption bands from adsorbed EG molecules can also be identified very clearly in the spectrum of Co(OH)2.32 The broad peak at 3440 cm−1 in CoO and metallic Co is attributed to the stretching vibration of OH from absorbed water, and the weak bands in the region of 1000–1800 cm−1 are derived from the adsorbed EG molecules. The strong absorption band at 495 cm−1 in CoO is assigned to the stretching vibrations of Co–O groups. For the metallic Co sample, there are no absorption bands in the region of 400–800 cm−1, indicating that there is no Co–O or Co–OH band in the sample. This result signifies that the metallic Co has a high purity, which is in agreement with the XRD pattern. Therefore, the FTIR results also confirm the formation of the as-prepared cobalt compounds.
The morphology and microstructure of the prepared samples were investigated by SEM (Fig. S1, ESI†) and TEM. Fig. 2(a)–(c) display the morphology of the as-obtained α-Co(OH)2, which presents a hexagonal in-plane symmetry with a lateral size of less than 1 μm. Additionally, the fringes observed from the magnified side-view can be well ascribed to the stacked layers, and the thickness of the platelets are around 30–100 nm. The typical EDS mapping of elements Co and O in an individual Co(OH)2 nanoplate displays well-defined hexagonal profiles, revealing that the elements are homogeneously distributed in the nanoplate (Fig. 2(h)). By contrast, the CoO product displays a spherical structure with a diameter ranging from 0.1 to 0.5 μm (Fig. S1, ESI†). From the TEM images of CoO in Fig. 2(d)–(f), it can be found that the microspheres possess a uniform outline and solid structure. The selected area electron diffraction (SAED) pattern displays a set of well-arranged diffraction spots from fcc CoO crystals, indicating a high phase purity and crystallinity of the CoO microspheres. The element spatial distribution among the CoO microspheres is also presented by EDS mappings as displayed in Fig. 2(h), and the outlines of both Co and O show obvious spherical profiles at the edge part. SEM images of the metallic Co sample in Fig. S1(e and f) (ESI†) reveal that the bulky metallic Co is composed of a large number of small particles with irregular shapes.
 | ||
| Fig. 2 TEM images of Co(OH)2 (a)–(c) and CoO (d)–(f), (g) a typical selected area electron diffraction (SAED) pattern of a CoO microsphere and (h) elemental mappings for Co and O in Co(OH)2 and CoO. | ||
XPS measurement was performed to analyze the chemical state of the elements Co and O in Co(OH)2 and CoO. The wide XPS survey spectra of the samples (in Fig. S2, ESI†) show the existence of Co and O, coupled with C derived from the absorbed organic solvent and intercalated acetate anions. The high-resolution spectrum of Co 2p in Fig. 3(a) shows two major peaks located at 780.6 and 796.5 eV accompanied by two shake-up satellite peaks at 785.4 and 802.7 eV, respectively. According to previous reports, the two peaks located at 796.5 and 780.6 eV can be assigned to the Co 2p1/2 and Co 2p3/2 spin orbit peaks of Co2+ in Co(OH)2.18,33Fig. 3(c) presents the Co 2p spectrum of CoO, through which we can see that there is no obvious difference between the Co 2p spectra of CoO and Co(OH)2. This result indicates that the Co ions are in the same valence state. However, with respect to the O 1s spectrum, those in Co(OH)2 and CoO present completely different profiles. The O 1s spectrum of Co(OH)2 in Fig. 3(b) presents a strong peak at 531.1 eV, and this peak can be well fitted by the presence of Co–OH bonds in Co(OH)2.21,34 By contrast, the O 1s spectrum from CoO in Fig. 3(d) split into two prominent peaks at 529.6 eV and 531.3 eV. The strong peak at 529.6 eV can be well indexed as oxygen bonded to the transition metal (O–Co–O) groups in the CoO crystalline structure.21,34 The small peak at 531.3 eV can be assigned to the Co–OH bond between CoO and absorbed water on the surface.
 | ||
| Fig. 3 XPS spectra of Co 2p (a) and O 1s (b) in Co(OH)2, and Co 2p (c) and O 1s (d) in the CoO sample. | ||
3.2. Process and mechanism for the formation of CoO microspheres and metallic Co
After cobalt acetate was reacted in the mixture of EG and water for 1 hour at 200 °C, an intermediate product with a pink color was obtained, and XRD analysis proved that it was α-Co(OH)2, instead of CoO. In view of this, we believe that the CoO microspheres and metallic Co prepared in the same medium for different reaction times were derived from the transformation of the pre-formed α-Co(OH)2 precursor. However, the α-Co(OH)2 produced in pure EG cannot accomplish this phase evolution process, even extending the reaction time for 24 hours. Therefore, the presence of water in the reaction solution was proved to be very significant for the phase evolution process. To understand the role of water, the α-Co(OH)2 products obtained in the different solutions were investigated in detail for a direct comparison.As displayed in Fig. 4(a), the intermediate α-Co(OH)2 obtained in the mixture solution for 1 hour reaction (denoted as Co(OH)2–HC) presents a large hexagonal in-plane symmetry with a lateral size up to 5 μm, which is much larger than the α-Co(OH)2 prepared in pure EG (denoted as Co(OH)2–C) (Fig. S1, ESI†). From Fig. 4(b), we can see that the diffraction peaks of Co(OH)2–HC shift to low angles, implying its larger d-spacing of adjacent planes. Specifically, the d-spacing of the (003) plane is calculated to be 0.915 nm, which is also larger than that of Co(OH)2–C (0.828 nm). We believe that this phenomenon is caused by the different species intercalated in the interlayer region. Water is a highly polar solvent compared with EG, indicating that it can easily form hydrogen bonds with acetate anions, EG molecules and Co(OH)2 layers. Therefore, the Co(OH)2 prepared in the mixture solution has a large amount of intercalated interlayer species, leading to a high degree of interlayer expansion.
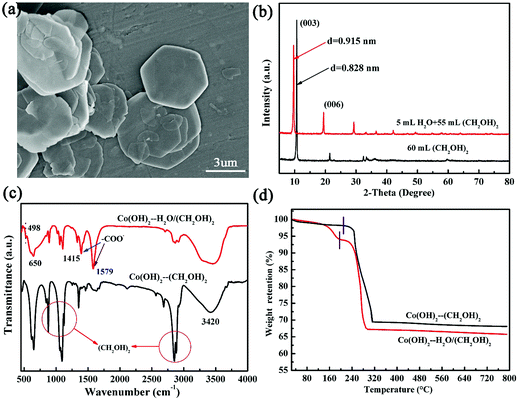 | ||
| Fig. 4 (a) Typical SEM image of α-Co(OH)2 prepared in EG and deionized water for 1 hour, and (b) XRD patterns, (c) FTIR spectra and (d) TG curves for the α-Co(OH)2 prepared in different solutions. | ||
To prove the above assumption, FTIR and TG investigations were carried out as shown in Fig. 4(c) and (d), respectively. In addition to the shared absorption bonds from OH and Co–OH groups (Fig. 4(c)), Co(OH)2–HC presents two strong absorption bands at 1579 and 1415 cm−1, corresponding to the symmetric and asymmetric stretches of –COO− from acetate groups.6 By contrast, the absorption bands from acetate groups in Co(OH)2–C are not so evident compared with the absorption bands from EG molecules. Based on this fact, we believe that the larger d-spacing of Co(OH)2–HC originates from the intercalation of more acetate groups between the layers. This assumption can be further evidenced by the fact that compared with Co(OH)2–C, the weight loss of Co(OH)2–HC increased from 3% to 7% below 190 °C in air, and the gradual mass loss at this temperature range is attributed to the removal of adsorbed species on the external surfaces and interlayer regions. The dominant mass loss at the subsequent stage is ascribed to the thermal decomposition of Co(OH)2 in air. We can find that Co(OH)2–HC decomposes over a temperature range of 190 to 270 °C, while Co(OH)2–C occurs at a higher temperature of 210 to 290 °C, indicating that Co(OH)2–HC has a lower thermal stability compared with Co(OH)2–C. From these results, we believe that water plays an important role in expanding Co(OH)2 interlayers, which leads to an unstable structure and reduces its thermal stability.
XRD patterns of the obtained time-dependent products during the reaction process in Fig. 5(a) reveal that the intensity of the diffraction signals from Co(OH)2 decreases with time and they completely disappeared after 8 hours of reaction. By contrast, the diffraction peaks belonging to CoO are strengthened, indicating that there is a phase transition process from Co(OH)2 to CoO. With the reaction time prolonged, the transformation from CoO to metallic Co then occurs, as is displayed in curve (e) in Fig. 5(a). Finally, all CoO will be converted into metallic Co with a high purity. Previous investigations have revealed that several nanostructured metals such as Pt, Ag, Ru, Ni, Cu, Co etc. can be obtained via EG-mediated synthesis processes.35–39 For instance, Sun et al. prepared Ag nanowires by reducing AgNO3 with EG.36 Kim synthesized Co metal by reacting cobalt acetate with NaOH in EG and oleic acid solutions.38 In this work, we believe that the CoO is reduced to metallic Co by EG with enough reaction time.
 | ||
| Fig. 5 XRD patterns and FTIR spectra of the intermediate products obtained at different reaction times in EG/water: 1 h (a), 3 h (b), 6 h (c), 8 h (d), 12 h (e) and 18 h (f). | ||
The transition process can also be reflected in the FTIR spectra in Fig. 5(b). The Co(OH)2 precursor shows a broad bond at about 650 cm−1 from the bending vibrations of Co–OH groups on the Co(OH)2−x hydrotalcite-like layers in curve (a). As the reaction time increased, the intensity of the band at 650 cm−1 was decreased, but the bond at about 495 cm−1 belonging to stretching vibrations of Co–O was strengthened. This process was ascribed to the dehydration of the pre-formed Co(OH)2 precursors.9 When the dehydration process finished, the bond at 650 cm−1 disappeared completely as shown in curve (d). With the reaction time further prolonged, the bond at 495 cm−1 was also decreased due to the reduction of Co–O to metallic cobalt. Finally, the bond vanished, indicating that CoO was completely reduced to metallic Co. The above results clearly demonstrate that the whole transition process involves Co(OH)2, CoO and metallic Co. The process undergoes three major sequential stages, including the formation of the Co(OH)2 precursor, dehydration of Co(OH)2 to form CoO, and reduction of CoO to metallic Co. It should be mentioned that the above processes are strongly related to the reaction time.
The SEM images in Fig. 6 clearly show the morphology evolution process from hexagonal Co(OH)2 plates to CoO microspheres and then metallic Co with increasing reaction time. After 1 hour reaction, the obtained Co(OH)2 presented a hexagonal plate-like morphology in Fig. 6(a). As the reaction proceeded, some solid particles with irregular shape increased accompanied by the breakdown of the Co(OH)2 plates, as shown in Fig. 6(b). Thereafter, numerous microspheres started to appear on the surfaces of the Co(OH)2 plates after 4 hours of reaction (Fig. 6(c)). The coexistence of damaged Co(OH)2 plates and CoO microspheres shown in Fig. 6(d) suggested a link between them. In Fig. 6(e), the closely packed particles, coupled with separating microspheres clearly showed the conversion from Co(OH)2 plates to CoO microspheres. As the process continued, more and more CoO microspheres formed in Fig. 6(f). When the conversion process was finished, uniform microspheres with smooth surfaces were obtained as shown in Fig. 6(g), indicating that the final CoO held a completely different morphology from its precursor. As the reaction continued, a mixture of microspheres and bulk particles, which had been proved to be CoO and metallic Co by XRD, was obtained (Fig. 6(h)). When this reduction process was finished, metallic Co bulk particles as shown in Fig. 6(i) were obtained.
 | ||
| Fig. 6 SEM images of the obtained intermediate products obtained at different reaction times: 1 h (a), 2 h (b), 4 h (c), 6 h (d, e), 7 h (f), 8 h (g), 14 h (h) and 18 h (i). | ||
On the basis of the above investigations, the observed phase evolution process can be briefly explained as follows. First, OH− derived from the hydrolysis of CH3COO− and Co2+ formed α-Co(OH)2 precursors. But these pre-formed α-Co(OH)2 products with a large size and d-spacing of adjacent planes were unstable in the mixture solution at 200 °C. In the following stage, dehydration of the α-Co(OH)2 precursor occurred, resulting in the generation of small CoO crystal nuclei. In the nucleation process, the Co(OH)2 served as a growth base for the CoO crystal nuclei. With the mass transport and Ostwald ripening process proceeding, the CoO nuclei aggregated into spherical particles with an fcc crystal structure, and they grew larger and smoother to reduce the Gibbs free energy of the system.27,28 The formed CoO microspheres caused etching of the hexagonal Co(OH)2 plates leading to the damage of the plate structures, as displayed in Fig. 6(d). With a suitable reaction period, for us 8 hours, all of the Co(OH)2 will be transformed into CoO with a regular microsphere morphology. In the final stage, the formed CoO microspheres will be further reduced by EG under 200 °C.35 Finally, all the CoO microspheres were reorganized and reduced to form bulk metallic Co particles. Nevertheless, how the crystal structural transformation occurring is an open question.
3.3. Lithium storage properties of the CoO microspheres
Cyclic voltammetry (CV) was employed to investigate the detailed electrochemical reaction processes, and the CV curves at the scan rate of 0.1 mV s−1 are shown in Fig. 7(a). The strong irreversible reduction peak at 0.437 V in the first cathodic scan is ascribed to the reduction of CoO to metallic Co and initial formation of the solid electrolyte interface (SEI) layer generated from electrolyte decomposition.26,39–42 The peak at around 2.1 V is connected with the oxidation of Co to CoO. In the subsequent two cycles, the CV curves of the second and the third cycles overlap very well, indicating a good cycle reversibility and stability of the electrode. A pair of redox peaks can be clearly observed, and they are generally attributed to the conversion between CoO and metallic Co that can be expressed by: CoO + 2Li+ + 2e− = Co0 + Li2O.26 In the anodic process, the broad peak at about 2.25 V is assigned to the oxidation reaction of Co to CoO. The small potential shift to a higher value compared to the first cycle can be assigned to the structural reorganization of the electrode material. The reduction peaks located at about 1.35 and 1.02 V correspond to the lithium insertion of CoO and reduction of CoO, respectively.14,26,40 | ||
| Fig. 7 (a) CV curves at a scan rate of 0.1 mV s−1, (b) rate performance, (c) cycle performance and (d) charge/discharge voltage profiles at the current density of 100 mA g−1 of the CoO microspheres. | ||
Fig. 7(b) demonstrates the rate capability from the current density of 100 mA g−1 to 1000 mA g−1. At 100 mA g−1, the first discharge capacity is up to 1236 mA h g−1, and this result is usually attributed to the interfacial lithium storage, formation of the SEI layer, and irreversible reactions with the electrolyte.43,44 From Fig. 7(b), it can be seen that the specific capacities of the CoO microspheres decline with increasing current density. The discharge capacities are 803, 676, 609, and 520 mA h g−1 at the current densities of 100, 200, 500, and 1000 mA g−1, respectively. It should be pointed out that the obtained discharge capacity of the CoO microspheres at 100 mA g−1 is higher than its theoretical value of 716 mA h g−1. This result has also been reported in previous literature, and the extra capacity is usually attributable to the formation of SEI films and other side reactions.43–47 When the current density returns to its initial value of 100 mA g−1 after 40 cycles, the discharge capacity of the CoO microspheres recovers to 733 mA h g−1, illustrating that the CoO microspheres have a good stability.
To analyze the cycling stability, galvanostatic charge/discharge tests were carried out at the current density of 100 mA g−1, as presented in Fig. 7(c). The CoO microsphere electrode suffers from a fast capacity fading, and after 70 cycles at 100 mA g−1, it can only deliver a discharge capacity of 453 mA h g−1 with 51.2% preservation of the second cycle. However, the reversibility of capacity is very stable with a Columbic efficiency close to 100% over the 70 cycles from the second cycle, indicating that the formed SEI during the first cycle is very favorable and stable. The discharge and charge profiles of the CoO microsphere at different cycles were investigated at a current density of 100 mA g−1 as presented in Fig. 7(d). A very long plateau at about 0.75 V (vs. Li+/Li) can be clearly observed in the first discharge curve. In the charge curve, the electrode shows an inclined plateau near 2.1 V, which corresponds well with the oxidation peak in the CV curve. The electrode exhibits discharge and charge capacities of 1236 mA h g−1 and 881 mA h g−1 in the first cycle, with a Columbic efficiency of 71.3%. The low Columbic efficiency is caused by the irreversible reaction processes during the first cycle. As the number of cycles is extended, the charge/discharge plateaus decrease gradually, because the volume expansion and structural collapse during the Li+ insertions and desertions in the CoO microspheres lead to an irreversible capacity decrease.
CoO materials with various unique morphologies such as nanocages, porous microspheres, nanowires, nanodisks and so on have been reported to be used as electrode materials for lithium ion batteries.14,40,46,48,49 Compared with these CoO materials, the specific capacity of the CoO microspheres in this work is not very outstanding. The poor electrochemical performances of the obtained CoO microspheres may be attributed to their compacted solid spherical structure and low specific surface area. To confirm this assumption, N2 adsorption–desorption isotherm measurements were applied to study the specific surface area and porous characteristics of the CoO sample (in Fig. S3, ESI†). The results indicated that the CoO microspheres have a low BET specific surface area of 8.29 m2 g−1, and the hysteresis loop in the isotherm is not very evident, indicating the limited existence of porous channels. The low specific surfaces area of the CoO microspheres offers a low electrode/electrolyte contact area, and the compacted solid structure is not beneficial for the permeation and diffusion of the electrolyte.50 As a result, the usage efficiency of the CoO microspheres is very limited due to a large dead body. Besides, the volume expansion and structural collapse during the Li+ insertions and desertions caused irreversible damaged to the CoO microsphere structure.
3.4. Magnetic properties of the CoO microspheres and metallic Co
CoO with a rock-salt cubic structure is antiferromagnetic in nature because it exhibits 180° Co–O–Co interactions, in which antiferromagnetic coupling is favored.12 However, according to previous reports, rock-salt cubic CoO with certain unique morphology and nano-size may show weak ferromagnetism due to ionic defects in the nanocrystal structures.12,51,52 The magnetic properties of the prepared CoO microspheres in this work were investigated by a superconducting quantum interference device magnetometer, and the result is displayed in Fig. 8(a). It can be clearly seen that the hysteresis loop of the CoO microspheres measured at 300 K shows a linear increase of the magnetization with the increase in magnetic field, suggesting an obvious antiferromagnetic behavior. This result can be ascribed to the large size of the CoO microspheres and their highly crystalline structure. | ||
| Fig. 8 Magnetic-field dependence of magnetization measured at 300 K for the CoO microspheres (a), and metallic Co (inset is the expanded hysteresis loop) (b). | ||
The obtained metallic Co converted from the fcc CoO microspheres exhibits a typical ferromagnetic behavior at room temperature as shown in Fig. 8(b). According to previous literature, the saturation magnetization of bulk Co with fcc structure is 161 emu g−1, and that of bulk Co with hcp structure is 168 emu g−1.2,3,53 The saturation magnetization of the as-prepared metallic Co in this work is measured to be 162 emu g−1, which is very close to the value of fcc Co. In the XRD analysis section, we have demonstrated that in addition to the peaks from fcc Co, very small peaks from hcp Co can also be discerned in the obtained Co product. As a result, the magnetic test result matches well with the XRD measurement of the obtained metallic Co. Moreover, the metallic Co shows a high coercivity value of 76.54 Oe, indicating that the metallic Co prepared by our method can be used for magnetic applications.
4. Conclusions
In summary, we have successfully prepared uniform CoO microspheres and metallic cobalt with a high purity by reacting cobalt acetate with a mixture solvent of EG and deionized water in a one-step hydrothermal process. CoO microspheres were transformed from pre-formed α-Co(OH)2 precursors. The morphologies and microstructures of the α-Co(OH)2 precursor were completely changed during the conversion process. By extending the reaction time, the reduction of CoO microspheres by EG at 200 °C will generate metallic Co. Water in the solvent was found to be very significant for the phase evolution processes. For the same reaction in pure EG without water, only α-Co(OH)2 could be obtained. The phase evolution processes from Co(OH)2 hexagonal plates to CoO microspheres, and then to metallic Co in the hydrothermal conditions were investigated in detail via a series of time-dependent experiments. This finding signifies a novel method to prepare uniform CoO microspheres with a high purity and metallic Co from cobalt acetate in solution. The obtained CoO microspheres showed some potential for application in lithium storage. Magnetic measurements demonstrate that the as-synthesized CoO microspheres show an antiferromagnetic behavior, but the metallic Co exhibits a ferromagnetic characteristic.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Natural Science Foundation of China (No. 81472961), Zhejiang Provincial Natural Science Foundation of China (No. LY18E020003) and Fundamental Research Funds for the Central Universities (lzujbky-2016-114).References
- Y. J. Zhao, X. W. Zhang, X. M. Xu, Y. Z. Zhao, H. P. Zhou, J. B. Li and H. B. Jin, Mater. Chem. Phys., 2017, 201, 235–240 CrossRef CAS.
- L. P. Zhu, W. D. Zhang, H. M. Xiao, Y. Yang and S. Y. Fu, J. Phys. Chem. C, 2008, 112, 10073–10078 CAS.
- K. M. Nam, J. H. Shim, H. Ki, S. Choi, G. Lee, J. K. Jang, Y. Jo, M. H. Jung, H. Song and J. T. Park, Angew. Chem., Int. Ed., 2008, 47, 9504–9508 CrossRef CAS PubMed.
- X. M. Xu, Y. J. Zhao, J. B. Li, H. B. Jin, Y. Z. Zhao and H. P. Zhou, Mater. Res. Bull., 2015, 72, 7–12 CrossRef CAS.
- X. T. Yuan, H. X. Ge, X. Wang, C. L. Dong, W. J. Dong, M. S. Riaz, Z. W. Xu, J. X. Zhang and F. Q. Huang, ACS Energy Lett., 2017, 2, 1208–1213 CrossRef.
- K. Y. Ma, W. J. Zhao, J. P. Cheng, F. Liu and X. B. Zhang, J. Colloid Interface Sci., 2016, 468, 238–246 CrossRef CAS PubMed.
- V. Gupta, T. Kusahara, H. Toyama, S. Gupta and N. Miura, Electrochem. Commun., 2007, 9, 2315–2319 CrossRef CAS.
- Z. F. Huang, Y. Zhao, Y. H. Song, Y. W. Li, G. J. Wu, H. J. Tang and J. Z. Zhao, RSC Adv., 2016, 6, 80059–80064 RSC.
- X. C. Shen, S. Dai, C. L. Zhang, S. Y. Zhang, S. M. Sharkey, G. W. Graham, X. Q. Pan and Z. M. Peng, Chem. Mater., 2017, 29, 4572–4579 CrossRef CAS.
- B. Liu, L. Ma, L. C. Ning, C. J. Zhang, G. P. Han, C. J. Pei, H. Zhao, S. Z. Liu and H. Q. Yang, ACS Appl. Mater. Interfaces, 2015, 7, 6109–6117 CAS.
- K. Vojisavljević, S. Wicker, I. Can, A. Benčan, N. Barsan and B. Malič, Adv. Powder Technol., 2017, 28, 1118–1128 CrossRef.
- X. M. He, X. Y. Song, W. Qiao, Z. W. Li, X. Zhang, S. M. Yan, W. Zhong and Y. W. Du, J. Phys. Chem. C, 2015, 119, 9550–9559 CAS.
- Y. Yu, C. H. Chen, J. L. Shui and S. Xie, Angew. Chem., Int. Ed., 2005, 44, 7085–7089 CrossRef CAS PubMed.
- Y. M. Sun, X. L. Hu, W. Luo and Y. H. Huang, J. Mater. Chem., 2012, 22, 13826–13831 RSC.
- T. Ling, D. Y. Yan, Y. Jiao, H. Wang, Y. Zheng, X. L. Zheng, J. Mao, X. W. Du, Z. P. Hu, M. Jaroniec and S. Z. Qiao, Nat. Commun., 2016, 7 Search PubMed.
- M. Nidhin, K. J. Sreeram and B. U. Nair, Chem. Eng. J., 2012, 185-186, 352–357 CrossRef CAS.
- L. J. Zhang, P. Hu, X. Y. Zhao, R. L. Tian, R. Q. Zou and D. G. Xia, J. Mater. Chem., 2011, 21, 18279–18283 RSC.
- L. H. Zhuo, J. C. Ge, L. H. Cao and B. Tang, Cryst. Growth Des., 2008, 9, 1–6 Search PubMed.
- L. Qiao and M. T. Swihart, Adv. Colloid Interface Sci., 2017, 244, 199–266 CrossRef CAS PubMed.
- Y. Z. Shao, J. Sun and L. Gao, J. Phys. Chem. C, 2009, 113, 6566–6572 CAS.
- J. Yang, H. W. Liu, W. N. Martens and R. L. Frost, J. Phys. Chem. C, 2010, 114, 111–119 CAS.
- W. S. Seo, J. H. Shim, S. J. Oh, E. K. Lee, N. H. Hur and J. T. Park, J. Am. Chem. Soc., 2005, 127, 6188–6189 CrossRef CAS PubMed.
- X. L. Huang, R. Z. Wang, D. Xu, Z. L. Wang, H. G. Wang, J. J. Xu, Z. Wu, Q. C. Liu, Y. Zhang and X. B. Zhang, Adv. Funct. Mater., 2013, 23, 4345–4353 CrossRef CAS.
- D. Y. Guo, F. F. Chen, W. Zhang and R. Cao, Sci. Bull., 2017, 62, 626–632 CrossRef.
- A. Indra, P. W. Menezes, C. Das, C. Gobel, M. Tallarida, D. Schmeiβer and M. Driess, J. Mater. Chem. A, 2017, 5, 5171–5177 CAS.
- X. L. Zhou, Y. R. Zhong, M. Yang, Q. Zhang, J. P. Wei and Z. Zhou, ACS Appl. Mater. Interfaces, 2015, 7, 12022–12029 CAS.
- H. W. Yu, X. Y. Li, Y. N. Zhu, Z. Q. Hao and X. Y. Zeng, J. Phys. Chem. C, 2017, 121, 12469–12475 CAS.
- S. J. Zhu, L. L. Lu, Z. W. Zhao, T. Wang, X. Y. Liu, H. J. Zhang, F. Dong and Y. X. Zhang, J. Phys. Chem. C, 2017, 121, 9394–9401 CAS.
- L. Yu, H. B. Wu and X. W. Lou, Acc. Chem. Res., 2017, 50, 293–301 CrossRef CAS PubMed.
- M. Cheng, S. B. Duan, H. S. Fan and R. M. Wang, CrystEngComm, 2016, 18, 6849–6859 RSC.
- X. H. Liu, R. Z. Ma, Y. Bando and T. Sasaki, Adv. Funct. Mater., 2014, 24, 4292–4302 CrossRef CAS.
- X. W. Xie, P. J. Shang, Z. Q. Liu, Y. G. Lv, Y. Li and W. J. Shen, J. Phys. Chem. C, 2010, 114, 2116–2123 CAS.
- S. S. Chu, C. Yang, X. Xia, J. D. Wang, Y. L. Hou and X. T. Su, New J. Chem., 2016, 40, 2722–2729 RSC.
- G. X. Chen, Y. Zhao, G. Fu, P. N. Duchesne, L. Gu, Y. P. Zheng, X. F. Weng, M. S. Chen, P. Zhang, C. W. Pao, J. F. Lee and N. F. Zheng, Science, 2014, 344, 495–498 CrossRef CAS PubMed.
- H. R. Yue, Y. J. Zhao, X. B. Ma and J. L. Gong, Chem. Soc. Rev., 2012, 41, 4218–4244 RSC.
- Y. G. Sun, Y. D. Yin, B. T. Mayers, T. Herricks and Y. N. Xia, Chem. Mater., 2002, 14, 4736–4745 CrossRef CAS.
- Y. Chen, K. Y. Liew and J. L. Li, Mater. Lett., 2008, 62, 1018–1021 CrossRef CAS.
- C. W. Kim, H. G. Cha, Y. H. Kim, A. P. Jadhav, E. S. Ji, D. I. Kang and Y. S. Kang, J. Phys. Chem. C, 2009, 113, 5081–5086 CAS.
- Y. Z. Qin, Q. Li, J. Xu, X. Wang, G. X. Zhao, C. K. Liu, X. Yan, Y. Z. Long, S. S. Yan and S. D. Li, Electrochim. Acta, 2017, 224, 90–95 CrossRef CAS.
- L. Chang, K. Wang, L. G. Huang, Z. S. He, H. B. Shao and J. M. Wang, J. Alloys Compd., 2017, 725, 824–834 CrossRef CAS.
- X. L. Huang, R. Z. Wang, D. Xu, Z. L. Wang, H. G. Wang, J. J. Xu, Z. Wu, Q. C. Liu, Y. Zhang and X. B. Zhang, Adv. Funct. Mater., 2013, 23, 4345–4353 CrossRef CAS.
- R. F. Zhao, X. Shen, Q. H. Wu, X. Zhang, W. L. Li, G. Gao, L. Y. Zhu, L. B. Ni, G. W. Diao and M. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 24662–24670 CAS.
- J. F. Li, S. L. Xiong, X. W. Li and Y. T. Qian, Nanoscale, 2013, 5, 2045–2054 RSC.
- J. Sun, C. X. Lv, F. Lv, S. Chen, D. H. Li, Z. Q. Guo, W. Han, D. J. Yang and S. J. Guo, ACS Nano, 2017, 11, 6186–6193 CrossRef CAS PubMed.
- L. Yu, L. Zhang, H. B. Wu, G. Q. Zhang and X. W. Lou, Energy Environ. Sci., 2013, 6, 2664–2671 CAS.
- J. Jiang, J. P. Liu, R. M. Ding, X. X. Ji, Y. Y. Hu, X. Li, A. Z. Hu, F. Wu, Z. H. Zhu and X. T. Huang, J. Phys. Chem. C, 2010, 114, 929–932 CAS.
- H. Wu, M. Xu, Y. Y. Wang and G. F. Zheng, Nano Res., 2013, 6, 167–173 CrossRef CAS.
- H. Guan, X. Wang, H. Q. Li, C. Y. Zhi, T. Y. Zhai, Y. Bandob and D. Golberg, Chem. Commun., 2012, 48, 4878–4880 RSC.
- D. J. Lee, S. H. Yu, H. S. Lee, A. Jin, J. Lee, J. E. Lee, Y. E. Sung and T. Hyeon, J. Mater. Chem. A, 2017, 5, 8744–8751 CAS.
- G. X. Wang, Y. M. Sui, M. N. Zhang, M. Xu, Q. X. Zeng, C. Liu, X. M. Liu, F. Du and B. Zou, J. Mater. Chem. A, 2017, 5, 18577–18584 CAS.
- K. Deori and S. Deka, CrystEngComm, 2013, 15, 8465–8474 RSC.
- H. G. Shi and X. M. He, J. Phys. Chem. Solids, 2012, 73, 646–650 CrossRef CAS.
- Y. P. Bao and K. M. Krishnan, J. Magn. Magn. Mater., 2005, 293, 15–19 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7cp06868a |
| This journal is © the Owner Societies 2018 |
