Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Gold-catalyzed [4+3]- and [4+2]-annulations of 3-en-1-ynamides with isoxazoles via novel 6π-electrocyclizations of 3-azahepta trienyl cations†
Sovan Sundar
Giri
and
Rai-Shung
Liu
 *
*
Department of Chemistry, National Tsing-Hua University, Hsinchu, Taiwan, Republic of China. E-mail: rsliu@mx.nthu.edu.tw
First published on 19th February 2018
Abstract
New gold-catalyzed [4+3]-annulations of 3-en-1-ynamides with isoxazoles afford 4H-azepines efficiently; this process involves 6π electrocyclizations of gold-stabilized 3-azaheptatrienyl cations. In the presence of Zn(OTf)2, the resulting 4H-azepines undergo skeletal rearrangement to furnish substituted pyridine derivatives. We subsequently develop new catalytic [4+2]-annulations between the same 3-en-1-ynamides and isoxazoles to deliver substituted pyridine products using Au(I)/Zn(II) catalysts. This work reports the first success of the 6π electrocyclizations of heptatrienyl cations that are unprecedented in literature reports.
Introduction
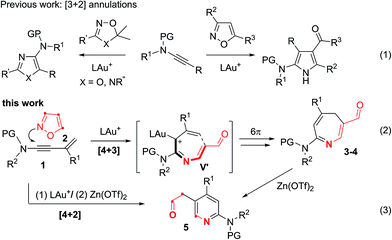
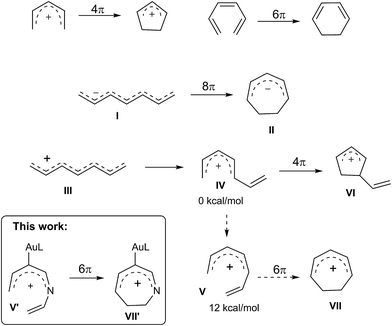
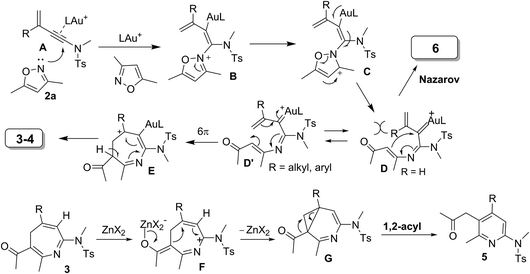
Electrocyclizations of acyclic conjugated π-motifs are powerful tools to access five-, six- and seven-membered carbocycles;1 prominent examples include Nazarov cyclizations of pentadienyl cations2 and 6π electrocyclizations of trienes,3 which have found widespread applications in organic synthesis.In the context of seven-carbon π-motifs, heptatrienyl anions I undergo facile 8π electrocyclizations via rapid interconversions among various anion configurations (Scheme 1).4 In contrast, heptatrienyl cations III5 exclusively undergo Nazarov reactions because of the difficulties of forming all σ-cis configured cations V that have a high energy state.5b 1-Aza- and 1-oxaheptatrienyl cations6 were also reported to follow Nazarov cyclizations. The realization of a 6π electrocyclization of conjugated seven-membered cations is formidable but challenging. This work reveals the first success of such seven-membered cyclizations of gold-stabilized 3-azaheptatrienyl cations V′ to form azacyclic products 3–4via a new C–C bond formation.
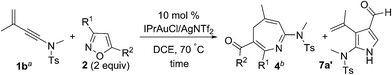
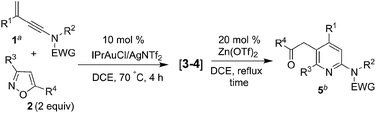
The advent of gold catalysis has inspired new annulations between alkynes and poor nucleophiles.7 N–O containing nucleophiles serve as useful building blocks to construct valuable azacyclic frameworks.7 Ye and Hashmi reported interesting [3+2]-annulations of isoxazoles or benzisoxazoles with electron-rich ynamides, yielding substituted pyrrole derivatives through aza-Nazarov cyclizations of the key intermediate [eqn (1)].7,8 These [3+2]-annulations were extensively expanded to other N–O heterocycles including benzisoxazoles, 1,2,4-oxadiazoles, 1,4,2-dioxazoles and 4,5-dihydro-1,2,4-oxadiazoles, yielding additional five-membered azacycles as depicted in [eqn (1)].9 Here, we report two distinct [4+3]- and [4+2]-annulations between 3-en-1-ynamides and isoxazoles using varied catalysts. An Au(I) catalyst alone delivers 4H-azepines 3–4 through 6π electrocyclizations of intermediates V′ [eqn (2)] whereas a combined action of Au(I)/Zn(II) on the same reactants furnishes highly functionalized pyridines 5 [eqn (3)]. With our convenient synthesis, the synthetic utility of new 4H-azepines 3–4 is also reported.10
Results and discussion
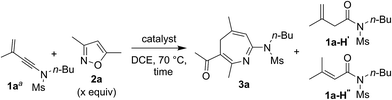
We examined the reactions of 3-methyl-3-en-1-ynamide 1a with 3,5-dimethylisoxazole 2a using various gold catalysts. Heating this mixture (1a/2a = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio) in hot DCE with 5 mol% LAuCl/AgNTf2 [L = p(t-Bu)2(o-biphenyl) and IPr] afforded a [4+3]-annulation product, 4H-azepine 3a, in 64% and 75% yields respectively (Table 1, entries 1–2). Under these conditions, a low loading (1.2 equiv.) of 3,5-dimethylisoxazole 2a gave 3a in a decreased yield, ca. 62% (entry 3). With a 10 mol% catalyst, IPrAuCl/AgNTf2 gave a clean reaction, yielding desired 3a up to 91% (entry 4). We tested other phosphine ligands such as PPh3 and P(OPh)3, yielding desired 3a in satisfactory yields (78–81%, entries 5–6). Other counter anions such as OTf− and SbF6− were also effective in producing 3a in 85–88% yields (entries 7–8). AgNTf2 alone was not active at all (entry 9).
2 ratio) in hot DCE with 5 mol% LAuCl/AgNTf2 [L = p(t-Bu)2(o-biphenyl) and IPr] afforded a [4+3]-annulation product, 4H-azepine 3a, in 64% and 75% yields respectively (Table 1, entries 1–2). Under these conditions, a low loading (1.2 equiv.) of 3,5-dimethylisoxazole 2a gave 3a in a decreased yield, ca. 62% (entry 3). With a 10 mol% catalyst, IPrAuCl/AgNTf2 gave a clean reaction, yielding desired 3a up to 91% (entry 4). We tested other phosphine ligands such as PPh3 and P(OPh)3, yielding desired 3a in satisfactory yields (78–81%, entries 5–6). Other counter anions such as OTf− and SbF6− were also effective in producing 3a in 85–88% yields (entries 7–8). AgNTf2 alone was not active at all (entry 9).
| Entry | Catalyst [mol%] | x | Time [h] | Yieldb [%] | ||
|---|---|---|---|---|---|---|
| 1a | 3a | 1a–H′/1a–H′′ | ||||
| a [1a] = 0.15 M. b Product yields are reported after separation from a silica column. c L = p(t-Bu)2(o-biphenyl). d IPr = 1,3-bis(diisopropylphenyl)-imidazol-2-ylidene. Ms = methanesulfonyl, DCE = 1,2-dichloroethane, and Tf = trifluoromethanesulfonyl. | ||||||
| 1c | LAuCl/AgNTf2 [5] | 2 | 3 | 20 | 64 | — |
| 2d | IPrAuCl/AgNTf2 [5] | 2 | 7 | 12 | 75 | 7 [2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] 1] |
| 3 | IPrAuCl/AgNTf2 [5] | 1.2 | 7 | 23 | 62 | 5 [1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] 1] |
| 4 | IPrAuCl/AgNTf 2 [10] | 2 | 3 | — | 91 | Trace |
| 5 | PPh3AuCl/AgNTf2 [10] | 2 | 3.5 | — | 81 | 5 [1.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] 1] |
| 6 | [PhO]3PAuCl/AgNTf2 [10] | 2 | 3.5 | — | 78 | 13 [1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] 1] |
| 7 | IPrAuCl/AgSbF6 [10] | 2 | 2.5 | — | 85 | 6 [1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] 1] |
| 8 | IPrAuCl/AgOTf [10] | 2 | 2 | — | 88 | Trace |
| 9 | AgNTf2 [10] | 2 | 15 | 33 | — | 11 |
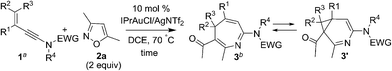
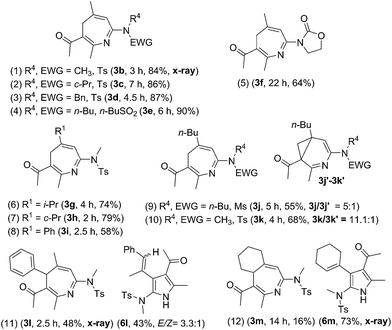
Suitable substituents of 3-en-1-ynamides 1 are crucial to achieve 6π cyclizations of 3-azaheptatrienyl cations V′ [eqn (2)]. We tested the reactions on 3-en-1-ynes 1b–1m bearing a C(3)-substituent to circumvent aza-Nazarov cyclizations as reported in Ye's work.7 Herein, only entries 9 and 10 showed the presence of 3-azanorcaradienes 3′. We examined these [4+3]-annulations on 3-methyl-3-en-1-ynamides 1b–1e bearing various sulfonamides NTsR4 (R4 = Me, cyclopropyl, benzyl and N(n-C4H9)(−SO2Bu)), affording the desired 4H-azepines 3b–3e in high yields (84–90%, Table 2, entries 1–4). Nevertheless, this new annulation becomes less efficient for 3-en-1-ynamide 1f bearing an oxazolidin-2-one to yield product 3f in 64% yield (entry 5).
We altered the C(3)-substituents as in substrates 1g–1i; their resulting products 3g–3h (R1 = isopropyl and cyclopropyl) were obtained in 74–79%, and 3i (R1 = Ph) with only 58% yield (entries 6–8). Notably, when a long n-butyl group was present as in species 1j and 1k, their corresponding reactions afforded compounds 3j/3j′ = 5/1 and 3k/3k′ = 11.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively, in 55% and 68% yields (entries 9–10). For E-configured trisubstituted 3-en-1-yne 1l (R1 = Me, R2 = Ph and R3 = H), 4H-azepine 3l and pyrrole 6l were obtained in equal proportions (entry 11). When a cyclohexenyl group was present for alkene as in species 1m, pyrrole product 6m was dominant over azepine 3m (entry 12). Accordingly, preferable 3-en-1-ynes comprise a small R2 or R3 substituent whereas R1 must be substituted. Herein, the structures of 4H-azepines 3b and 3l, and pyrrole species 6m were confirmed with X-ray diffraction.11
1, respectively, in 55% and 68% yields (entries 9–10). For E-configured trisubstituted 3-en-1-yne 1l (R1 = Me, R2 = Ph and R3 = H), 4H-azepine 3l and pyrrole 6l were obtained in equal proportions (entry 11). When a cyclohexenyl group was present for alkene as in species 1m, pyrrole product 6m was dominant over azepine 3m (entry 12). Accordingly, preferable 3-en-1-ynes comprise a small R2 or R3 substituent whereas R1 must be substituted. Herein, the structures of 4H-azepines 3b and 3l, and pyrrole species 6m were confirmed with X-ray diffraction.11
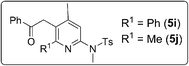
Isoxazoles of a wide scope are compatible with these [4+3]-annulations, as depicted in Table 3. The reaction of unsubstituted isoxazole 2b with model 3-en-1-ynamide 1b afforded the desired 4H-azepine 4a in 84% yield, together with pyrrole 7a′ in only 8% yield (entry 1). Mono-substituted 3-methyl or 5-methyl isoxazoles 2c and 2d are also suitable for these annulations to afford compounds 4b and 4c in 75% and 87% yields, respectively (entries 2–3). We prepared additional 3,5-disubstituted isoxazoles 2e–2i with R1 = alkyl and phenyl, and R2 = alkyl; their annulations proceed smoothly to produce desired 4d–4h in 69–85% yields (entries 4–8). For di-substituted isoxazoles 2j and 2k bearing R2 = Ph, 4H-azepines 4i and 4j were obtained in 61% and 71% yields respectively, together with their rearrangement products 5i and 5j in 15–30% yields (entries 9–10). Compounds 4a and 5i were characterized by X-ray diffraction.11
| Entry | (R1, R2) | 2 | Time [h] | Yield [%] | 4 |
|---|---|---|---|---|---|
| a [1b] = 0.15 M. b Product yields are reported after separation from a silica column. | |||||
| (1) | H, H | 2b | 4 | 84 | 4a (X-ray) |
| 8 | 7a′ | ||||
| (2) | H, Me | 2d | 3 | 75 | 4b |
| (3) | Me, H | 2c | 3 | 87 | 4c |
| (4) | Et, Et | 2e | 6 | 85 | 4d |
| (5) | n-Bu, n-Bu | 2f | 7 | 81 | 4e |
| (6) | Me, n-Bu | 2g | 3 | 82 | 4f |
| (7) | n-Bu, c-Pr | 2h | 2 | 77 | 4g |
| (8) | Ph, n-Bu | 2i | 4 | 69 | 4h |
| (9) | Ph, Ph | 2j | 6.5 | 61 | 4i |
| 30 | 5i (X-ray) | ||||
| (10) | Me, Ph | 2k | 4 | 71 | 4j |
|
15 | 5j | |||
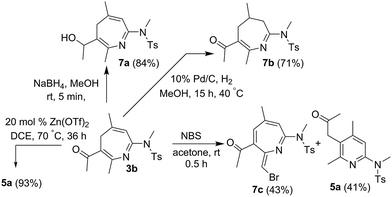
Our convenient synthesis of 4H-azepines provides new synthetic utilities; several new functionalizations are depicted in Scheme 2. NaBH4-reduction of species 3b delivered an alcohol derivative 7a in 84% yield. Selective hydrogenation of the same species afforded 2-aza-1,3-dien-5-one 7b in 71% yield. A final treatment of 4H-azepine 3b with NBS in acetone afforded compound 7c, of which the molecular structure was determined by 1H NOE spectra.
The Lewis-catalyzed rearrangement of 4H-azepines 3–4 to substituted pyridines 5 [eqn (3)] is unprecedented in 4H-azepine chemistry.10 We undertook such novel [4+2]-annulations between 3-en-1-ynamides 1 and isoxazoles 2 using Au(I)/Zn(II) in a relay series, as depicted in Table 4. In the reactions of various 3-substituted 3-en-1-ynamides 1 (R1 = methyl, n-butyl, cyclopropyl and isopropyl) with 3,5-dimethylisoxazole 2a, substituted pyridines 5a–5d were obtained in satisfactory yields (51–73%, entries 1–4). In entry 1, if the reaction was performed with combined Au(I)/Zn(II) catalysts in a non-relay operation, compounds 5a and 3b were isolated in 35% and 28% yields respectively. For 3-en-1-ynamide 1a bearing a NMs(n-butyl), the corresponding product 5e was obtained in 63% yield (entry 5). We tested the reactions on 3,5-disubstituted isoxazoles 2e–2f & 2h bearing all alkyl substituents, producing desired 5f–5h in good yields (69–78%, entries 6–8). For such disubstituted isoxazoles bearing R4 = Ph, the reactions afforded the desired pyridine derivatives 5i and 5j in 75–80% yields (entries 9–10). The molecular structures of compounds 5a and 5i were characterized by X-ray diffraction.11
| Entry | (R1, R2, EWG) | 1 | (R3, R4) | 2 | Time [h] | Yield [%] | 5 |
|---|---|---|---|---|---|---|---|
| a [1] = 0.15 M. b Product yields are reported after separation from a silica column. c The value in parentheses is reported using a mixture of IPrAuCl/AgNTf2 (10 mol%) and Zn(OTf)2 (20 mol%) in hot DCE (70 °C, 48 h); 3b was also isolated in 28% yield. | |||||||
| (1) | Me, Me, Ts | 1b | Me, Me | 2a | 19 | 73 (35)c | 5a (X-ray) |
| (2) | n-Bu, Me, Ts | 1k | Me, Me | 2a | 33 | 64 | 5b |
| (3) | c-Pr, Me, Ts | 1h | Me, Me | 2a | 20 | 56 | 5c |
| (4) | i-Pr, Me, Ts | 1g | Me, Me | 2a | 15 | 51 | 5d |
| (5) | Me, n-Bu, Ms | 1a | Me, Me | 2a | 28 | 63 | 5e |
| (6) | Me, Me, Ts | 1b | n-Bu, n-Bu | 2f | 19 | 78 | 5f |
| (7) | Me, Me, Ts | 1b | Et, Et | 2e | 16 | 69 | 5g |
| (8) | Me, Me, Ts | 1b | nBu, c-Pr | 2h | 20 | 75 | 5h |
| (9) | Me, Me, Ts | 1b | Ph, Ph | 2j | 24 | 80 | 5i (X-ray) |
| (10) | Me, Me, Ts | 1b | Me, Ph | 2k | 30 | 75 | 5j |
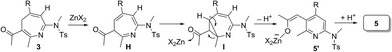
Scheme 3 rationalizes the crucial roles of substituents of 3-en-1-ynamides in the chemoselectivity that relies on two conformational structures DversusD′. The N-attack of isoxazole at gold-π-ynamide A is expected to form a gold-carbene D′, which can be visualized as a gold-stabilized cycloheptatrienyl cation. Conformation D is favorable with R = H, which prefers aza-Nazarov reactions.12 When a C(3)-substituent is present (R = alkyl and aryl), all σ-cis configured species D′ are the preferable geometry to induce novel 6π electrocyclizations. This ring closure is expected to proceed through an attack of enamide at the alkenylgold moiety that is also visualized as a gold-stabilized cation. Additional C(4)-substituents render the formation of cations D′ difficult, thus yielding pyrrole 6 as byproducts. A loss of an acidic proton from seven-membered cations E is expected to yield azepines 3–4. 4H-Azepines 3–4 bear an enone conjugated with a triene; this extensive conjugation is very stable to impede a 6π electrocyclization of their triene moieties unless a Lewis acid is present. Zn(OTf)2 likely coordinates with the carbonyl of 4H-azepine 3 to generate a 2-azapentadienyl cation F bearing a zinc enolate, further enabling an intramolecular cyclization to generate species G. A 1,2-acyl shift14 of species G delivers the observed product 5.13
Conclusions
In summary, this work describes new gold-catalyzed [4+3] annulations15 of 3-substituted 3-en-1-ynamides with isoxazoles to form 4H-azepines. A relay catalysis is also developed with Au(I)/Zn(II) catalysts to achieve [4+2] annulations from the same reactants. The mechanisms of gold-catalyzed [4+3] annulations involve unprecedented 6π electrocyclizations of 3-azacycloheptatrienyl cations to form 4H-azepines 3–4 efficiently. Control experiments confirm that 4H-azepines 3–4 are catalyzed by Zn(OTf)2 to undergo new rearrangement reactions to form substituted pyridine derivatives.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors thank the Ministry of Science and Technology and the Ministry of Education, Taiwan, for supporting this work.Notes and references
- (a) S. Sankararaman, in Pericyclic Reactions-A Textbook. Reactions, Applications and Theory, Wiley-VCH, New York, 2005, ch. 6, pp. 393–398 Search PubMed; (b) C. M. Beaudry, J. P. Malerich and D. Trauner, Chem. Rev., 2005, 105, 4757 CrossRef CAS PubMed; (c) P. V. R. Schleyer, J. I. Wu, F. P. Cossio and I. Fernandez, Chem. Soc. Rev., 2014, 43, 4909 RSC; (d) M. Bian, L. Li and H. Ding, Synthesis, 2017, 49, 4383 CrossRef CAS; (e) E. C. Taylor and I. J. Turchi, Chem. Rev., 1979, 79, 181 CrossRef CAS; (f) F. D. Proft, P. K. Chattaraj, P. W. Ayers, M. T. Sucarrat, M. Elango, V. Subramanian, S. Giri and P. Geerlings, J. Chem. Theory Comput., 2008, 4, 595 CrossRef PubMed; (g) N. Jana and G. Driver, Org. Biomol. Chem., 2015, 13, 9720 RSC.
- (a) W. T. Spencer III, T. Vaidya and A. J. Frontier, Eur. J. Org. Chem., 2013, 3621 CrossRef PubMed; (b) R. L. Davis and D. L. Tantillo, Curr. Org. Chem., 2010, 14, 1561 CrossRef CAS; (c) T. N. Grant, C. J. Rieder and F. G. West, Chem. Commun., 2009, 5676 RSC; (d) M. J. Riveira, L. A. Marsili and M. P. Mischne, Org. Biomol. Chem., 2017, 15, 9255 RSC; (e) N. Shimada, C. Stewart and M. A. Tius, Tetrahedron, 2011, 67, 5851 CrossRef CAS PubMed.
- (a) V. A. Guner, K. N. Houk and I. W. Davis, J. Org. Chem., 2004, 69, 8024 CrossRef CAS PubMed; (b) R. V. Essen, D. Frank, H. W. Sunnemann, D. Vidovic, J. Magull and A. D. Meijere, Chem.–Eur. J., 2005, 11, 6583 CrossRef PubMed; (c) N. A. Magomedov, P. L. Ruggiero and Y. Tang, J. Am. Chem. Soc., 2004, 126, 1624 CrossRef CAS PubMed; (d) E. N. Marvell, G. Caple, B. Schatz and W. Pippin, Tetrahedron, 1973, 29, 3781 CrossRef CAS; (e) J. R. Otero, J. Org. Chem., 1999, 64, 6842 CrossRef; (f) P. E. Tessier, N. Nguyen, M. D. Clay and A. G. Fallis, Org. Lett., 2005, 7, 767 CrossRef CAS PubMed; (g) C. L. Benson and F. G. West, Org. Lett., 2007, 9, 2545 CrossRef CAS PubMed; (h) G. A. Barcan, A. Patel, K. N. Houk and O. Kwon, Org. Lett., 2012, 14, 5388 CrossRef CAS PubMed.
- (a) R. B. Bates, W. H. Delnes, D. A. McCombs and D. E. Potter, J. Am. Chem. Soc., 1969, 91, 4608 CrossRef CAS; (b) K. Marx and W. Eberbach, Chem.–Eur. J., 2000, 6, 2063 CAS; (c) M. Reisser and G. Mass, J. Org. Chem., 2004, 69, 4913 CrossRef CAS PubMed; (d) T. Hübner, Dissertation, University of Freiberg, 1987 Search PubMed; (e) A. Arany, D. Bendell, P. W. Groudwater, I. Garnett and M. Nyerges, J. Chem. Soc., Perkin Trans. 1, 1999, 2605 RSC.
- (a) N. C. Deno, C. U. Pittman and J. O. Turner, J. Am. Chem. Soc., 1965, 87, 2153 CrossRef CAS; (b) O. N. Faza, C. S. López, R. Alvarez and A. R. de Lera, Chem.–Eur. J., 2009, 15, 1944 CrossRef CAS PubMed.
- D. Alickmann, R. Fröhlich, A. H. Maulitz and E. U. Wurthwein, Eur. J. Org. Chem., 2002, 1523 CrossRef CAS.
- (a) A. H. Zhou, Q. He, C. Shu, Y. F. Yu, S. Liu, T. Zhao, W. Zhang, X. Lu and L. W. Ye, Chem. Sci., 2015, 6, 1265 RSC; (b) X. Y. Xiao, A. H. Zhou, C. Shu, F. Pan, T. Li and L. W. Ye, Chem.–Asian J., 2015, 10, 1854 CrossRef CAS PubMed; (c) W. B. Shen, X. Y. Xiao, Q. Sun, B. Zhou, X. Q. Zhu, J. Z. Yan, X. Lu and L. W. Ye, Angew. Chem., Int. Ed., 2017, 56, 605 CrossRef CAS PubMed; (d) L. Li, T. D. Tan, Y. Q. Zhang, X. Liu and L. W. Ye, Org. Biomol. Chem., 2017, 15, 8483 RSC.
- (a) H. Jin, L. Huang, J. Xie, M. Rudolph, F. Rominger and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2016, 55, 794 CrossRef CAS PubMed; (b) H. Jin, B. Tian, X. Song, J. Xie, M. Rudolph, F. Rominger and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2016, 55, 12688 CrossRef CAS PubMed.
- (a) Z. Zeng, H. Jin, J. Xie, B. Tian, M. Rudolph, F. Rominger and A. S. K. Hashmi, Org. Lett., 2017, 19, 1020 CrossRef CAS PubMed; (b) M. Chen, N. Sun, H. Chen and Y. Liu, Chem. Commun., 2016, 52, 6324 RSC; (c) W. Xu, G. Wang, N. Sun and Y. Liu, Org. Lett., 2017, 19, 3307 CrossRef CAS PubMed.
- For synthesis of nH-azepines (n = 2 or 4), see selected examples: (a) U. Gockel, U. Hartmannsgruber, A. Steigel and J. Sauer, Tetrahedron Lett., 1980, 21, 599 CrossRef; (b) I. R. Dunkin, A. E. Ayeb and M. A. Lynch, J. Chem. Soc., Chem. Commun., 1994, 1695 RSC; (c) K. Satake, R. Okuda, M. Hashimoto, Y. Fujiwara, H. Okamoto, M. Kimura and S. Morosawa, J. Chem. Soc. Perkin Trans 1, 1994, 1753 RSC; (d) Y. Luo and J. Wu, Chem. Commun., 2011, 47, 11137 RSC.
- Crystallographic data of compounds 3b, 3l, 4a, 5a, 5i, and 6m were deposited in Cambridge Crystallographic Data Center: 3b: CCDC 1589549, 3l: CCDC 1589562, 4a: CCDC 1589561, 5a: CCDC 1589558, 5i: CCDC 1589559 and 6m CCDC 1589560.†.
- For the aza-Nazarov cyclizations, see: (a) D. A. Klumpp, Y. Zhang, M. J. O'Connor, P. M. Esteves and L. S. de Almeida, Org. Lett., 2007, 9, 3085 CrossRef CAS PubMed; (b) Z. X. Ma, S. He, W. Song and R. P. Hsung, Org. Lett., 2012, 14, 5736 CrossRef CAS PubMed; (c) R. L. Sahani and R. S. Liu, Angew. Chem., Int. Ed., 2017, 56, 12736 CrossRef CAS PubMed; (d) S. K. Pawar, R. L. Sahani and R. S. Liu, Chem.–Eur. J., 2015, 21, 10843 CrossRef CAS PubMed; (e) C. Shu, Y. H. Wang, C. H. Shen, P. P. Ruan, X. Lu and L. W. Ye, Org. Lett., 2016, 18, 3254 CrossRef CAS PubMed.
- As suggested by one reviewer, an alternative mechanism is also possible for the Zn(II)-catalyzed rearrangement; this process involves an isomerization of initial species 3 to an unconjugated iminoyl ketone H, followed by a 6π-cyclization to generate species I. A subsequent Zn(II)-catalyzed aromatization of species I is expected to yield the final product 5. In this process, species H is relatively higher than 3 in energy, but its feasibility is not excluded
 .
. - (a) S. M. Wang and L. Zhang, Org. Lett., 2006, 8, 4585 CrossRef CAS PubMed; (b) G. Li, G. Zhang and L. Zhang, J. Am. Chem. Soc., 2008, 130, 3704 Search PubMed; (c) S. B. Wagh and R. S. Liu, Chem. Commun., 2015, 51, 15462 RSC; (d) R. Chaudhuri, A. Das, H. Y. Liao and R. S. Liu, Chem. Commun., 2010, 46, 4601 RSC.
- For reviews of gold-catalyzed cycloaddition or annulation reactions of alkynes, see (a) D. J. Gorin, B. D. Sherry and F. D. Toste, Chem. Rev., 2008, 108, 3351 CrossRef CAS PubMed; (b) A. S. K. Hashmi, Chem. Rev., 2007, 107, 3180 CrossRef CAS PubMed; (c) F. López and J. L. Mascareñas, Beilstein J. Org. Chem., 2011, 7, 1075 CrossRef PubMed; (d) C. Aubert, L. Fensterbank, P. Garcia, M. Malacria and A. Simonneau, Chem. Rev., 2011, 111, 1954 CrossRef CAS PubMed; (e) D. Garayalde and C. Nevado, ACS Catal., 2012, 2, 1462 CrossRef CAS; (f) M. E. Muratore, A. Homes, C. Obradors and A. M. Echavarren, Chem.–Asian J., 2014, 9, 3066 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1589549, 1589562, 1589561, 1589558, 1589559 and 1589560. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8sc00232k |
| This journal is © The Royal Society of Chemistry 2018 |