Assembly of hepatitis E vaccine by ‘in situ’ growth of gold clusters as nano-adjuvants: an efficient way to enhance the immune responses of vaccination†
Hai
Wang
a,
Yanping
Ding
a,
Shishuai
Su
a,
Duojia
Meng
b,
Ayeesha
Mujeeb
a,
Yan
Wu
*a and
Guangjun
Nie
*a
aCAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, National Center for Nanoscience and Technology (NCNST), Beiyitiao, Zhongguancun, Beijing 100190, China. E-mail: wuy@nanoctr.cn; niegj@nanoctr.cn
bChangchun Institute of Biological Products Co., Ltd, 3456 Xian road, Changchun city, 130062, China
First published on 17th June 2016
Abstract
Vaccine-based immunotherapy plays an integral role in the development of present and future clinical therapies. Despite the success, there is still a great need to improve the efficiency and safety of vaccines. Nanoparticles have been widely used for improving the efficacy of vaccines by encapsulating the vaccines or using nanoparticles as immune adjuvants. However, the methods for the preparation of nanoparticles are complex with a relatively low encapsulation efficiency of protein vaccine inside the nanocarriers and/or undefined physiochemical properties. Here, we report a new method of preparation of a vaccine by the “in situ” growth of gold clusters in the hepatitis E vaccine (HEVA). The gold cluster grafted HEVA (HEVA/Au) can be easily obtained and there is no loss of HEVA during the preparation process. More importantly, the “in situ” prepared HEVA/Au can not only enhance its immune responses in vivo, but also reduce the potential toxicity of HEVA. Furthermore, the intrinsic fluorescence of gold clusters enables the HEVA to be traceable, which may open a way to track the dynamic behavior of vaccines and further help to optimize an individual therapeutic regimen for immunotherapy.
Conceptual insightsThe development of safer and more effective vaccines is a continuous challenge. The unique physicochemical properties of nanoparticles offer great opportunity to improve the immune-response of vaccine via nano-adjuvant or nanoparticle-based enhanced delivery. However, the application of nanotechnology-based strategy is restrained by the complex methodology and limited efficacy. In this study, we introduced a novel strategy via growing gold nanoclusters within the monomers of hepatitis E vaccine (HEVA). The formation of HEVA aggregates via gold clusters (HEVA/Au) gives rise to high potency in inducing antibody response and significantly enhances the immune response in vivo. Moreover, the gold clusters not only aggregated the vaccine, but also significantly reduced the potential toxicity of HEVA. Compared with traditional techniques, our strategy via simply introducing gold clusters to protein interfaces of monomers highlights the important potential application of gold cluster-based nanotechnology in immunotherapy. Considering the facts that protein vaccine plays an integral role in the development of present and future clinical therapies, this novel method provides great opportunity for developing next-generation vaccines, empowered by nanotechnology. |
Immunotherapy is leading to dramatic advancements in the development of future clinical therapies against various diseases.1,2 Vaccines, containing pathogen-derived antigens, are an essential type of immunotherapy for disease prevention and treatment.3–5 Although great progress has been achieved in the past few decades, improvement in the effectiveness and safety is critically required for the development of the next-generation vaccines. One promising strategy is nanotechnology-based engineering of vaccines which has shown enhanced safety and immune responses in vivo.6–10 Among those previous studies, the strategies mainly utilized nanocarriers for the delivery of vaccines to target immune cells, or simply tailored functional nanoparticles as immune adjuvants.11–15 However, the complex methodology for nanoparticle preparation and/or low encapsulation efficiency of protein vaccines severely restricted their clinical applications.6
An alternative strategy for the elevation of the vaccine-induced immune responses is to form protein vaccine aggregates. The aggregated protein vaccines possess high potency in inducing antibody responses compared with the monomeric form.16,17 Although this phenomenon has been known for a long time, it is still not widely used as of today due to the limited methods for aggregating the protein vaccines and preserving their efficacy at the same time. In the present study, we developed a simple method for the generation of hepatitis E vaccine (HEVA) aggregates by growing gold (Au) clusters within the vaccine molecules. Hepatitis E virus (HEV) infection can lead to fulminant hepatitis.18 According to the World Health Organization (WHO), about 20 million people are infected by hepatitis E virus every year, and 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people die from the hepatitis E related diseases.19 Fortunately, the first HEVA was approved in China in 2011.20 However, the safety and efficacy profile of the HEVA still challenges its approval worldwide. Considering the fact that a huge population is infected every year, further improvement of the safety and efficacy of HEVA is urgently needed. Herein using our strategy has successfully led to the formation of the HEVA aggregates via gold (Au) clusters within the HEVA monomers. The Au cluster aggregated HEVA (HEVA/Au) has shown both enhanced safety and augmented immune response, holding great promise for future development.
000 people die from the hepatitis E related diseases.19 Fortunately, the first HEVA was approved in China in 2011.20 However, the safety and efficacy profile of the HEVA still challenges its approval worldwide. Considering the fact that a huge population is infected every year, further improvement of the safety and efficacy of HEVA is urgently needed. Herein using our strategy has successfully led to the formation of the HEVA aggregates via gold (Au) clusters within the HEVA monomers. The Au cluster aggregated HEVA (HEVA/Au) has shown both enhanced safety and augmented immune response, holding great promise for future development.
Au clusters have been reported to form inside various proteins including bovine serum albumin, insulin, and ferritin.21,22 Since it was our intention to construct an intermolecular complex of HEVA, we hypothesized that small monomeric HEVA subunits (molecular weight ∼20 kD) were inclined to aggregate through the formation of intermolecular Au clusters, resulting in enhanced immune responses of vaccines. To generate Au clusters, we incubated the HEVA (50 mg mL−1) with HAuCl4 (10 mM) at pH 8 for the adsorption of Au ions onto the Au nucleation sites on HEVA. Amino acids with reducibility (e.g., histidine, His, and cysteine, Cys) in the vaccine could change Au3+ to Au1+ or Au0 and served as nucleation sites for efficiently binding with Au ions.23 Indeed, HEVA/Au could be easily and quickly obtained by mixing HEVA and HAuCl4 for several minutes.
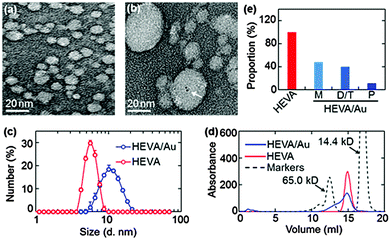
To characterize the HEVA and HEVA/Au, samples negatively stained by uranyl acetate were observed by transmission electron microscopy (TEM). Compared with pure HEVA (Fig. 1a), HEVA/Au re-assembled to form protein aggregates with different sizes and Au clusters were localized inside the vaccine complex (Fig. 1b). A wide size distribution of HEVA/Au was also observed using dynamic light scattering (DLS) analysis in comparison to that of HEVA (Fig. 1c). These results suggested that some HEVA aggregated during the formation of the intermolecular Au clusters. To quantitatively determine the components of the HEVA aggregates, we analyzed the molecular weights of HEVA and HEVA/Au using analytical size-exclusion gel chromatography. As shown in Fig. 1d and e, pure HEVA revealed a single absorbance peak with a molecular weight of approximately 20 kD. In contrast, HEVA/Au was very heterogeneous, with 48% in the monomeric state, 40% as dimers or trimers, and 12% as pentamers (Fig. 1d and e).
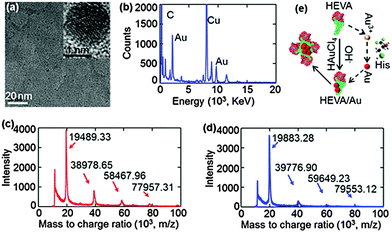
Next, we confirmed and characterized the formation of Au clusters within the HEVA aggregates. TEM clearly showed the presence of Au clusters within several nanometers (Fig. 2a), which was further confirmed using energy-dispersive X-ray spectroscopy (Fig. 2b). To quantify the number of Au ions which could be reduced and attached to each HEVA, matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF) was applied to detect the molecular weights of HEVA and HEVA/Au. A major peak at about 20 kD was observed in both the HEVA and HEVA/Au spectra, which indicated the molecular weight of the corresponding monomer (Fig. 2c and d). Interestingly, the molecular weight of the HEVA/Au monomer was larger than that of HEVA, and the variation of 393.95 Da was equivalent to the molecular weight of two Au ions (molecular weight, 196.97 Da). Meanwhile, we found that multiple aggregates of HEVA and HEVA/Au could be measured. The variation of 798.25, 1181.27, and 1595.81 Da in the molecular weight of dimer, trimer and tetramer between HEVA and HEVA/Au suggested 4, 6, and 8 Au ions in each protein complex, respectively, further confirming that each HEVA could reduce and bind to two Au ions. These results demonstrated that Au clusters were successfully synthesized in the HEVA complex and each HEVA absorbed two Au ions.
According to our data and the current understanding of Au cluster formation,24 we proposed a schematic illustration of the Au assembly process in the HEVA protein complex. As shown in Fig. 2e, after two Au ions were reduced and bound to the monomeric HEVA, more Au ions on the HEVA were incorporated to form Au clusters and the HEVA simultaneously aggregated. The aggregates of HEVA were supposed to enhance the immune response.
We further evaluated the biochemical and physical properties of HEVA/Au. Both HEVA/Au and HEVA revealed a major absorbance peak at 280 nm (Fig. S1, see the ESI†). The secondary structure of HEVA/Au was analyzed by circular dichroism (CD) spectroscopy. HEVA/Au and HEVA showed similar CD spectra, indicating that the secondary structure of HEVA/Au did not change during the formation of Au clusters (Fig. S2, see the ESI†). The maintenance of the secondary conformation of HEVA is very critical for retaining the immune ability of HEVA. Moreover, DLS analysis showed that the surface charges of the HEVA/Au and HEVA were also the same (Fig. S3, see the ESI†).
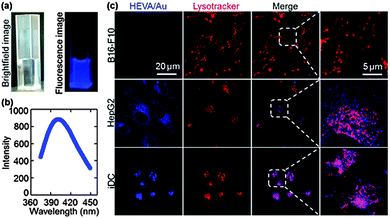
A key step in the understanding of the functionality of vaccines is the determination of their fate and the dynamic interaction with targeted cells and molecules.15,25–27 The intrinsic fluorescence properties of Au clusters enabled tracing of HEVA/Au in cells and in vivo. Under 365 nm UV light, the HEVA/Au solution exhibited a strong blue fluorescence (Fig. 3a). The photoluminescence emission spectra of HEVA/Au showed the absorption peak at about 410 nm (Fig. 3b). The emission quantum yield ϕf was about 6%. Since the Au clusters had no effect on the physiochemical properties of HEVA (Fig. S1–S3, see the ESI†), we further evaluated the in vitro subcellular localization and in vivo biodistribution of HEVA/Au. As shown in Fig. 3c, when we incubated the HEVA/Au with three different cell lines (B16-F10, HepG2, and iDC) for 24 h, only a small fraction of HEVA/Au was internalized into B16-F10 cells, whereas significantly more HEVA/Au was taken up by HepG2 cells, and more importantly, iDC cells showed the most intense fluorescence intensity of HEVA/Au. Therefore, HEVA/Au could be easily taken up by liver cells (HepG2) and immune cells (iDC), indicating that HEVA/Au may specifically target certain types of cells and are internalized into those cells efficiently. We also found that the HEVA/Au was mainly localized in cytosol and lysosome compartments (Fig. 3c).
To evaluate the in vivo distribution, we intravenously injected the HEVA/Au into mice and various organs were harvested for ex vivo imaging. As shown in Fig. S4 (see the ESI†), the HEVA/Au was mainly distributed in the liver, heart, kidney, and lymph node, and little was observed in spleen. Although detailed investigation is needed to further identify the interactions between HEVA/Au and various potential targets, the Au cluster grafted HEVA offers a promising strategy to understand the biological behavior of HEVA both in vitro and in vivo, with a minimal disturbance of immune interactions.
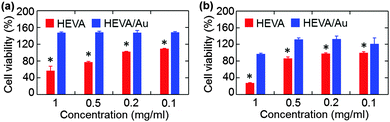
We next evaluated whether the Au cluster-mediated aggregation of HEVA could affect its biological functions. We examined the potential toxicity of HEVA/Au to HepG2 cells and iDC cells. As shown in Fig. S5a and b (see the ESI†), neither HEVA nor HEVA/Au showed obvious cytotoxicity at low concentrations which were below the clinical dosage. However, apparent cytotoxicity was observed when the concentration of HEVA was increased to or above 0.1 mg mL−1 (Fig. 4a and b). In contrast, HEVA/Au did not show any cytotoxic effect even at the concentration of 1 mg mL−1, indicating the improved safety profile of HEVA/Au. The accumulation of HEVA in targeted organs such as liver and heart (Fig. S4, see the ESI†) remains a concern for its clinical application, possibly resulting in concentration-dependent toxicity. Our current results suggested that the HEVA/Au complex could greatly reduce the potential toxicity of this vaccine although the exact mechanism still needs further exploration.
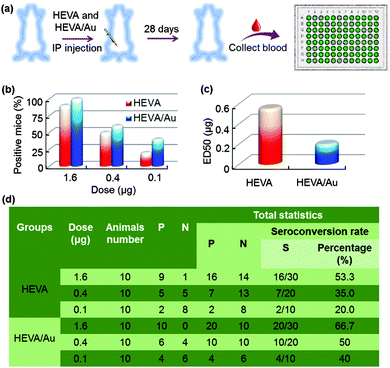
We further investigated the immunotherapeutic effects of HEVA or HEVA/Au in vivo. Mice were intraperitoneally injected with different dosages (1.6, 0.4 or 0.1 μg each mouse) of HEVA or HEVA/Au (n = 10/group). After four weeks, sera were collected for the detection of the hepatitis E virus antibody by ELISA (Fig. 5a). The cutoff value was determined to be 2.1 times of the absorbance value in the control group. Mice were considered to show positive response only if the absorbance value was higher than the cutoff value, while negative response indicated that the absorbance value was lower than the cutoff value. Strikingly, the number of positive mice in the HEVA/Au-treated group was much higher than that of the HEVA-treated group at all dosages (Fig. 5b). The median effective dose (ED50) for HEVA was about two times of that for HEVA/Au (Fig. 5c, 0.2 μg for HEVA/Au compared to 0.55 μg for HEVA). Detailed information is summarized in Fig. 5d. These results suggested that the formation of HEVA aggregates could strongly enhance the immune responses. According to previous studies, the vaccine aggregates influenced the Th1/Th2 immune response, which in turn enhanced the immunotherapy efficacy.5,14 Compared to previous studies (encapsulations of vaccine in the nanocarriers, or using nanoparticles as immune adjuvants), our current strategy via the formation of intermolecular Au clusters showed both enhanced safety profile and therapeutic efficacy.
Conclusions
In summary, apart from previously reported nanotechnology-based immunotherapies, the current Au cluster grafted vaccines showed some interesting and important features including: (a) simple preparation method; (b) improved safety and immunotherapeutic efficacy; and (c) in vitro and in vivo visualization and tracking capacity. The HEVA aggregates formed during the growth of Au clusters may contribute to the enhanced immune response by influencing the Th1/Th2 immune response.14 We also have to point out that the current strategy holds the potential for application in many other protein-based vaccines. By labelling the vaccine with fluorescent Au clusters, this method can further help to understand the molecular mechanism of vaccines for inducing immune responses. Although the underlying mechanism of the reduced toxicity of HEVA by Au clusters has not been defined, we have shown a promising novel strategy for the development of efficient and safe vaccines against the spreading of hepatitis E virus.Methods
Materials, cells and animals
HAuCl4 was purchased from Bailingwei (Beijing). The HEVA was produced and purified by Changchun Institute of Biological Products (Changchun, China). The cell counting kit-8 (CCK-8) was purchased from Dojindo Molecular Technologies (Rockville, MD, USA). Lyso-Tracker Red, fetal bovine serum (FBS) and penicillin/streptomycin were obtained from Invitrogen (Carlsbad, CA, USA). F-12K and DMEM cell culture media were purchased from ATCC (Manassas, VA, USA). All other chemicals were purchased from Sigma (St Louis, MO, USA). The human liver hepatocellular carcinoma cell line HepG2 was obtained from ATCC (Manassas, VA, USA) and cultured in a DMEM containing 10% FBS. An immature mouse dendritic cell line (iDC) was kindly provided by Prof. Honglin Xu (National Vaccine and Serum Institute, China) and cultured in RPMI-1640 medium supplemented with 10% FBS. NIH mice (female, 6–8 weeks) were provided by the Changchun Institute of Biological Products Co., Ltd. All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC).Preparation of HEVA/Au
First, 1 mL of the HEVA solution (50 mg mL−1) was fully mixed with 0.5 mL of HAuCl4 solution (10 mM). After the pH was adjusted to 8, the solution was further incubated for 10 min at room temperature. The resulted HEVA/Au was collected by centrifugation at 1000g using an ultrafilter device. After washing three times with distilled water, the concentration of HEVA/Au was measured using a Bicinchoninic Acid Kit (Thermo Scientific™, MA, USA).Characterization of HEVA and HEVA/Au
The morphologies of HEVA and HEVA/Au were obtained by depositing the sample on a carbon-coated copper grid, and then examined using a transmission electron microscope (TEM, Tecnai G2 20 S-TWIN, FEI, USA). UV/Vis absorbance and fluorescence spectra were recorded on a Lambda 950 UV/Vis spectrophotometer (Perkin-Elmer) and a LS 55 fluorescence spectrometer. The size distribution was determined by using a ZetaSizer Nano series Nano-ZS (Malvern Instruments Ltd, Malvern, UK) with a measuring range from 0.6 nm to 6 μm. The molecular weights of HEVA and HEVA/Au were analyzed using matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS) (Bruker, Germany). 10 μL of each sample was spotted on the target plate and allowed to dry completely. The MS detection range was within 500–2000 Da.In vitro cell viability and cell imaging study
The HepG2 and iDC cells were seeded in 96-well plates (5000 cells per well) at 37 °C overnight. After the cells were incubated with HEVA or HEVA/Au (0.1, 0.4, 1.6, 3.2, 100, 200, 500, and 1000 μg mL−1) in fresh medium for 24 h, the cell viability was examined using a CCK-8. For cell imaging, B16-F10, HepG2 and iDC cells were seeded in glass bottom dishes (Nunc, DX, Danmark). When the cells were 70% confluent, they were incubated with HEVA/Au for 24 h at 37 °C. After washing three times with PBS, the cells were observed using confocal microscopy (LSM710, Carl Zeiss, Germany).Ex vivo imaging and biodistribution
For ex vivo imaging of the HEVA/Au biodistribution, mice were intravenously injected with 100 μg mL−1 of HEVA/Au in 100 μL saline. After 6 h, major organs (heart, liver, spleen, lung, and kidney) were excised for ex vivo imaging using a Maestro™ in vivo imaging system (Cambridge Research & Instrumentation, Woburn, MA, USA).In vivo immunotherapy
Sixty NIH mice were randomly divided into six groups (n = 10/group) and were treated with HEVA or HEVA/Au at different concentrations (1.6, 0.4, and 0.1 μg per mouse) via intraperitoneal injection. After 28 days, blood was collected and analyzed for antibody using a hepatitis E ELISA kit (Changchun Institute of Biological Products, China). Briefly, HEVA antibody and saline were used as positive control and negative control, respectively. The samples were diluted 10 times and added into the 96-well plate for 30 min at 37 °C. Then, each sample was washed four times and incubated with the secondary antibody solution for 30 min. After washing five times, the samples were incubated with the 3,3′,5,5′-tetramethylbenzidine (TMB) substrate for 19 min in the dark. After the reaction was stopped, the absorbance at 450 nm was measured using a micro-reader (PerkinElmer VICTORX3, USA).Statistical analysis
All data are represented as mean ± standard deviation (SD) from at least three independent experiments. Data were analyzed by the Student’s t-test for comparison of two groups and one-way ANOVA followed by the post hoc test for multiple groups.Acknowledgements
This work was supported by grants from MoST 973 (2012CB934004), the NSFC (31325010, 21320102003, 81472850, and U1505228), and the Key Research Program of the Chinese Academy of Sciences (KGZD-EW-T06).References
- C. G. Figdor, I. J. M. de Vries, W. J. Lesterhuis and C. J. Melief, Nat. Med., 2004, 10, 475–480 CrossRef CAS PubMed.
- J. Bousquet, R. Lockey and H.-J. Malling, J. Allergy Clin. Immunol., 1998, 102, 558–562 CrossRef CAS PubMed.
- S. J. Danishefsky and J. R. Allen, Angew. Chem., Int. Ed., 2000, 39, 836–863 CrossRef CAS PubMed.
- J. Banchereau and A. K. Palucka, Nat. Rev. Immunol., 2005, 5, 296–306 CrossRef CAS PubMed.
- A. Singh and N. A. Peppas, Adv. Mater., 2014, 26, 6530–6541 CrossRef CAS PubMed.
- L. Zhao, A. Seth, N. Wibowo, C.-X. Zhao, N. Mitter, C. Yu and A. P. Middelberg, Vaccine, 2014, 32, 327–337 CrossRef PubMed.
- R. Klippstein and D. Pozo, Nanomedicine, 2010, 6, 523–529 CrossRef CAS PubMed.
- X. Wang, C. Sun, P. Li, T. Wu, H. Zhou, D. Yang, Y. Liu, X. Ma, Z. Song and Q. Nian, Adv. Mater., 2015, 28, 694–700 CrossRef PubMed.
- C. Qiao, J. Liu, J. Yang, Y. Li, J. Weng, Y. Shao and X. Zhang, Biomaterials, 2016, 85, 1–17 CrossRef CAS PubMed.
- L. Zhang, Z. Zeng, C. Hu, S. L. Bellis, W. Yang, Y. Su, X. Zhang and Y. Wu, Biomaterials, 2016, 77, 307–319 CrossRef CAS PubMed.
- A. des Rieux, V. Fievez, M. Garinot, Y.-J. Schneider and V. Préat, J. Controlled Release, 2006, 116, 1–27 CrossRef CAS PubMed.
- J. Panyam and V. Labhasetwar, Adv. Drug Delivery Rev., 2003, 55, 329–347 CrossRef CAS PubMed.
- S. L. Demento, S. C. Eisenbarth, H. G. Foellmer, C. Platt, M. J. Caplan, W. M. Saltzman, I. Mellman, M. Ledizet, E. Fikrig and R. A. Flavell, Vaccine, 2009, 27, 3013–3021 CrossRef CAS PubMed.
- M. Zhu, X. Tian, X. Song, Y. Li, Y. Tian, Y. Zhao and G. Nie, Small, 2012, 8, 2841–2848 CrossRef CAS PubMed.
- I. Dimier-Poisson, R. Carpentier, T. T. L. N'Guyen, F. Dahmani, C. Ducournau and D. Betbeder, Biomaterials, 2015, 50, 164–175 CrossRef CAS PubMed.
- A. S. Rosenberg, AAPS J., 2006, 8, E501–E507 CrossRef PubMed.
- J. C. Jones, E. W. Settles, C. R. Brandt and S. Schultz-Cherry, Vaccine, 2011, 29, 7696–7703 CrossRef CAS PubMed.
- S. B. Park, Nature, 2012, 491, 21–22 CrossRef CAS PubMed.
- S. A. Baylis, J. Blümel, S. Mizusawa, K. Matsubayashi, H. Sakata, Y. Okada, C. M. Nübling, K.-M. O. Hanschmann and H. C. S. Group, Emerging Infect. Dis., 2013, 19, 729 CrossRef PubMed.
- J. Zhang, X.-F. Zhang, S.-J. Huang, T. Wu, Y.-M. Hu, Z.-Z. Wang, H. Wang, H.-M. Jiang, Y.-J. Wang and Q. Yan, N. Engl. J. Med., 2015, 372, 914–922 CrossRef CAS PubMed.
- J. Xie, Y. Zheng and J. Y. Ying, J. Am. Chem. Soc., 2009, 131, 888–889 CrossRef CAS PubMed.
- C. Sun, Y. Yuan, Z. Xu, T. Ji, Y. Tian, S. Wu, J. Lei, J. Li, N. Gao and G. Nie, Bioconjugate Chem., 2015, 26, 193–196 CrossRef CAS PubMed.
- C. Sun, H. Yang, Y. Yuan, X. Tian, L. Wang, Y. Guo, L. Xu, J. Lei, N. Gao and G. J. Anderson, J. Am. Chem. Soc., 2011, 133, 8617–8624 CrossRef CAS PubMed.
- C. L. Liu, H. T. Wu, Y. H. Hsiao, C. W. Lai, C. W. Shih, Y. K. Peng, K. C. Tang, H. W. Chang, Y. C. Chien and J. K. Hsiao, Angew. Chem., Int. Ed., 2011, 50, 7056–7060 CrossRef CAS PubMed.
- M. G. Panthani, T. A. Khan, D. K. Reid, D. J. Hellebusch, M. R. Rasch, J. A. Maynard and B. A. Korgel, Nano Lett., 2013, 13, 4294–4298 CrossRef CAS PubMed.
- K. Ekaterina, L. J. Caproni and J. S. Tregoning, PLoS One, 2015, 10, e0130375 CrossRef PubMed.
- F. Li, J. Wang, S. Sun, H. Wang, Z. Tang and G. Nie, Small, 2015, 11, 1954–1961 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6nh00087h |
| This journal is © The Royal Society of Chemistry 2016 |