Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nanomaterials-modified reverse osmosis membranes: a comprehensive review
Mahmoud A. Ahmed
 *ab,
Safwat A. Mahmoud
c and
Ashraf A. Mohamed
*ab,
Safwat A. Mahmoud
c and
Ashraf A. Mohamed
 a
a
aChemistry Department, Faculty of Science, Ain Shams University, Cairo-11566, Egypt. E-mail: mahmoudmahmoud_p@sci.asu.edu.eg
bVeolia Water Technologies, Cairo 11835, Egypt
cPhysics Department, Faculty of Science, Northern Border University, Arar, 13211, Saudi Arabia
First published on 12th June 2024
Abstract
Because of its great efficiency and widespread application, reverse osmosis (RO) is a popular tool for water desalination and purification. However, traditional RO membranes have a short lifespan due to membrane fouling, deterioration, decreased salt rejection rate, and the low water flux with aging. As a result, membrane modification has received a lot of attention recently, with nanomaterials being extensively researched to improve membrane efficacy and lifespan. Herein, we present an in-depth analysis of recent advances of RO membranes modification utilizing nanomaterials. An overview of the various nanomaterials used for membrane modification, including metal oxides, zeolites, and carbon nanomaterials, is provided. The synthesis techniques and methods of integrating these nanomaterials into RO membranes are also discussed. The impacts of nanomaterial change on the performance of RO membranes are addressed. The underlying mechanisms responsible for RO membrane enhancements by nanomaterials, such as improved surface hydrophilicity, reduced membrane fouling via surface repulsion and anti-adhesion properties, and enhanced structural stability, are discussed. Furthermore, the review provides a critical analysis of the challenges and limitations associated with the use of nanomaterials to modify RO membranes. Overall, this review provides valuable insights into the modification of RO membranes with nanomaterials, providing a full grasp of the benefits, challenges, and future prospects of this challenging topic.
1. Introduction
The global water crisis threatens human beings, economies, and ecosystems worldwide. Access to clean and safe water is a fundamental human need, yet a significant portion of the global population still lacks this vital resource. Population expansion, urbanization, climate change, water pollution, unsustainable agricultural practices, inadequate water infrastructure, and water conflicts all contribute to the global water crisis. Understanding the complexities of this crisis and developing innovative mitigation strategies is crucial for ensuring water security and sustainable development. Scientists and researchers are constantly looking for sustainable and cost-effective tools to obtain clean and potable water.1,2 The majority of the Earth's water, approximately 97%, is found in the form of saline water in oceans and seas, making it a vast and untapped resource that could help alleviate the water crisis.3 Consequently, desalination has emerged as a highly efficient and cost-effective solution to the global water scarcity problem. However, in areas where access to brackish or seawater is limited, wastewater reclamation provides a viable solution to the water crisis.4 RO desalination has become a widely adopted process for producing clean water, with numerous sources contributing to its feed, with about 60% coming from seawater, 20% from brackish water, and the remaining from other sources including rivers, and wastewaters.5Desalination methods can be categorized into two main groups based on their separation mechanisms: phase-change and membrane-based processes. Phase-change methods involve the continuous cycling of evaporation and condensation to eliminate salts from water and are characterized by high capital and operational costs and are acknowledged for their intensive energy consumption, mainly reliant on thermal energy derived basically from fossil fuels.6–9 On the other hand, membrane-based desalination relies on specialized membranes, which act as filters to allow water to pass through while retaining salts and minerals, e.g., reverse osmosis, forward osmosis, nanofiltration, electrodialysis, membrane distillation, and capacitive deionization methods.
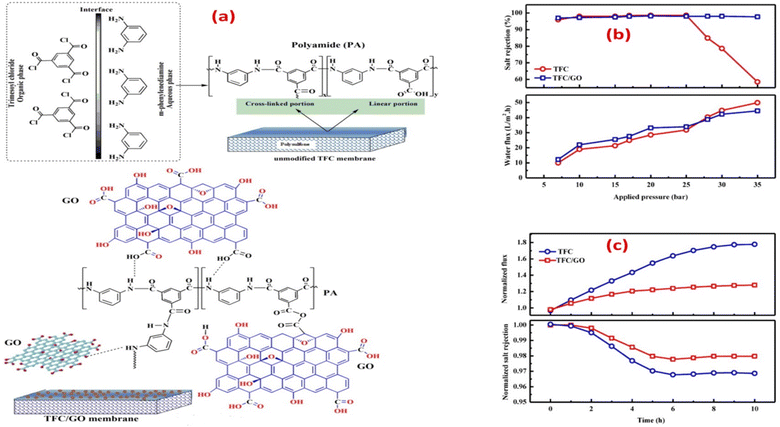
Currently, RO processes are the most widely used, accounting for over 65% of desalination plants globally. This is attributed to its simplicity, low-energy consumption, separation efficiency, stable product quality, and relatively low operational costs. The first thin-film composite (TFC) membrane was develop using an interfacial polymerization reaction,10 such as the combination of trimesoyl chloride (TMC) and m-phenylene diamine (MPD) or piperazine (PIP), as shown in Fig. 1a.11 A conventional polyamide (PA)-based RO membrane typically comprises three layers: a very thin active layer made of aromatic PA, a support layer with small pores, and a layer of nonwoven fabric, as shown in Fig. 1b. These layers have approximate thicknesses of 200 nm, 20–50 μm, and 120–150 μm, respectively.12,13 The polyester support layer alone cannot offer a suitable surface for the PA active layer due to its uneven and porous structure. To overcome this issue, a microporous polysulfone interlayer is put between the selective layer and the support layer.14 This extra layer protects the ultra-thin selective layer from high-pressure compression.15 The PA layer is responsible for providing selectivity, with typical salt rejection of about 99%.16 TFC membranes have a high selectivity and water flux; however, its use in RO processes faces several challenges that significantly impact their overall performance.17
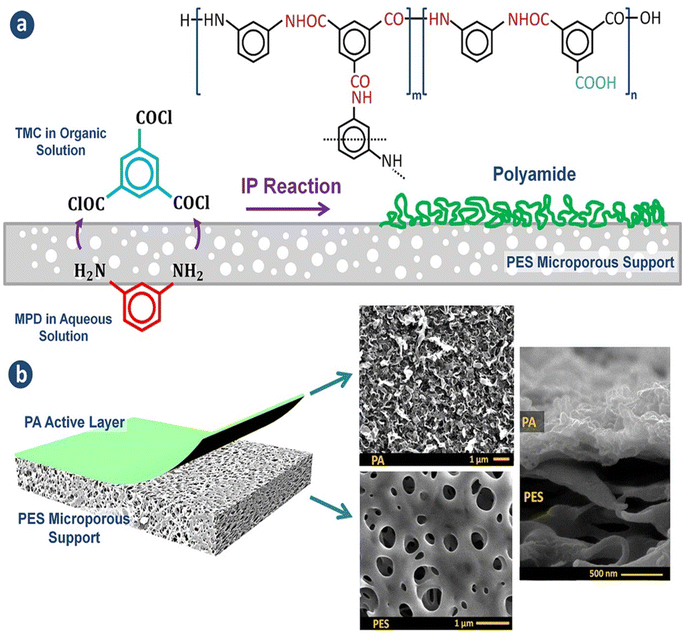 | ||
| Fig. 1 (a) The interfacial polymerization process between TMC and MPD on a microporous support layer. (b) Structure of the fabricated TFC membranes with the top and cross-sectional morphologies.148 | ||
One major compromise that RO membranes face is the trade-off between membrane permeability and salt rejection/selectivity. Finding a balance between high permeability and excellent salt rejection/selectivity is crucial in designing efficient RO membranes. Furthermore, fouling is a persistent issue that degrades RO membranes' performance. Membrane fouling occurs when organic substances, mineral scales, colloids, and biofilms accumulate on the membrane's surface or within its pores.18 This process is complex and is controlled by factors such as the membrane's surface hydrophilicity, roughness, and charge, as well as feed water quality and operational parameters.19,20 A more hydrophilic and smoother membrane is less susceptible to fouling as it discourages foulants from attaching.21 Hydrophilic materials are more resistant to fouling because they can undergo hydration, mainly via hydrogen bonding. Conversely, surface roughness provides more area and surface defects for foulants to adhere to membrane surfaces, increasing the likelihood of fouling.22 Managing these variables is critical for minimizing membrane fouling. One of the most convenient approaches for preventing biofouling in RO units is the chlorination of feed water.23 However, the use of chlorine can have detrimental effects on the polyamide barrier layer, which is a critical component of the TFC-RO membrane.23 Chlorine attacks the polyamide layer through several mechanisms Fig. 2.24 One mechanism involves the substitution of the hydrogen in the amide group with a chlorine atom.25 Further, chlorine can cause rapid N-chlorination followed by intramolecular rearrangement, leading to the migration of chlorine to the aromatic ring.26 Another mechanism is the direct replacement of chlorine atoms for the aromatic ring of m-phenylene diamine.24,27 Moreover, chlorine promotes the hydrolysis of the amide group, resulting in the formation of amido and carboxylic groups. Oxidation of the membrane by chlorine degrades performance, specifically water permeability and salt rejection.28,29
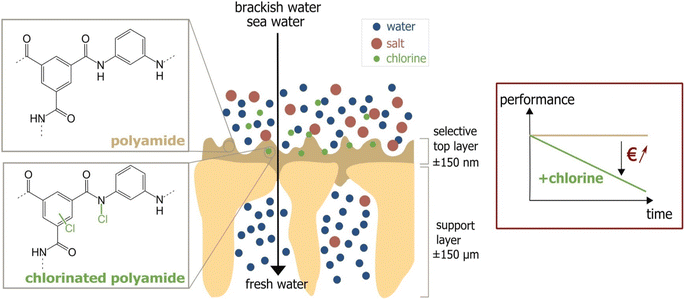 | ||
| Fig. 2 Chlorine attack on PA membrane and the performance decline due to the attack. Reprinted with the permission of ref. 23, copyright 2024, 2024 Elsevier. | ||
Therefore, to address the challenges that RO membranes face, scientists have extensively researched the use of numerous materials and strategies to enhance membrane properties. Among these, the incorporation of nanomaterials derived from metal oxides, zeolites, and carbon-based nanoparticles into RO membranes have emerged as intriguing enhancement option. Nanomaterials have been proved to improve multiple aspects of RO membranes, including water permeability, chlorine resistance, antifouling potential, and antimicrobial characteristics.30 Furthermore, nanomaterials have been found to improve the mechanical strength and thermal stability of RO membranes. This performance enhancement can be attributed to mechanisms such as increased surface area, improved interfacial interactions, alteration of surface features, and enhanced structural integrity provided by the incorporated nanoparticles.31–33 For instance, carbon-based nanomaterials, including graphene, carbon nanotubes, and metal organic frameworks (MOF) have showed potential in improving RO membrane performance. These nanomaterials possess high surface area, and unique porosity and adsorption capabilities. They can act as molecular sieves, allowing water transport while effectively excluding salts and pollutants. Carbon-based nanomaterials, for instance, can reduce fouling potential and extend membrane lifespan. Additionally, carbon-based nanomaterials have excellent mechanical strength and chemical stability, which improve the durability and lifespan of the modified membranes. They can withstand harsh operating conditions, such as high pressures and varying pH levels, making them suitable for industrial-scale RO applications. Further, the microporous structure of zeolite nanoparticles selectively allows water molecules to flow through while hindering the transport of larger molecules, assuring effective rejection of pollutants and reducing the likelihood of fouling.34 Furthermore, metal oxide nanoparticles can exhibit photocatalytic properties, enabling the degradation of organic pollutants that contribute to fouling. This photocatalytic activity effectively reduces fouling and maintains the membrane's permeability and salt rejection capabilities over prolonged operation. Moreover, Silica's nanoparticles remarkable affinity for water makes it an appealing material for improving the hydrophilicity, water permeability, and efficiency of RO membranes.31
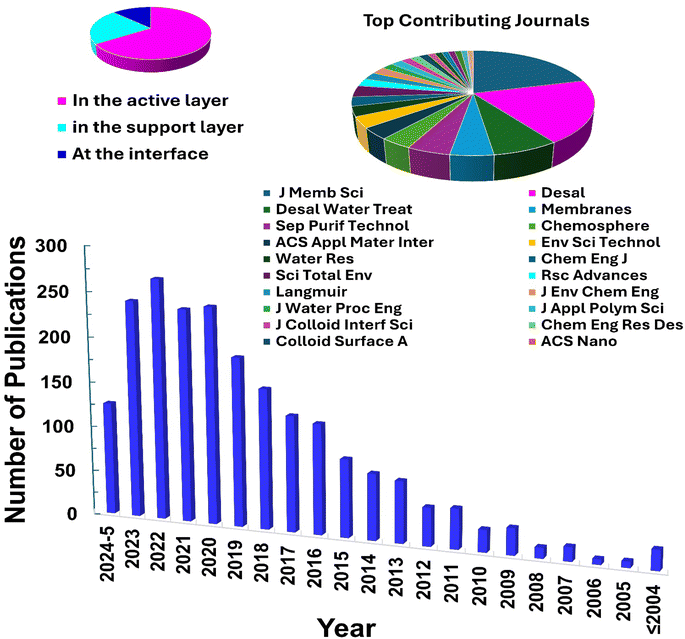
The characteristics of nanocomposite membranes undergo significant alterations based on the nature, concentration, chemical properties, and size of the incorporated nanomaterials.33 Consequently, it is possible to customize the properties of nanocomposite membranes according to the specific nanomaterial employed.33 Nanomaterials can be integrated into thin film composite (TFC) membranes through various primary approaches, as shown in Fig. 3.33 Firstly, nanomaterials can be dispersed within the organic/water phase of polyamide (PA) monomers during their interfacial polymerization (IP) reaction whereby the nanomaterials become randomly captured and enveloped by the active PA matrix, resulting in TFNa membranes (the suffix “a” means: within the active PA layer).35,36 Alternatively, nanomaterials can be dispersed within the substrate matrix through the process of phase inversion, resulting in the formation of TFNs membranes with nanocomposite substrates (the suffix “s” means: within the substrate).37 Lastly, nanomaterials can be uniformly coated or deposited onto the substrate surface before the IP reaction, yielding TFNi membranes with nanomaterial interlayer (the suffix “i” means: at the interface of substrate).38 These various incorporation methods play a significant role in the development of TFC membranes and are important aspects to consider when examining their performance. The escalating interest in the advancement of nanomaterial-modified RO membranes is clearly apparent from the growing number of research studies published, as demonstrated in Fig. 4. This Figure shows the major contribution of TFNa over both TFNs and TFNi membranes, as well as the major contributing journals to this interesting topic. This observation underscores the necessity to analyze and review the existing literature on this topic.
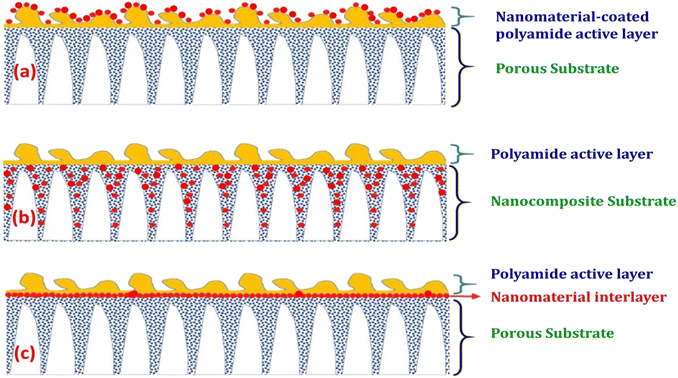 | ||
| Fig. 3 Schematic illustrations demonstrating typical structures of nanomaterial-modified PA TFC membranes: (a) TFC membrane with nanomaterial-coated PA surface layer, (b) TFC membrane with nanocomposite substrate and (c) TFC membrane with nanomaterial interlayer. Reprinted with the permission of ref. 32, copyright 2024, 2024 Elsevier. | ||
This review offers a thorough and in-depth analysis of recent advancements in the field of nanomaterials-modified RO membranes. The article systematically explores the synthesis methods employed to incorporate carbonaceous and inorganic-based nanomaterials into membranes, along with the characterization techniques used to assess their properties. Moreover, in-depth characterization methods for evaluating the structural, morphological, and physicochemical properties of modified membranes will also be addressed. The impact of these modifications on critical performance parameters, such as water permeability, salt rejection, fouling resistance, and membrane stability, will be discussed in detail. This review will also address the challenges associated with scaling up these modifications from the laboratory to the industrial scale and will identify future research directions to promote further advancements in the field. By critically analyzing the existing literature and providing valuable insights, this review aims to contribute to the development of innovative and effective RO membrane technologies for sustainable water treatment.
2. Nanomaterials and nanocomposites-enhanced polymeric membranes
Various nanomaterials can be used as fillers to enhance water permeability and salt rejection rates of polymeric TFC membrane for NF and RO applications. These nanofillers can be tailored based on various factors that operate collectively, including composition, size, shape, porosity, hydrophilicity, the interfacial polymerization method employed, and the membrane layer where these nanofillers have been incorporated, as well as its preparation method. Various nanofiller shapes have been identified including nanospheres, nanotubes, nanosheets, nanocrystals, nano-bowl, nano-cubes, hollow particles, and core shell shaped nanoparticles.39,40 Moreover, the researchers interestingly compared the performance of non-porous and porous nanofillers having various compositions, sizes, inclusion membrane layer, interfacial polymerization process, and operated under different conditions.39,40Furthermore, the effect of nanofiller positions on TFC membrane performance was interestingly established.41 Membranes can be classed as TFNa, TFNs, or TFNi based on where nanomaterials are incorporated, i.e., in the active layer, the support layer, or at the interface between the two. The researchers found that TFNa membranes for RO and FO applications and TFNi membranes for NF were more effective in overcoming the trade-off between water permeability and selectivity. Furthermore, compared to conventional nanomaterials, the use of porous 1D or 2D nanofillers, such as nanosheets, nanotubes, hollow and mesoporous NPs, in the fabrication of TFNi and TFNs membranes gave superior water permeability through their pores and channels, as well as enhanced selectivity due to their hydrophilicity.41 Nevertheless, nano-bowl-shaped nanofillers with concave cavities reduced mass transfer resistance of water molecules and shortened their flow-through pathways. It is worth noting that these factors were not studied using monovariate optimizations due to the practical limitations involved in researching each factor separately while keeping all other factors constant. The current review discusses some representative examples of specially tailored nanofillers with fruitful characteristics for their potential performance in modifying TFC membranes.
2.1 Carbon nanomaterial
Carbon based nanomaterials are attractive for membrane modification owing to their porous structures, biocidal activities and hydrophilic properties. A variety of carbon nanomaterials, such as carbon nanotubes (CNTs), graphene oxides (GO) and metal organic framework (MOF) have been used to modify various membranes. Table 1 Shows the performance evaluation of several TFN reverse osmosis membranes with incorporation various carbon-based nanoparticle.| Modifier type | Test conditions TDS (ppm), temperature, pressure | Flux L m−2 h−1 | Rejection % | Remarks | Ref. |
|---|---|---|---|---|---|
| GO | 2000, 25.1, 25 °C 20.6 | 39 to 59.4 | 95.7 to 93.8 | GO nanosheet interlayer spacing has acted as water conduits, increasing water permeability in the process | 149 |
| CNT | 2000, 25 °C, 15.5 bar | 40.9 to 51.3 | >97 | The mechanical properties of the polymer nanocomposite are enhanced by the incorporation of CNTs | 90 |
| Due to its smoother surface, TFN RO membrane exhibits less fouling potential and improved cleaning recovery efficiency | |||||
| Oxidized MWCNTs | 2000, 25 °C, 15.5 bar | 20.3 to 28.9 | >97.4 | By including oxidized MWCNTs in the polyamide layer of the RO membranes, the fouling resistance was increased | 150 |
| CNT | 2000, 25 °C, 15.5 bar | 37.2 to 43.5 | 97.5 to 95.4 | The hydrophobic nanochannels of CNTs and good-dispersed states in the PA formed through interactions between PA and CNTs are credited with the excellent membrane performance (high salt rejection and water flux) and enhanced stability of the PA membranes containing CNTs | 89 |
| MWNTs | 2000, 25 °C, 15.5 bar | 14.8 to 28.05 | The results demonstrated the ability he electron-rich MWNTs for protection of active sites in MPD form attack by chlorine | 56 | |
| GO | 1000, 25 °C, 15.5 bar | 45 to 59.5 | >97 | For the duration of the assessed exposure time, GO layer increases chlorine resistance | 40 |
| GO | 2000, NA, 20 bar | 17.2 to 31.8 | 97.9 to 98.7 | GO improve resistance of the membrane towered chlorine by preserving its structure. Moreover, the results showed that the enhancing antibacterial activities of RO PI-GO TFN membranes due to oxidative stress mechanism | 151 |
| UiO-66 | 2000, 25 °C, 15 bar | 36.7 to 56.8 | 99 to 99.35 | The incorporation of UiO-66 into the membrane showed a marginal increase in rejection and ∼a 50% increase in water flux compared to the pristine membrane under the brackish water condition. Further, in a simulated seawater test, the UiO-66 modified membrane enhanced the boron rejection by ∼11% owing to its significant porous structure which gives extra pathways for water movement, as well as powerful adsorption to boron which leads to further pore narrowing impact | 152 |
| ZIF-8 | 2000, 25 °C, 15.5 bar | 40.8 to 61.2 | 98.9 to 99.2 | The chemical alterations and the contribution of extra water transport channels made possible by ZIF-8 inclusion, according to the obtained results, are responsible for the increase in water permeance | 102 |
| ZIF-8 | 2000, NA, 15.5 | 19.8 to 52.3 | 98.1 to 98.5 | Compared to the pure PA surface, the TFN surface was less crosslinked and more hydrophilic. For the impacts of filler on the shape and characteristics of the TFN membrane surface, an encapsulating mechanism was proposed | 153 |
| ZIF-8 | 2000, NA, 15.5 bar | 13.9 to 21.3 | 98.4 to 99.4 | The results demonstrated that ZIF-8 can significantly improve the polyamide fouling resistance and, in this study, the modified membrane reduced the fouling by more than 75% | 154 |
| MIL-101 (Cr) | 2000, NA, 16 bar | 24.3 to 35.2 | 99.3 to 99.1 | MIL-101 (Cr)'s porous structures can provide direct water channels for water molecules to go through fast in the thick selective PA layer, enhancing the membranes' water permeance | 101 |
 | ||
| Fig. 5 The proposed antimicrobial mechanisms of GO. Reprinted with the permission of ref. 55, copyright 2024, RSC. | ||
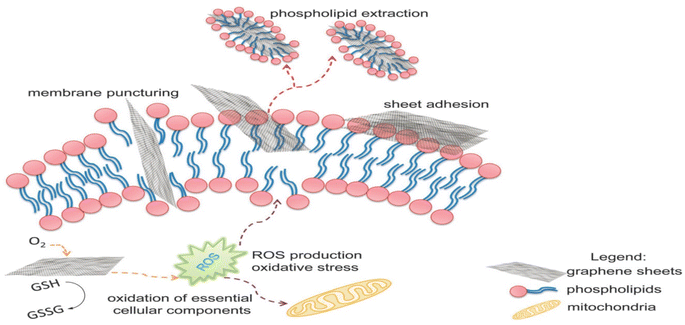
Functional groups, such as carboxyl, hydroxyl, and epoxide groups, present on GO nanosheets enhance the negative charge of the membrane surface. This strengthens the electrostatic repulsion between extracellular polymeric substances (EPS) and microorganisms, decreasing microbial adhesion to the membrane surface.56 The presence of hydrophilic groups increases the membrane's affinity towards water molecules, reducing interfacial tension and enhancing the membrane's wetting properties. As a result, water molecules can penetrate and flow more easily through the membrane, leading to increased water flux and improved permeate flow. The highly interconnected pores and channels formed by the GO nanosheets provide more and more direct pathways for water molecules to travel through the membrane.57,58 Furthermore, the addition of GO nanoparticles to the membrane matrix improves its mechanical strength and stability, particularly under high trans-membrane pressures, enhancing the long-term performance of the membrane.59 Furthermore, incorporating GO into the membrane structure can enhance chlorine resistance due to its unique properties. GO acts as a barrier that effectively hinders the penetration of chlorine molecules into the membrane matrix and reduces its reactivity with –NH2 groups of MPD moieties of the membrane's PA layer.60,61 In another mechanism, the oxygen-containing functional groups of GO, such as hydroxyl and epoxide groups, provide preferential sites for chlorine molecules to react, where chlorine atoms can form covalent bonds with oxygen atoms on GO, resulting in chlorine–oxygen bonds that are more stable than chlorine–carbon bonds typically found in polymeric membrane material upon chlorination.62,63
Thus, the distinctive properties of GO nanosheets, including their hydrophilicity, chemical durability, and rapid water permeation, were used to develop a dual-function barrier coating for PA-TFC membranes.29 This conformal coating of GO effectively enhanced the membrane's surface hydrophilicity and reduced its roughness, leading to improved proteins' fouling resistance. Moreover, due to the chemically inert nature of GO nanosheets, the coating layer also served as a chlorine barrier, preventing membrane degradation when exposed to chlorine, while maintaining high salt rejection capability.29 On the other hand, reducing the polymer solution concentration used in the PA support layer preparation enhanced the TFC membrane water flux but resulted in a porous sub-surface structure that degraded the support layer's mechanical strength.64 Therefore, the researchers incorporated GO nanosheets into the sublayer, which enhanced the mechanical strength, porosity, and the water flux. These enhancements were affected by the GO nanosheets wt% and thickness, where higher content and thicker nanosheets had negative effects on the mechanical strength. The dispersion and surface area of the nanofillers also played important roles in their incorporation into the polymer matrix.
According to the findings, incorporating GO nanosheets with a thickness of 1.5 nm and 0.9 wt% dose yielded the maximum mechanical strength. The incorporation of GO did not affect the polyamide layer, as the active layer roughness and thickness were retained, and the network structure of the surface layer remained unchanged. Furthermore, the impact of incorporating GO during interfacial polymerization on membrane performance was examined, Fig. 6a.65 Namely, the membrane's water flux, salt rejection, and resistance to chlorine were assessed under various operating conditions, as shown in Fig. 6b and c, where TFC/GO membrane exhibited consistent salt rejection, whereas the pristine TFC membrane experienced a sharp decrease in salt rejection at high pressures due to deformation. Additionally, the TFC/GO showed enhanced chlorine resistance, Fig. 6c, as evidenced by higher normalized salt rejection and stable normalized flux, which can be attributed to the hydrogen bonding between the oxygen-bearing functional groups of GO and the secondary amide groups in PA, offering protection against chlorine. Furthermore, prior to interfacial polymerization, GO was added to the aqueous MPD solution to be embedded into the PA layer, and the resulting GO-TFN membranes showed 98% improvement in water anti-biofouling based on biovolume changes, as well as 80% increase in permeability features.66 These enhancements can be attributed to changes in surface charge, roughness, hydrophilicity, and PA layer thickness resulting from GO incorporation. In addition, the GO-TFN also exhibited a high salt rejection even when exposed to challenging conditions of 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm h chlorination with a NaOCl solution, which can be attributed to the protection of PA-amide groups from chlorine attack. The study also emphasized the importance of GO concentration and size in influencing the performance of GO–TFN membranes.66 Furthermore, modification of RO membranes with GO improved surface properties, making them smoother and more hydrophilic, as well as reduced CaSO4 scaling and biofouling, demonstrating its potential for enhanced RO membrane performance.67 In another study, incorporating GO into the PA layer of TFC membrane had no effect on the membrane's permeability and salt rejection; however, it resulted in a noteworthy decline of viable E. coli cells, reducing them by 64.5% within one hour.68 GO induced membrane damage, mediated by physical disruption, charge transfer, formation of reactive oxygen species and lipid extraction from the cell membrane, as evidenced by SEM imaging, where most bacterial cells attached to the GO–TFC membrane appeared to be shrunk or flattened with compromised integrity compared to the control-TFC membrane.68
000 ppm h chlorination with a NaOCl solution, which can be attributed to the protection of PA-amide groups from chlorine attack. The study also emphasized the importance of GO concentration and size in influencing the performance of GO–TFN membranes.66 Furthermore, modification of RO membranes with GO improved surface properties, making them smoother and more hydrophilic, as well as reduced CaSO4 scaling and biofouling, demonstrating its potential for enhanced RO membrane performance.67 In another study, incorporating GO into the PA layer of TFC membrane had no effect on the membrane's permeability and salt rejection; however, it resulted in a noteworthy decline of viable E. coli cells, reducing them by 64.5% within one hour.68 GO induced membrane damage, mediated by physical disruption, charge transfer, formation of reactive oxygen species and lipid extraction from the cell membrane, as evidenced by SEM imaging, where most bacterial cells attached to the GO–TFC membrane appeared to be shrunk or flattened with compromised integrity compared to the control-TFC membrane.68
 | ||
| Fig. 6 (a) Synthesis of PA with and without the addition of GO during interfacial polymerization; salt rejection and water flux of TFC and TFC/GO membranes at different (b) feed pressures, and (c) chlorine exposure time at a 500 ppm Cl2-dose. Reprinted with the permission of ref. 65, copyright 2024, Elsevier. | ||
For instance, incorporating MWCNTs into the PA layer of a TFC membrane resulted in enhanced chlorine resistance that was attributed to the electron-rich MWCNTs protection of the amide linkage.77 Furthermore, TMC solutions in n-hexane were mixed with MPD solutions containing modified CNTs to prepare PA thin films via controlled interfacial polymerization (IP) process, resulting in the formation of well-dispersed states.78 In that work, CNTs were previously treated for several hours (x) at predetermined settings with various mixtures of sulphuric and nitric acids to impart oxygen bearing functionalities to the modified CNTx. Among the prepared membranes, PA-CNT4 demonstrated enhanced durability, water flux, and chemical resistance compared to the pristine PA membranes. These improved performance features were attributed to the presence of hydrophobic nanochannels provided by the CNTs. Additionally, incorporating CNTs into PA films of TFC membranes improved water permeability, leading to reduced energy consumption, high anti-fouling potential, and improved recovery efficiency during the cleaning stage due to the smoother surfaces.79 Furthermore, the effect of varying amounts of CNTs on the performance of PA membranes was examined.75 The experimental findings demonstrated promising results, with notable improvements observed in salt rejection, high water flux, and remarkable anti-biofouling properties, as well as enhanced durability. However, when higher amounts of CNTs were incorporated, the anti-biofouling characteristics of the PA–CNT membrane coated with polyvinyl alcohol (PVA) were reduced. This decline in anti-biofouling efficacy was attributed to the formation of aggregated CNT clusters within the membrane. The superior anti-biofouling properties observed in the PA–CNT–PVA membrane were primarily attributed to the inherent antimicrobial properties of the CNTs. To validate this, both confocal laser scanning microscopy (CLSM) imaging and cell viability tests were conducted, providing evidence of the membrane's anti-biofouling efficacy.75
Layer to enhance its resistance to fouling and chorine as well.73 Experimental tests conducted using inorganic (Ca(HCO3)2) and BSA-protein foulants demonstrated that the TFN membrane experienced a lower decline in water flux and exhibited reduced irreversible fouling, as shown in Fig. 7a and b, respectively. These improvements were primarily attributed to the enhanced electrostatic repulsion between the foulants and the membrane surface, as well as the improved hydrophilicity of the TFN membrane, which minimized foulant adhesion. Furthermore, the TFN membrane also exhibited high resistance to chlorine, which can be attributed to the MWCNTs electron-rich nature that provide protection to MPD active sites, and hence hindering the chlorine attack (Fig. 7c and d).73 Furthermore, incorporation of raw and oxidized MWCNTs reduced inorganic-, organic- and bio-fouling owing to the increased membrane's negative charge and smooth surfaces.80 Additionally, the fabricated membrane exhibited twice the lifespan in comparison to existing commercially available membranes.80 Results reflected that the hydrophilicity, as well as water flux, improved with increasing concentration of oxidized MWCNTs compared to bare PA or raw MWCNTs/PA. Despite their lower hydrophilicity, the membranes with raw MWCNTs exhibited significant improvements in water flux and salt rejection due to their proper dispersion within the polyamide matrix. However, at higher concentrations of both MWCNT types, the water flux decreased. Assessment of fouling behavior in a 24 hours test using BSA/salt solution demonstrated that membranes with all concentrations of MWCNTs, whether raw or oxidized, exhibited better antifouling performance compared to unmodified membranes. Finally, optimized concentrations played a crucial role in achieving superior membrane characteristics.80
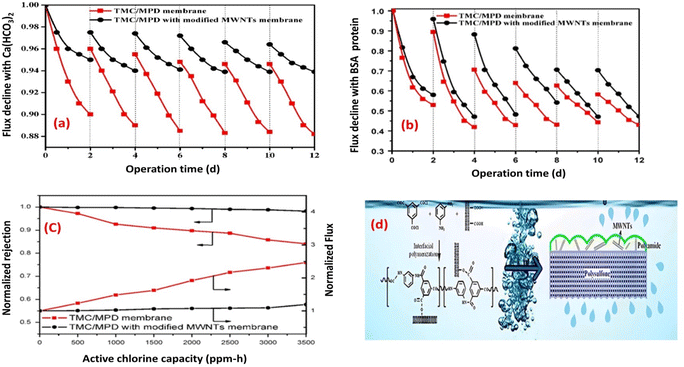 | ||
| Fig. 7 Flux decline for TMC/MPD and TMC/MPD/MWNTs membranes due to (a) Ca(HCO3)2, (b) BSA-protein foulant, (c) RO performance under the active chlorine, and (d) schematic fabrication of TMC/MPD/MWNTs membrane. Reprinted with the permission of ref. 73, copyright 2024, Elsevier. | ||
In another investigation,81 the researchers follow the efficiently reduced protein adhesion and antifouling capabilities of MWCNT–PA by employing a multifaceted approach combining molecular dynamics (MD) simulations and experimental studies. MD simulations, suggested that the incorporation of MWCNTs within the PA framework gives rise to weaker interactions between BSA proteins and membrane surfaces. This weakened interaction can be attributed to three key factors: a more rigid PA structure resulting in reduced molecular conformity with BSA, enhanced hydrophilicity, and smoother morphology leading to the formation of an interfacial water layer.81 Similarly, a fabricated MWCNTs–PA exhibited resistance to chlorine attacks that as ascribed to the electronic-rich nature of the highly sp2-hybridized MWCNTs and the existence abundance of sacrificial organic functionalities on nanotubes. Moreover, the physical crosslinking effect of MWCNT also contributes to the membrane's resistance by preventing the formation of pinholes. It should be noted that PA with a significant degree of crosslinking is generally more resistant to chlorine attack.82
The enhanced performance of reverse osmosis (RO) membranes upon incorporating metal–organic framework (MOF) materials can be attributed to several key mechanisms. Firstly, MOFs can provide a high density of active sites for selective interaction with water molecules, leading to improved water permeability. The porous structure of MOFs allows for the formation of nanoscale channels, increasing the overall water flux through the membrane.89,90 The permeation characteristics of a MOF-TFN are significantly altered by the size of the pores of a MOF.89 Furthermore, MOFs can enhance the salt rejection capabilities of RO membranes. Additionally, by functionalizing the MOF materials with specific functional groups or modifying the ligands, the selectivity towards certain ions can be increased.
For instance, this selectivity can be especially valuable in applications like seawater desalination, where high salt rejection rates are crucial for obtaining fresh water. Thus, the addition of myristic acid (MA) to zirconium-based porphyrinic MFOs (PCN-222) reduces pore size and results in improved rejection compared to pristine MOFs.89 Additionally, MOFs can enhance the stability and durability of RO membranes. The incorporation of MOFs into the polymeric matrix of the membrane can improve its mechanical strength and resistance to fouling.78,79 Moreover, the high thermal stability of MOFs allows for their application in harsh operating conditions, such as high temperatures or corrosive environments. This expands the range of potential applications for RO membranes, making them suitable for a broader range of industries and processes.
Furthermore, the researchers examined the potential of a microporous and hydrophobic hybrid zeolitic imidazole frameworks substance (ZIF-8) as filler in TFC-RO membranes. They observed a remarkable enhancement in water permeance of up to 162% while keeping an impressive NaCl rejection rate of 98%. Additionally, the selective layer's surface, when integrated with ZIF-8, exhibited more hydrophilicity and reduced crosslinking compared to the pristine polyamide (PA).79,91 Additionally, to enhance the water permeability, and the support layer's porosity and hydrophilicity, researchers developed a polysulfone (PSf) layer incorporating a sulfuric acid-treated HKUST-1 MOF, [Cu3(1,3,5-benzenetricarboxylate)2·(H2O)3]n.91,92 The overall surface roughness of the TFC was affected by the support layer's porosity and hydrophilicity. By improving these features in the support layer, they successfully produced a TFC-RO membrane with reduced roughness which resulted in enhanced resistance to fouling and improved water flux compared to a TFC-RO with a bare PSf, whilst maintaining its excellent salt rejection capability.93 The rodlike structure of PCN-222 MOFs has been successfully fabricated and incorporated into TFC RO.89 Furthermore, the PCN-222 was modified with MA after synthesis, allowing for the adjustment of the MOF pore size (Fig. 8a). Membranes fabricated using both bare and modified PCN-222 MOF demonstrated slight reductions in selectivity and significant increase in water flux compared to bare PA membranes. Specifically, the modified PCN-222 -TNF membranes (MA-10) exhibited an almost 100% enhancement in flux while maintaining salt rejection above 95% (Fig. 8b).89 The authors proposed that the existence of hydrophobic MA chains hinders the infiltration of MPD into the pores of the PCN-222 nanoparticles in suspension, leading to a reduction in pore penetration by the polyamide (Fig. 8a). This causes an increase in pore volume available for transport, resulting in higher flux and lower salt rejection compared to the control membrane (Fig. 8b). Additional mechanisms that could contribute to the enhanced flux in the PA/PCN-222 include interfacial movement occurring at the surface of the MOF aggregates, as well as transport taking place within the interstitial channels of the MOF aggregates.89
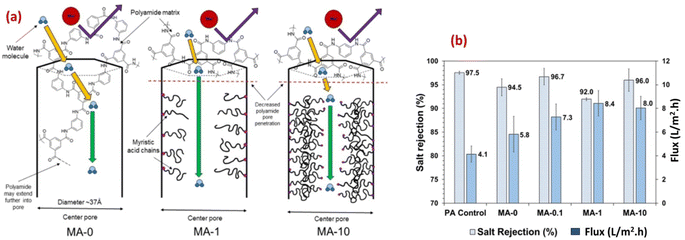 | ||
| Fig. 8 (a) Schematics of water transport through the pores of zirconium-based porphyrinic MOFs (PCN-222 nanorods) with increasing modifier loading (myristic acid, MA, at 0, 0.1, 1 and 10%), and (b) salt rejection and water flux results for MA-modified and pristine membrane reprinted with the permission of ref. 89, copyright 2024, American Chemical Society. | ||
In another investigation, a microporous and hydrophilic hybrid MOFs, MIL-101 (Cr), was incorporated into a TCF RO membrane, resulting in improved overall membrane performance.90 This enhanced performance can be attributed to changes in membrane characteristics such as roughness, crosslinking extent, morphology, and wettability. Moreover, doped MIL-101 (Cr) also creates direct channels within the PA layer of the membrane. At a concentration of 0.05 w/v% of MIL-101 (Cr), water permeability increased by up to 44% while keeping NaCl salt rejection over 99.91 Overall, the integration of metal–organic framework materials into RO membranes can significantly enhance their performance. MOFs offer improved water permeability, higher salt rejection rates, enhanced stability, and increased durability.
2.2 Incorporation of inorganic nanomaterials
The inclusion of inorganic nanomaterials into the polymer matrix has gained attention due to their hydrophilic characteristics, which can enhance a range of physical and biomedical benefits, such as optical, mechanical, catalytic, and antimicrobial membrane properties.94,95 Through the addition of inorganic NPs, the permeability of pure polyamide membranes can be enhanced by facilitating water transport through a direct pathway or modifying the membrane structure.21 Consequently, the development of PA-TFC reverse osmosis membranes incorporating inorganic nanomaterials like pure zeolites, metal oxides, and metal nanoparticles has resulted in improved separation performance.96–98 Table 2. Shows the performance evaluation of several TFN reverse osmosis membranes with incorporation various inorganic-nanoparticle.| Modifier type | Test conditions TDS (ppm), temperature, pressure | Flux | Rejection % | Remarks | Ref. |
|---|---|---|---|---|---|
| Mesoporous SiO2 NPs | 2000, NA, 16 bar | 19 to 53.0 | >96 | Mesoporous silica produces hydrophilic pores in RO membranes' thin film layer, enhancing water diffusion and improving water flux | 155 |
| TiO2 NPs | 2000, NA, 15.5 bar | 33.6 to 40 | 99.7 to 99.8 | Low-concentration TiO2 in the synthesized TFNC membrane improved its resistance to organic fouling | 136 |
| TiO2 NPs | 2000, 25 °C, 15.2 bar | 21.5 to 24.3 | >97 | The thermal stability and anti-biofouling of TFN membranes were enhanced by the incorporation of TiO2 | 50 |
| Fluorinated silica | 2000, 25 °C, 15.5 bar | 39.3 | 96.0 to 98 | Incorporation of hydrophobic fluorinated silica increasing membrane hydrophobicity and decreased salt passage | 156 |
| Pours SiO2 | 2000, 25 °C, 20.6 bar | 28.5 to 46.6 | 98 to 97.5 | Interior pores, due to their narrow flow routes, significantly enhance water flux, as evidenced by the disparities in water flux between two membranes | 157 |
| Non porous SiO2 | 2000, 25 °C, 20.6 bar | 28.5 to 35.8 | 98.0 to 97.0 | ||
| rGO/TiO2 | 2000, NA, 15.0 bar | 34.3 to 51.3 | 97 to 99.45 | The addition of rGO/TiO2 improved the hydrophilicity, negative surface charge, and roughness of the polyamide layer of the RO membrane, which improved water permeability, salt rejection, antifouling, and chlorine resistance | 29 |
| Due to TiO2 nanoparticles' increased affinity for water, the rGO/TiO2/RO membranes' increased hydrophilicity may be the cause of this improved permeability | |||||
| SiO2 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, NA, 44 bar 000, NA, 44 bar |
31 to 49 | >90.0 | The integration of SiO2 nanoparticles reduces the fouling tendency of the polyamide active thin layer, making it acceptable due to its hydrophilic properties | 30 |
| CeO2 | 2000, NA, 16 bar | 29.4 to 49.3 | ≥98 | By limiting the adherence of hydrophobic foulants, CeO2 embedded membranes demonstrated improved fouling resistance | 158 |
| Aluminum doped ZnO | 2000, 25 °C, 15.5 bar | 26 to 32 | >98.0 | The structure and hydrophilicity of membranes, influenced by hydrophilic nano-ZnO, significantly influence their filtration properties, leading to higher water flux in hybrid membranes compared to TFC membranes | 147 |
| AgNPs | 2000, NA, 20 bar | NA | 95 to −98 | The enhancement in rejection can be assigned to Donnan and size exclusion mechanism | 147 |
| NaY zeolite | 2000, NA, 15.5 bar | 39.6 to 74.2 | 98.5 to 98.8 | The incorporation of NaY zeolite exhibits a molecular sieve effect, allowing water molecules to pass preferentially while rejecting solute molecules, due to their homogeneous porosity structure and 0.74 nm pore size | 55 |
When RO is modified with zeolite, the enhanced performance can be attributed to several mechanisms. The negatively charged molecular sieve and super-hydrophilic zeolite will facilitate the selective movement of water by establishing specific pathways while giving effective solute rejection by utilizing a combination of Donnan and steric exclusion mechanisms.96 Zeolites contain pores with sizes ranging from approximately 0.3–0.8 nm. These pore sizes are positioned between the diameters of hydrated salt ions, such as Na+ (0.72 nm), and water molecules (0.27 nm).104 Taking advantage of the principle of size exclusion, zeolites are expected to facilitate the preferential permeation of water while impeding the passage of salt ions.
For instance a 12–16% improvement in water flow with zeolite-TFN sea water reverse osmosis (SWRO) membranes was observed compared to unmodified SWRO membranes with similar salt removal rates.105 The researchers attributed this enhancement to both molecular sieving and the formation of defects in the TFN-SWRO membranes, as well as the low degree of crosslinking due to the presence of zeolite despite of its low concentration (0.2 wt%).105 Further, incorporating sodium-aluminum-silicate zeolite (NaA-NPs) into the active PA layer of TFC-RO membranes resulted in more hydrophilic, smoother, and more negatively charged membrane surfaces owing to the characteristics of NaA zeolite which results in improved salt rejection and enhanced antifouling features.96 The highest zeolite loading content (0.5%) led to a reduction in contact angle from about 70° to 40° and increased permeability while maintaining solute rejection. This improvement can be attributed to the preferential flow paths for water permeation provided by NaA zeolite, along with steric and Donnan exclusion mechanisms.96
Additionally, two types of zeolite, hydrophilic (FAU, Al/Si = 1.0) and hydrophobic (MFI, Al/Si = 0), were incorporated into RO membranes.106 Both FAU and MFI membranes demonstrated a remarkable salt rejection rate of 100%, with improved membrane permeability reaching 720 L m−2 h−1 bar−1 for a membrane thickness below 3.5 nm, that is approximately 100 times higher than the permeability of commercial RO membranes.106 Additionally, the FAU membrane showed a lower pressure drop due to its hydrophilic nature, while the hydrophobic MFI zeolite membrane displayed a higher pressure drop attributed to capillary resistance.106
In addition, nonthermal glow discharge plasma was used to incorporate clinoptilolite zeolite into the polyamide (PA) layer of the TFC-RO membrane and the resulting composite membrane demonstrated significant enhancement in water flux and fouling recovery ratio when compared to the unmodified membrane.20 Moreover, the researchers investigated the impacts of incorporating nano-NaX zeolite into PA membranes, and observed several improvements in the surface features of the nanocomposite.107 These enhancements included a reduction in root mean square (RMS) surface roughness, an increase in free energy of liquid–solid interface, and a decrease in contact angle. Furthermore, the addition of nano-NaX zeolite resulted in larger pore size, a thinner film thickness, higher thermal stability, and improved water flux compared to pure PA membranes.107 Additionally, TFN membranes integrated with NaY zeolite nanoparticles were successfully fabricated on polysulfone supports through interfacial polymerization of MPD and TMC.108 The experimental findings revealed that increasing the reaction time during IP process resulted in higher salt rejection, indicating a denser zeolite-polyamide layer, along with enhancing the water flux from 39.63 to 74.32 L m−2 h−1, while maintaining a salt rejection of 98.8% under optimized conditions. Through post-treatment of the TFN membranes using a solution composed of camphor sulfonic acid-triethylamine salt, sodium lauryl sulfate, and glycerol, the water flux was further enhanced to 86.05 L m−2 h−1, with a salt rejection of 98.4%. Comparatively, the water flux of the post-treated TFN membranes containing NaY zeolite nanoparticles was twice as high as that of a pristine TFC membrane without zeolite nanoparticles.108
For instance, the researchers examined the utilization of SiO2 NPs as nanofillers in PA membranes to improve their performance. They created TFN membranes using SiO2 NPs of various sizes (10–20 nm) and ratios. The inclusion of SiO2 NPs led to structural modifications in the membranes, with higher concentrations of SiO2 resulting in a more extensive porous network. The incorporation of SiO2 NPs led to a considerable improvement in permeability, increasing it by 58%. When compared to the pristine membrane, the modified membranes with SiO2 demonstrated higher permeability and lower flux decline ratio, while filtering organic matter solutions. This investigation offers valuable insights into the potential of SiO2 NPs as a viable strategy for enhancing the performance of PA membranes.119 For instance, the incorporation of SiO2 NPs into RO membranes led to enhanced thermal stability, as indicated by a minimal decrease in salt rejection of less than 2.5% after heat treatment at 95 °C for 180 minutes.120 Furthermore, the membranes demonstrated excellent resistance to chlorine exposure, with a salt rejection decrease of less than 2% even after being exposed to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm h of NaOCl.120 Further, TFN membranes were fabricated by IP process of MPD and TMC organic solution on silicon nitride/polyethersulfone (PES) composite substrate, as shown in Fig. 9.118 Researchers have found that by introducing amino functionalization to silica (SNPs) using p-aminophenol, the compatibility of silica NPs with the PA layer can be enhanced. This modification also reduces the occurrence of agglomeration during the interfacial polymerization process, thereby facilitating the successful production of high-quality TFN membranes. The TFN shows considerably improved flux, compared to the pristine TFC membrane, along with higher thermal stability.118
000 ppm h of NaOCl.120 Further, TFN membranes were fabricated by IP process of MPD and TMC organic solution on silicon nitride/polyethersulfone (PES) composite substrate, as shown in Fig. 9.118 Researchers have found that by introducing amino functionalization to silica (SNPs) using p-aminophenol, the compatibility of silica NPs with the PA layer can be enhanced. This modification also reduces the occurrence of agglomeration during the interfacial polymerization process, thereby facilitating the successful production of high-quality TFN membranes. The TFN shows considerably improved flux, compared to the pristine TFC membrane, along with higher thermal stability.118
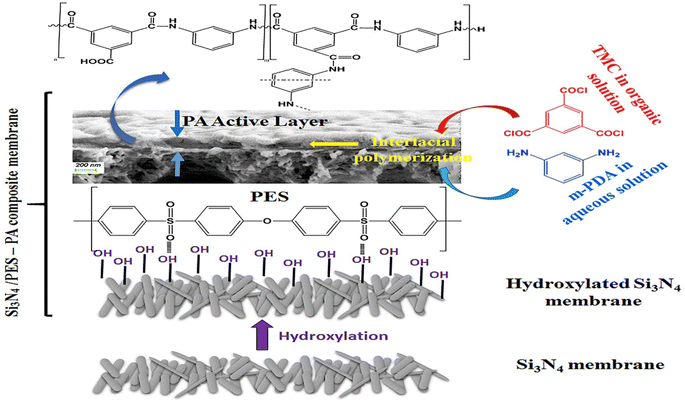 | ||
| Fig. 9 TFC synthesis based on Si3N4/polyethersulfone (PES) composite membrane. Reprinted with the permission of ref. 118, copyright 2024, Elsevier. | ||
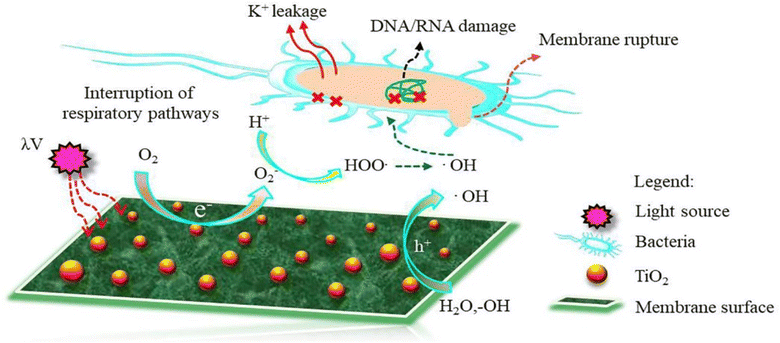 | ||
| Fig. 10 Proposed mechanism of ROS formation on the surface of TiO2 NPs-modified TFC membrane. Reprinted with the permission of ref. 135, copyright 2024, Elsevier. | ||
For instance, PA/TiO2 TFN membranes were synthesized using an in situ interfacial polymerization,126 where two different approaches were utilized to incorporate TiO2 nanoparticles into the membranes: dispersing them in the dodecane organic phase of TMC or the aqueous phase of MPD. The synthesized PA–TiO2 TFN membranes exhibited a significant enhancement in the permeate flux that increased from 33.6 to 40 L m−2 h−1, indicating improved water productivity. Additionally, there was a slight increase in salt rejection, rising from 99.75% to 99.82%. Moreover, the TFN membrane with a lower TiO2 concentration demonstrated improved resistance to organic fouling.126
Furthermore, the impact of incorporating TiO2 NPs, at different NP concentrations (0.01, 0.05, 0.2, and 0.5% w/v) into PA on the rejection of organic matter (OM) and salts as well as permeate flux, was assessed.119 The inclusion of TiO2 nanofillers in the PA membranes enhances their porosity, hydrophilicity, and consequently, their permeability that increased by 24% compared to membranes without NPs, where the rejection performance and fouling behavior of the membranes were assessed using salts (MgSO4 and NaCl) and OM (humic acid [HA] and tannic acid [TA]). In addition, sub-10 nm TiO2 NPs were incorporated, at different doses, into the PA layer to create TFN.127 The researchers found that these TFN membranes exhibited enhanced thermal stability and antifouling properties compared to pristine membranes. The number of E. coli colonies that formed over the UV-illuminated membranes was counted, Fig. 11. The results revealed that the exposure of TiO2-incorporated TFN membranes to UV light significantly reduced the viability of E. coli.127 However, despite its effectiveness, the use of TiO2-based antifouling membranes is hindered by the requirement of costly UV irradiation.123 This has limited their practical application.123 However, there has been considerable research directed toward the development of visible-light responsive-TiO2 that utilizes the localized surface plasmon resonance (LSPR) of metal nanoparticles, specifically gold (Au) and silver (Ag) nanoparticles.123,128
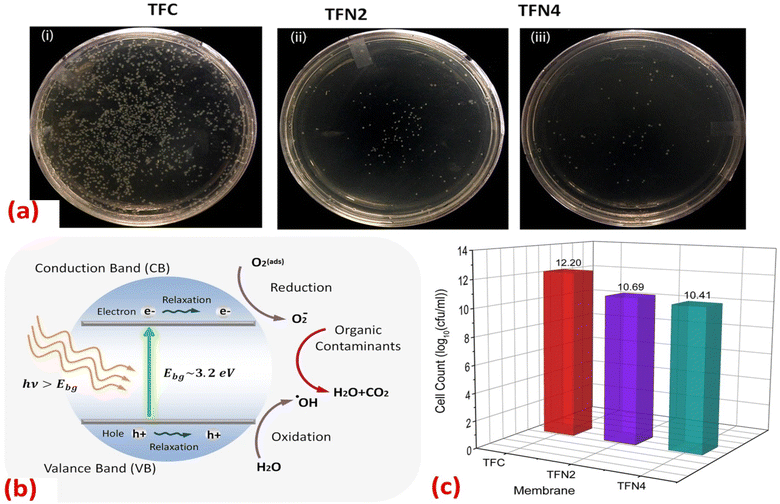 | ||
| Fig. 11 (a) Images of the E. coli colonies formed in the plate of UV-treated (i) TFC, (ii) TFN2 (0.012 wt% TiO2) and (iii) TFN4 (0.024 wt% TiO2) membranes; (b) mechanism for photocatalytic activity of TiO2 NPs under UV irradiation; (c) number of E. coli colonies counted on the plate of TFC, TFN2 and TFN4 membranes after 30 minutes of UV irradiation.127 | ||
Overall, the enhanced performance of RO membranes modified with TiO2 is driven by its photocatalytic activity, improved hydrophilicity, antimicrobial properties, and structural reinforcement. These mechanisms collectively enable the membrane to resist fouling, prevent microbial growth, increase water permeability, and extend its lifespan.
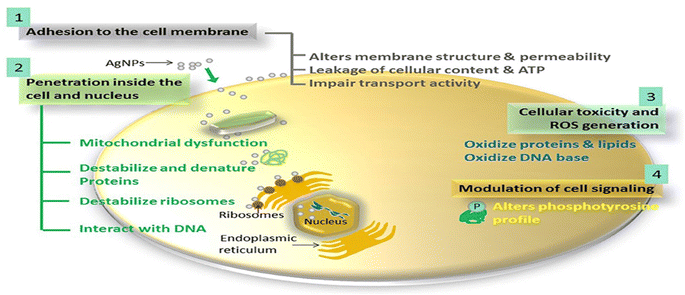 | ||
| Fig. 12 The proposed antimicrobial mechanisms of Ag NPs.130 | ||
For instance, the antibacterial characteristics of a covalently bonded cysteamine-modified TFC membrane incorporating Ag-NPs was investigated.138 Compared to the pristine TFC membrane, the Ag-NPs-grafted TFC membrane exhibited slightly lower salt rejection but a higher water flux and potent antibacterial properties against E. coli, evidenced by the absence of bacterial growth on the membrane surface. The antimicrobial mechanism of Ag-NPs can be attributed to their ability to disrupt bacterial cell metabolism. The antimicrobial effect of Ag+ ions involves the inactivation of membrane proteins followed by binding to bacterial DNA, which interferes with DNA replication.138 The facile loading of Ag-NPs onto TFC-RO membranes was achieved through the reaction of a reducing agent with a silver salt on the surface of the membrane to yield a uniform Ag-NPs coverage with firm bonding to the membrane.97 These Ag-NPs demonstrated strong antibacterial activity, resulting in a decrease of over 75% in the number of viable bacteria attached to the membrane for different bacterial strains. Additionally, using confocal microscopy, the researchers observed that Ag-NPs effectively inhibited biofilm formation, leading to a significant decrease in total biomass, as well as reduced levels of EPS and both live and dead bacteria on the membrane.97 Furthermore, a study of Ag-NPs TFN membrane unveils the creation of nanochannels with an average size of approximately 2.5 nm around the Ag-NPs.139 The formation of these nanochannels can be attributed to the hydrolysis of TMC monomers and the subsequent termination of IP by the water layer surrounding each hydrophilic Ag-NPs, as shown in Fig. 13a. Importantly, these nanochannels significantly enhanced the water permeability of TFN membranes, nearly tripling it compared to the pristine membrane without nanochannels, Fig. 13b. Furthermore, the incorporation of Ag-NPs leads to improved rejection capabilities against various substances such as NaCl, boron, and small organic compounds like norfloxacin, propylparaben, and ofloxacin, Fig. 13. This enhanced rejection performance can be attributed to a combination of factors, including enhanced Donnan exclusion, improved size exclusion, and suppressed hydrophobic interaction.139
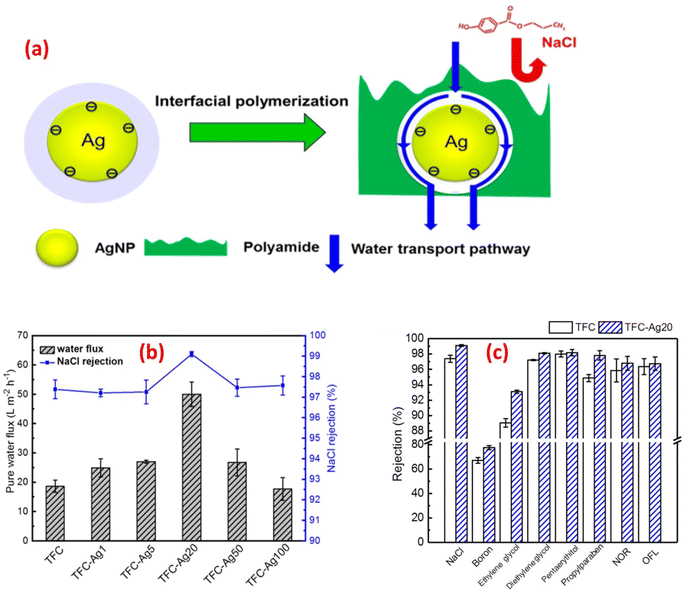 | ||
| Fig. 13 (a) Schematic diagram of Ag-NPs induced nanochannels in the polyamide layer for efficient water transport, (b) separation performance results of the control and TFC-Ag20 membranes, and (c) membrane rejection results of the control TFC and TFC-Ag20 membranes (the suffix Ag20 means prepared using 20 mM solution of AgNO3). Reprinted with the permission of ref. 147, copyright 2024, American Chemical Society. | ||
3. Characterization of polyamide layers
Characterization techniques play a vital role in studying the physicochemical properties of nanoparticles incorporated into/onto thin film composite (TFC) membranes.140 These techniques enable researchers to acquire valuable information about the morphology, composition, and interactions between nanoparticles and membrane surfaces. This overview section presents a classification of characterization techniques, highlighting their types and importance in providing essential information for assessing the performance of nanoparticles-modified membranes.3.1 Surface imaging techniques
Surface imaging techniques, such as atomic force microscopy (AFM), and scanning and tunneling electron microscopy (SEM, and TEM) are of significant importance in characterizing nanoparticles-modified RO membranes.141,142 These techniques provide valuable insights into the surface morphology, roughness, thickness, and distribution of nanoparticles on the membrane surface. This information is critical for understanding the interaction between nanoparticles and the membrane surface, assessing the uniformity and coverage of the modification layer, and evaluating the potential impact on membrane performance.AFM enables high-resolution imaging of the membrane surface, which allows for the assessment of nanoparticle adhesion, distribution, and stability. It provides information about the surface topography, roughness, and porosity, aiding in understanding the surface modifications caused by the incorporated nanoparticles. AFM offers detailed insights into the surface features at the nanoscale level, which are crucial for evaluating the impact of nanoparticle modifications on membrane performance. Additionally, AFM can measure the thickness of the nanoparticle layer, further assisting in optimizing the coating process. For instance, AFM analysis revealed that the PA membrane coated with 10% w/w graphene oxide (GO10-PA), displayed an average roughness (Ra) of approximately 21.5 nm. This value was significantly lower compared to the pristine PA membrane, which exhibited an Ra of approximately 46.5 nm (Fig. 14a and b).29 In another investigation, AFM images provided evidence showcasing that the surface roughness of membrane increased proportionally with the concentration of SiO2.31 Further, AFM analysis of the (PA/GO) composite revealed non-uniform coverage of GO NPs, with some areas showing folded GO NPs. Additionally, the surface roughness exhibited slight variations across different regions of the sample. These observations can be attributed to the uneven distribution of GO NPs within the PA matrix. Furthermore, due to the thickness of GO NPs ranging from 1.1 to 2.6 nm, the incorporation of GO NPs resulted in a slightly thicker membrane formation.60 In another study,62 it was observed that the surface modification of PA resulted in a smoother surface, evidenced by a decrease in roughness (Ra) from 66.4 nm to 50.3 nm. Similarly, AFM analysis showed that as the content of the reduced graphene oxide (rGO)-TiO2 nanocomposite increased, the TFN-RO membrane roughness gradually decreased.30
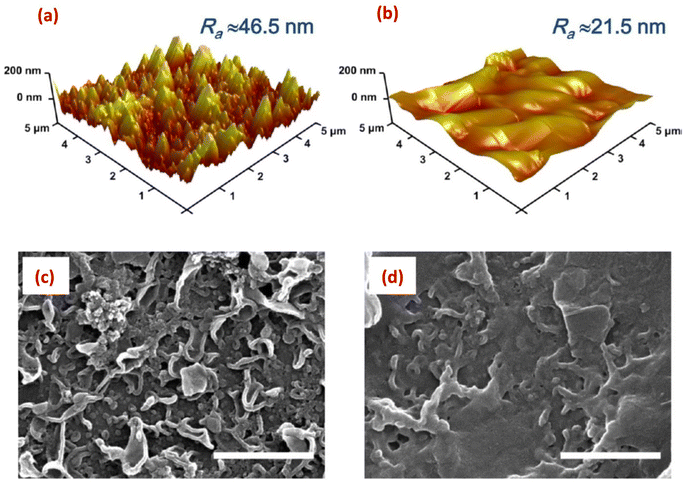 | ||
| Fig. 14 AFM height images of (a) the pristine, uncoated polyamide (PA) and (b) the GO10-coated PA membranes; and the SEM images of (c) the pristine PA and (d) the GO10-coated PA membranes, (scale bar = 1 μm). Reprinted with the permission of ref. 29, copyright 2024, American Chemical Society. | ||
SEM offers high-resolution imaging of the modified membrane surface, enabling researchers to scrutinize nanoscale features and assess the distribution of nanoparticles. It provides a larger field of view compared to AFM, allowing for the examination of a larger sample area. With SEM, the morphology, cross-sectional views, shape, and spatial arrangement of nanoparticles on the membrane surface can be observed, aiding in understanding the efficacy of the modification process.143,144 Thus, SEM was employed to examine the surface structure of SiO2-modified TFC membrane.145 The researchers observed a nodular pattern on the membrane surface, consisting of densely packed granular bumps, where the addition of SiO2 nanoparticles resulted in the formation of prominent ridge-like nanoribbons, clearly visible on the surface of the modified membrane. As the concentration of the added SiO2 NPs increased, the substrate surface exhibited an enhanced presence of these distinct structural characteristics, resembling a nanoribbon polyamide (PA) layer.145 In another study, SEM images revealed the existence of spherical 10–20 nm sized Ag-NPs on the membrane surface.138 Additionally, SEM images of the original PA membrane surface displayed a rugged topography characterized by ridges and valleys; however, when planar aminated graphene oxide (AGO) and GO nanosheets are introduced, they effectively smoothened the surface by filling in the valleys (Fig. 14c and d).29 Further, by utilizing SEM analysis, researchers could obtain high-resolution images of the layers cross-section, revealing important information about the layer-by-layer structure, thickness, and spatial arrangement of the nanomaterials in the modified RO membranes. Therefore, to examine the PA layer's cross-section, membrane samples are prepared by either removing the polyester backing layer and fracturing the polyamide support layers, or immersing the entire membrane in liquid nitrogen and then fracturing the polyamide support layer.146 Other studies have used SEM analysis to investigate the cross-section of TFN membranes.66,147–149
Additionally, SEM can be combined with energy-dispersive X-ray spectroscopy (EDS) to obtain elemental composition information, helping to identify the presence and distribution of nanoparticles on the membrane surface.127,141
3.2 Chemical composition analysis
Chemical composition analysis techniques, such as X-ray Photoelectron Spectroscopy (XPS) and Fourier Transform Infrared Spectroscopy (FTIR), provide in-depth insights into the elemental composition, chemical state, and bonding configuration of nanoparticles incorporated onto RO membranes. These techniques offer valuable information for understanding the surface modifications, compatibility, and reactivity of the modified membranes.XPS provides valuable information about the surface composition of modified RO membranes. By measuring the kinetic energy of photoelectrons emitted from the sample surface, XPS allows for the identification of elements present on the surface, their chemical states, and their binding energies.150 This information aids in determining the type and concentration of nanoparticles incorporated onto the membrane surface. Additionally, XPS can reveal changes in oxidation states, chemical functionalities, and the extent of nanoparticle dispersion. These insights are crucial for evaluating the distribution and stability of nanoparticle modifications, which impact the performance and longevity of the modified membranes. Moreover, XPS can detect subtle changes in the chemical environment surrounding the nanoparticles, providing valuable information about potential interactions with the membrane materials and the formation of chemical bonds. For instance, the XPS analysis of GO-modified PA demonstrated the formation of bonds between the TMC moieties of AP and GO NPs;60 however there was no clear evidence of covalent bonding between the MPD moieties of AP and GO NPs; nevertheless, it was assumed that H-bonding might be involved.60
FTIR spectroscopy provides information about the functional groups and molecular vibrations present in modified RO membranes. By measuring the absorption of infrared light, FTIR can identify the types of chemical bonds within the modified layer, including both surface-bound nanoparticles and the membrane matrix. Through analysis of FTIR spectra, changes in peak positions, intensities, or shifts can be correlated to chemical interactions occurring between nanoparticles and the membrane surface. These changes in chemical bonds and functional groups offer insights into modifications in surface properties, hydrophilicity, and compatibility with the aqueous environment. Furthermore, FTIR enables the identification of specific functional groups present in the modified membranes, facilitating the understanding of potential changes in surface chemistry and enabling the optimization of membrane performance. For instance, The FTIR analysis of GO-modified PA indicated that covalent bonds are indeed formed between the acyl chloride groups of TMC moieties and the functional groups of GO.60 This signifies a strong chemical interaction between these two components.60 Likewise, the presence of silica in the composite was evident from a pronounced peak in the infrared spectrum at approximately 1040–1060 cm−1, whose intensity increased with increasing the silica content of samples, indicating a clear correlation between the silica content and the strength of the interaction observed in the FTIR spectrum.151 Further, in GO-modified membranes, the existence of GO leads to peak broadening at 1585 cm−1 owing to combined contributions from graphene (G) band and vibrations of the polysulfone phenyl ring.68
The combined use of XPS and FTIR allows for a comprehensive analysis of the chemical composition and reactivity of modifications applied to RO membranes. These techniques provide detailed information about the incorporation, distribution, and stability of nanoparticles, as well as the potential modifications occurring at the membrane surface. Understanding these surface modifications and interfacial interactions is crucial for tailoring the functional properties of RO membranes, such as improved fouling resistance, enhanced flux rates, and selective ion rejection.
3.3 Contact angle measurement
Contact angle measurement assesses the membrane's surface energy, the wetting behavior and surface hydrophilicity or hydrophobicity of modified membranes. By placing a water droplet on the membrane surface, contact angle analysis determines the angle between the liquid–vapor interface and the solid surface. Modifications that enhance the hydrophilicity of RO membranes can improve water flux rates, reduce fouling propensity, and enhance overall membrane performance. For instance, the inclusion of GO NPs enhanced the membrane hydrophilicity, as demonstrated by a reduction in the contact angle.60 Namely, when 15 PA layers with 3 GO layers on top were assembled, the GO-modified PA sample (GO3/PA15) exhibited the highest GO NPs coverage on the PA surfaces and displayed the highest reduction of contact angle from 60 ± 2 to 20 ± 5 with an accompanying marginal increase in hydrophilicity.60 Similarly, the immobilization of Ag-NPs on the membrane led to a noticeable decrease in the contact angle, reducing it from 56.7 ± 2.2° to 32.2 ± 2.4°.138 In another investigation, the TFC membrane devoid of zeolite nanoparticles exhibited a relatively elevated contact angle of 68.4°.108 However, upon the introduction of zeolite, the contact angle of the modified membranes was reduced from 63.4° to 46.2° as the zeolite loading increased from 0.02 wt% to 0.2 wt%.1083.4 Zeta potential analysis
The characterization of modified RO membranes using zeta potential analysis is a valuable technique in assessing the surface charge and its impact on membrane performance. Zeta potential analysis also allows the assessment of surface charge changes due to membrane modifications, such as the incorporation of nanoparticles, functional groups, or surface coatings, all of which can alter the surface charge, and result in improved anti-fouling properties or enhanced selectivity. A higher magnitude of zeta potential indicates a greater electrostatic repulsion between the membrane surface and charged solutes, reducing fouling potential and promoting higher rejection of dissolved species. Furthermore, monitoring zeta potential changes over time can help assess the stability and durability of modified RO membranes. Variations in zeta potential can indicate changes in membrane properties, such as fouling accumulation or chemical degradation, providing valuable information for membrane maintenance and cleaning protocols. For instance, incorporation of 0.1 weight percent of multi-walled carbon nanotubes (MWCNTs) reduced the modified membrane's zeta potential and surface charge.733.5 Thermal stability
Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC) are two key techniques used in the characterization of nanoparticles-modified TFC membranes for reverse osmosis (RO) applications. These techniques provide valuable information on the thermal stability, decomposition behavior, and phase transitions of nanocomposite films as a function of temperature. During a typical TGA experiment, the sample is subjected to a controlled temperature ramp, and as the temperature increases, the weight loss due to volatilization, degradation, or combustion of the constituents is recorded. TGA enables the determination of the temperature at which weight loss begins, which indicates the onset of decomposition or degradation. Moreover, DSC is a technique used to analyze the thermal properties, phase transitions, and heat flow characteristics of materials as a function of temperature.152 It measures the difference in heat flow to or from a sample and a reference during controlled heating or cooling. This technique gives valuable data about melting temperature (Tm) glass transition temperature (Tg), and crystallization temperature (Tc) of various polymer types.152,153 For instance, using TGA to examine the thermal stability of TFC and TFN membranes revealed that the distribution of TiO2 NPs within the TFN membrane, produced a slightly higher onset temperature for intense degradation compared to the unmodified TFC membrane.127 Namely, the onset degradation temperature increased from 530 to 550 °C upon the incorporation of TiO2 NPs.127 Similarly, the TGA curves provided evidence supporting the correlation between the addition of SiO2 and a reduction in thermal decomposition. The results indicated that as the SiO2 content increased, the thermal decomposition of modified membranes decreased.151Overall, thermal stability data allows the determination of the degradation temperature, which is the temperature at which a significant weight loss occurs. This information helps in assessing the temperature limits that the nanocomposite film can tolerate during RO operations, ensuring its stability and preventing any detrimental effects on the film's properties.
4. Challenges in TFC membrane
Most research papers of TFN membranes focus on membrane modification with a given nanomaterial to prepare a novel membrane and study its improved performance characteristics. However, there is a need for a systematic monovariate exploration of incorporating one nanomaterial with various membrane preparation methods and in various locations to produce TFNa, TFNs and TFNi containing the same controlled size, porosity, and hydrophilicity NPs. An additional challenge is maximizing TFN membranes' performances while incorporating different nanomaterials with different features in various membrane positions via regulating the IP process.39–41 The primary challenge in incorporating nanomaterials in polyamide membrane fabrication is achieving a uniform dispersion of the nanofillers within the polymer matrix.154,155 Poor dispersion can lead to accumulation, uneven properties, and reduced membrane performance.33 Factors such as the surface chemistry of nanomaterials, shear forces during processing, and compatibility with the polymer matrix all influence dispersion quality. For instance, the dispersion of hydrophilic nanoparticles in non-polar chemical solvents is unstable.156 It is challenging for large hydrophilic nanoparticles in water to phase-transfer to a nonpolar organic solvent.156 Researchers are currently focusing on surface modification approaches for nanomaterials, including the employment of amine-functionalized or carboxylated carbon nanotubes (CNTs) and halloysite nanotubes (HNTs).156–159 Modifying the surface properties of nanoparticles can help minimize agglomeration in nonpolar solvents or polymer matrices.33 In addition to modifying the surface of nanofillers, novel nanomaterials such as MOFs and COFs can be created with specific pore structure and surface charge to promote even dispersion of nanofillers within the polymer matrix. Moreover, the intrinsic orientation of 1D and 2D nanofillers during the IP processes can be another challenge, e.g., most nanotubes because of gravitational forces prefer horizontal alignment, creating longer paths and higher resistance for horizontal nanochannels water flow. Otherwise, nano-bowls that are randomly dispersed create extra resistance for water flow.40,159,160 Additionally, horizontal nanochannels of nanosheets can be disrupted with long pressurization, and lead to TFN membrane performance deterioration. It is common knowledge that the longer the path, the greater the hydraulic resistance. 2D nanomaterials like COF have internal channels that allow water molecules to flow through with reduced distance, making them advantageous compared to traditional materials.40 Hence, the concept of the preferential pathway for water molecules requires further verification since it is only possible if the nanochannels are aligned toward the water flux direction and not blocked by the polymer matrix. So far, most studies on liquid separation reported the membrane performance results with only randomly arranged nanotubes. Furthermore, the trade-off between water permeability, salt rejection rate, fouling and Cl2-resistance should be carefully considered. The nanofillers toxicity and appreciable leaching rates from TNF membranes should also be avoided. Moreover, green synthesis of nanofillers and TFN membranes are preferred. Another significant challenge in incorporating nanomaterials in polyamide membrane fabrication is ensuring the stability and durability of the composite membrane under harsh operating conditions. Nanomaterials, such as carbon nanotubes, graphene oxide, or metal nanoparticles, can be susceptible to degradation, oxidation, or leaching, which can compromise the long-term performance of the membrane. Finally, the scalability and cost-effectiveness of incorporating nanomaterials in polyamide membrane fabrication are critical factors that need to be considered to meet industrial production requirements. Advanced manufacturing processes, such as electrospinning, 3D printing, and roll-to-roll coating, can be employed to scale up the production of nanocomposite membranes while maintaining cost-effectiveness.40,159,1605. Economic analysis
The economic aspects of modified RO membranes incorporating nanomaterials (graphene, CNTs, MOF, zeolites, TiO2, and SiO2, Ag NPs, etc.) for water treatment applications are crucial considerations in determining their feasibility and commercial viability. Nanomaterial-modified RO membranes offer improved performance characteristics, such as increased permeability, salt rejection, reduced cleaning requirements, antimicrobial properties, and enhanced mechanical, chemical, and physical stability. These advancements translate into several economic benefits, including higher water production rates, reduced energy consumption, improved water quality, and extended membrane lifespan. The increased productivity and efficiency of nanomaterial-modified membranes can result in cost savings over time, making them economically viable alternatives to traditional membranes. A research161 highlighted the important role of membrane modification in lowering specific energy consumption (SEC) during desalination processes across different water sources (Fig. 15a and b). The study demonstrated considerable energy savings of up to 80% in scenarios involving feed waters with low osmotic pressure, such as water reuse. However, the cost of nanomaterials is a significant consideration when assessing their economic feasibility. Additionally, nanomaterials may have limited availability and higher market costs due to their specialized nature and manufacturing constraints. These higher initial costs can pose a barrier to the widespread adoption of nanomaterial-modified membranes, particularly in large-scale applications.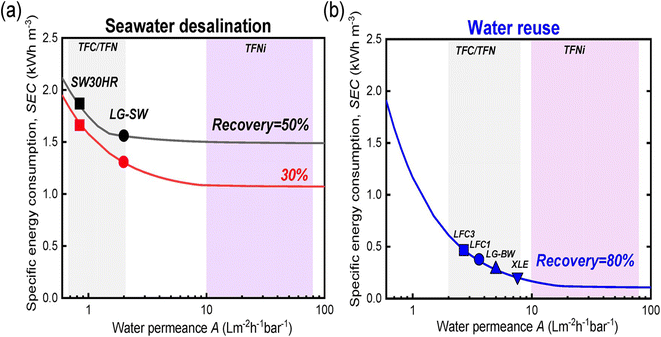 | ||
| Fig. 15 Relationship between specific energy consumption (SEC, kW h m−3) and product flow in the context of (a) seawater desalination and (b) water reuse. Reprinted with the permission of ref. 29, copyright 2024, American Chemical Society. | ||
For instance, metal oxides, silica, and zeolites are generally more economically viable due to their wide availability and competitive pricing. The use of metal oxides in RO membrane modification provides several economic advantages. They are readily available in bulk quantities, ensuring a steady supply and competitive pricing. Furthermore, the relatively low material costs associated with metal oxides make them economically viable for large-scale applications. The cost-effectiveness of these materials is particularly advantageous for water treatment facilities and industrial processes where long-term cost savings are essential. Similarly, zeolite nanoparticles are relatively cost-effective and readily available in the market, making them a cost-effective option for membrane modification.
On the other hand, carbon-based nanomaterials, namely graphene, single-walled carbon nanotubes (SWCNTs), and multi-walled carbon nanotubes (MWCNTs), hold significant potential for enhancing RO membrane performance. Their unique properties, including high permeability, hydrophilicity, and salt rejection rates, make them ideal candidates for improving desalination efficiency. However, the economic feasibility of carbon nanomaterials remains a challenge. The high cost of production and limited availability hinder their widespread adoption. In addition, the complex manufacturing processes required for these nanomaterials make large-scale production and local availability difficult. Further research and development are necessary to overcome these economic barriers and make carbon nanomaterials more cost-effective. Further, the use of silver coatings in RO membrane modification offers antimicrobial properties, effectively preventing biofouling. This reduces the need for frequent membrane cleaning and maintenance, leading to long-term cost savings. While silver is relatively more expensive compared to other materials, the potential economic benefits resulting from reduced cleaning requirements should be considered. Moreover, the integration of these nanomaterials into the RO membranes involves additional processing steps, which might increase manufacturing costs. Techniques such as physical blending, electrospinning, and chemical deposition methods require specific instrumentation, energy consumption, and skilled labor. Hence, an economic analysis could provide insights into the long-term monetary benefits of employing these modified membranes in terms of reduced operational expenses.
6. Conclusion and prospective
In conclusion, the incorporation of nanomaterials into RO membranes holds great promise for improving membrane performance and addressing challenges in water purification. Utilizing inorganic and porous organic material, TFN membranes can be fabricated through interfacial polymerization by dispersing NPs in the organic phase or the aqueous solution. The inclusion of nanomaterials in the PA layer not only creates nanochannels for enhanced water transport and consistent solute rejection but also changes membrane features such as hydrophilicity, surface roughness, cross-linking degree, and surface charge, which significantly impact membrane performance. Extensive research studies have demonstrated the positive impacts of these nanomaterials on various membrane features, including rejection rates, water flux, selectivity, chlorine resistance, and fouling resistance. The optimal dose of nanomaterials and suitable fabrication methods play vital roles in achieving the overall desired performance. Incorporating materials like SiO2 further enhances the mechanical strength and durability of the membrane, thereby extending its lifespan. Likewise, membranes containing CNTs, GO, SiO2 and TiO2 NPs exhibit versatility in RO applications due to their exceptional thermal, electrical, fouling resistance and mechanical stability, as well as their antibacterial and photocatalytic features. Additionally, it is worth noting that MOFs are particularly attractive porous nanomaterials that warrant intensive research attention in the fabrication of TFN membranes. However, there are still areas of concern and knowledge gaps in the existing literature that will require further investigation in the future:1. Tailored material design: future research can focus on developing and tailoring carbon nanomaterials, and other NPs with specific properties for membrane modification. This could involve exploring new synthesis methods, optimizing material composition, and engineering advanced structures at the nanoscale. By fine-tuning material properties, such as surface chemistry, pore size, porosity, and hydrophilicity, researchers can enhance membrane selectivity, permeability, and fouling resistance.
2. Mechanistic insights: gaining a deeper understanding of the mechanisms underlying the interaction between modified membranes and various pollutants is crucial for optimizing membrane performance.
3. Scaling up and commercial viability: to enable the practical implementation of NPs-modified membranes, researchers need to address scalability and cost-effectiveness. Further research should focus on developing scalable and cost-efficient synthesis methods for incorporating nanomaterials into large-area membranes. The durability and stability of modified membranes under realistic operating conditions must also be thoroughly evaluated to ensure long-term performance. Collaborations between academia and industry can facilitate the translation of research findings into commercially viable membrane products.
4. Environmental sustainability: as the field progresses, it is crucial to consider the broader environmental implications of modified membranes. Research should explore the life cycle analysis of these membranes, including the environmental impact of raw material extraction, synthesis processes, and end-of-life disposal. Additionally, efforts should be made to develop recycling and regeneration methods for used membranes to minimize waste generation.
Overall, the use of nanomaterials to modify reverse osmosis membranes holds promise for advancing membrane technology and addressing challenges in water treatment. Continued research and development in this field will contribute to the improvement of membrane science and play a critical role in providing sustainable and efficient solutions for water purification and desalination processes on a global scale.
Conflicts of interest
Authors declare no conflict of interest.Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at Northern Border University, Arar, KSA for funding this research work through the project number “NBU-FFR-2024-2292-02”References
- B. Chen, et al., Global land-water nexus: Agricultural land and freshwater use embodied in worldwide supply chains, Sci. Total Environ., 2018, 613, 931–943 Search PubMed.
- M. Adel, M. A. Ahmed and A. A. Mohamed, A facile and rapid removal of cationic dyes using hierarchically porous reduced graphene oxide decorated with manganese ferrite, FlatChem, 2021, 26, 100233 CrossRef CAS.
- A. Matin, et al., Fouling control in reverse osmosis for water desalination & reuse: Current practices & emerging environment-friendly technologies, Sci. Total Environ., 2021, 765, 142721 CrossRef CAS PubMed.
- V. G. Gude, Desalination and water reuse to address global water scarcity, Rev. Environ. Sci. Biotechnol., 2017, 16(4), 591–609 CrossRef.
- M. A. Ahmed, S. Amin and A. A. Mohamed, Fouling in reverse osmosis membranes: monitoring, characterization, mitigation strategies and future directions, Heliyon, 2023, 9(4), e14908 CrossRef PubMed.
- A. Ali, et al., Membrane technology in renewable-energy-driven desalination, Renew. Sustain. Energy Rev., 2018, 81, 1–21 CrossRef CAS.
- M. Qasim, et al., Reverse osmosis desalination: A state-of-the-art review, Desalination, 2019, 459, 59–104 CrossRef CAS.
- A. Cipollina, G. Micale and L. Rizzuti, Seawater desalination, Green Energy and Technology, Spinger-Verlag, Heidelberg, 2009 Search PubMed.
- P. Youssef, R. Al-Dadah and S. Mahmoud, Comparative analysis of desalination technologies, Energy Proc., 2014, 61, 2604–2607 CrossRef.
- J. E. Cadotte, et al., A new thin-film composite seawater reverse osmosis membrane, Desalination, 1980, 32, 25–31 CrossRef.
- N. Zhang, et al., Advanced thin-film nanocomposite membranes embedded with organic-based nanomaterials for water and organic solvent purification: A review, Sep. Purif. Technol., 2021, 269, 118719 CrossRef CAS.
- F. Liu, et al., A review: the effect of the microporous support during interfacial polymerization on the morphology and performances of a thin film composite membrane for liquid purification, RSC Adv., 2019, 9(61), 35417–35428 RSC.
- M. Adel, et al., Characterization of fouling for a full-scale seawater reverse osmosis plant on the Mediterranean sea: membrane autopsy and chemical cleaning efficiency, Groundw. Sustain. Dev., 2022, 16, 100704 CrossRef.
- D. Suresh, et al., Insights into biofouling in reverse osmosis membrane: A comprehensive review on techniques for biofouling assay, J. Environ. Chem. Eng., 2023, 110317 CrossRef CAS.
- K. P. Lee, T. C. Arnot and D. Mattia, A review of reverse osmosis membrane materials for desalination—Development to date and future potential, J. Membr. Sci., 2011, 370(1–2), 1–22 CrossRef CAS.
- M. Thomas and B. Corry, A computational assessment of the permeability and salt rejection of carbon nanotube membranes and their application to water desalination, Philos. Trans. R. Soc., A, 2016, 374(2060), 20150020 CrossRef PubMed.
- X. Song and et al., Coupling solar-driven interfacial evaporation with forward osmosis for continuous water treatment, in Exploration, Wiley Online Library, 2022 Search PubMed.
- D. Al-Halbouni, et al., Correlation of EPS content in activated sludge at different sludge retention times with membrane fouling phenomena, Water Res., 2008, 42(6–7), 1475–1488 CrossRef CAS PubMed.
- X. Huang, et al., Low-fouling antibacterial reverse osmosis membranes via surface grafting of graphene oxide, ACS Appl. Mater. Interfaces, 2016, 8(23), 14334–14338 CrossRef PubMed.
- M. Safarpour, et al., High flux and fouling resistant reverse osmosis membrane modified with plasma treated natural zeolite, Desalination, 2017, 411, 89–100 CrossRef CAS.
- G.-R. Xu, J.-N. Wang and C.-J. Li, Strategies for improving the performance of the polyamide thin film composite (PA-TFC) reverse osmosis (RO) membranes: Surface modifications and nanoparticles incorporations, Desalination, 2013, 328, 83–100 CrossRef CAS.
- N. Misdan, W. Lau and A. Ismail, Seawater Reverse Osmosis (SWRO) desalination by thin-film composite membrane—Current development, challenges and future prospects, Desalination, 2012, 287, 228–237 CrossRef CAS.
- Y.-H. Wang, et al., Chlorine disinfection significantly aggravated the biofouling of reverse osmosis membrane used for municipal wastewater reclamation, Water Res., 2019, 154, 246–257 CrossRef CAS PubMed.
- R. Verbeke, V. Gomez and I. F. Vankelecom, Chlorine-resistance of reverse osmosis (RO) polyamide membranes, Prog. Polym. Sci., 2017, 72, 1–15 CrossRef CAS.
- J. Glater, S.-k. Hong and M. Elimelech, The search for a chlorine-resistant reverse osmosis membrane, Desalination, 1994, 95(3), 325–345 CrossRef CAS.
- C. Liu, et al., Separation, anti-fouling, and chlorine resistance of the polyamide reverse osmosis membrane: From mechanisms to mitigation strategies, Water Res., 2021, 195, 116976 CrossRef CAS PubMed.
- Y.-N. Kwon, et al., Surface modification of a polyamide reverse osmosis membrane for chlorine resistance improvement, J. Membr. Sci., 2012, 415, 192–198 CrossRef.
- A. Antony, et al., Assessing the oxidative degradation of polyamide reverse osmosis membrane—Accelerated ageing with hypochlorite exposure, J. Membr. Sci., 2010, 347(1–2), 159–164 CrossRef CAS.
- W. Choi, et al., Layer-by-layer assembly of graphene oxide nanosheets on polyamide membranes for durable reverse-osmosis applications, ACS Appl. Mater. Interfaces, 2013, 5(23), 12510–12519 CrossRef CAS PubMed.
- M. Safarpour, A. Khataee and V. Vatanpour, Thin film nanocomposite reverse osmosis membrane modified by reduced graphene oxide/TiO2 with improved desalination performance, J. Membr. Sci., 2015, 489, 43–54 CrossRef CAS.
- A. Peyki, A. Rahimpour and M. Jahanshahi, Preparation and characterization of thin film composite reverse osmosis membranes incorporated with hydrophilic SiO2 nanoparticles, Desalination, 2015, 368, 152–158 CrossRef CAS.
- W. Yan, et al., Amino-modified hollow mesoporous silica nanospheres-incorporated reverse osmosis membrane with high performance, J. Membr. Sci., 2019, 581, 168–177 CrossRef CAS.
- N. Akther, et al., Recent advances in nanomaterial-modified polyamide thin-film composite membranes for forward osmosis processes, J. Membr. Sci., 2019, 584, 20–45 CrossRef CAS.
- N. Ma, et al., Zeolite-polyamide thin film nanocomposite membranes: Towards enhanced performance for forward osmosis, J. Membr. Sci., 2012, 405, 149–157 CrossRef.
- Y. Wen, et al., Thin-film nanocomposite membranes incorporated with water stable metal-organic framework CuBTTri for mitigating biofouling, J. Membr. Sci., 2019, 582, 289–297 CrossRef CAS.
- A. Tiraferri, C. D. Vecitis and M. Elimelech, Covalent binding of single-walled carbon nanotubes to polyamide membranes for antimicrobial surface properties, ACS Appl. Mater. Interfaces, 2011, 3(8), 2869–2877 CrossRef CAS PubMed.
- X. Wang, et al., Fabrication of nanofiltration membrane on MoS2 modified PVDF substrate for excellent permeability, salt rejection, and structural stability, Chem. Eng. J., 2021, 416, 129154 CrossRef CAS.
- Y. Wen, et al., Metal–organic framework nanosheets for thin-film composite membranes with enhanced permeability and selectivity, ACS Appl. Nano Mater., 2020, 3(9), 9238–9248 CrossRef.
- Y. T. Chew and W. F. Yong, Recent advances of thin film nanocomposite membranes: Effects of shape/structure of nanomaterials and interfacial polymerization methods, Chem. Eng. Res. Des., 2021, 172, 135–158 CrossRef CAS.
- W.-L. Li, et al., High-performance thin-film composite (TFC) membranes with 2D nanomaterial interlayers: An overview, Results Eng., 2024, 101932 CrossRef CAS.
- X. Wei, et al., A critical review on thin-film nanocomposite membranes enabled by nanomaterials incorporated in different positions and with diverse dimensions: Performance comparison and mechanisms, J. Membr. Sci., 2022, 661, 120952 CrossRef CAS.
- M. Adel, M. A. Ahmed and A. A. Mohamed, Synthesis and characterization of magnetically separable and recyclable crumbled MgFe2O4/reduced graphene oxide nanoparticles for removal of methylene blue dye from aqueous solutions, J. Phys. Chem. Solids, 2021, 149, 109760 CrossRef CAS.
- M. A. Ahmed, M. A. Ahmed and A. A. Mohamed, Facile adsorptive removal of dyes and heavy metals from wastewaters using magnetic nanocomposite of zinc ferrite@ reduced graphene oxide, Inorg. Chem. Commun., 2022, 144, 109912 CrossRef CAS.
- M. A. Ahmed, M. A. Ahmed and A. A. Mohamed, Adsorptive removal of tetracycline antibiotic onto magnetic graphene oxide nanocomposite modified with polyvinylpyrroilidone, React. Funct. Polym., 2023, 191, 105701 CrossRef CAS.
- M. A. Ahmed, M. A. Ahmed and A. A. Mohamed, Synthesis, characterization and application of chitosan/graphene oxide/copper ferrite nanocomposite for the adsorptive removal of anionic and cationic dyes from wastewater, RSC Adv., 2023, 13(8), 5337–5352 RSC.
- M. Adel, et al., Removal of heavy metals and dyes from wastewater using graphene oxide-based nanomaterials: A critical review, Environ. Nanotechnol., Monit. Manage., 2022, 18, 100719 CAS.
- M. Adel, M. A. Ahmed and A. A. Mohamed, Effective removal of cationic dyes from aqueous solutions using reduced graphene oxide functionalized with manganese ferrite nanoparticles, Compos. Commun., 2020, 22, 100450 CrossRef.
- J. Dai, et al., Electrochemical degradation of antibiotic enoxacin using a novel PbO2 electrode with a graphene nanoplatelets inter-layer: Characteristics, efficiency and mechanism, Chemosphere, 2022, 307, 135833 CrossRef CAS PubMed.
- M. A. Ahmed and A. A. Mohamed, Recent progress in semiconductor/graphene photocatalysts: synthesis, photocatalytic applications, and challenges, RSC Adv., 2023, 13(1), 421–439 RSC.
- H. Saleem and S. J. Zaidi, Nanoparticles in reverse osmosis membranes for desalination: A state of the art review, Desalination, 2020, 475, 114171 CrossRef CAS.
- H. Zhang, et al., Carboxyl-functionalized graphene oxide polyamide nanofiltration membrane for desalination of dye solutions containing monovalent salt, J. Membr. Sci., 2017, 539, 128–137 CrossRef CAS.
- H. Mittal, A. Maity and S. S. Ray, Effective removal of cationic dyes from aqueous solution using gum ghatti-based biodegradable hydrogel, Int. J. Biol. Macromol., 2015, 79, 8–20 CrossRef CAS PubMed.
- V. C. Sanchez, et al., Biological interactions of graphene-family nanomaterials: an interdisciplinary review, Chem. Res. Toxicol., 2012, 25(1), 15–34 Search PubMed.
- H. M. Hegab, et al., The controversial antibacterial activity of graphene-based materials, Carbon, 2016, 105, 362–376 CrossRef CAS.
- F. Perreault, A. F. De Faria and M. Elimelech, Environmental applications of graphene-based nanomaterials, Chem. Soc. Rev., 2015, 44(16), 5861–5896 RSC.
- W. Ying, et al., Extracellular polymeric substances (EPS) in a hybrid growth membrane bioreactor (HG-MBR): viscoelastic and adherence characteristics, Environ. Sci. Technol., 2010, 44(22), 8636–8643 CrossRef PubMed.
- S. Homaeigohar and M. Elbahri, Graphene membranes for water desalination, NPG Asia Mater., 2017, 9(8), e427 CrossRef CAS.
- D. W. Boukhvalov, M. I. Katsnelson and Y.-W. Son, Origin of anomalous water permeation through graphene oxide membrane, Nano Lett., 2013, 13(8), 3930–3935 CrossRef CAS PubMed.
- X. Wang, et al., Graphene oxide-based polymeric membranes for water treatment, Adv. Mater. Interfaces, 2018, 5(15), 1701427 CrossRef.
- M. Abbaszadeh, D. Krizak and S. Kundu, Layer-by-layer assembly of graphene oxide nanoplatelets embedded desalination membranes with improved chlorine resistance, Desalination, 2019, 470, 114116 CrossRef CAS.
- Q. Liu and G.-R. Xu, Graphene oxide (GO) as functional material in tailoring polyamide thin film composite (PA-TFC) reverse osmosis (RO) membranes, Desalination, 2016, 394, 162–175 CrossRef CAS.
- F. Shao, et al., Graphene oxide modified polyamide reverse osmosis membranes with enhanced chlorine resistance, J. Membr. Sci., 2017, 525, 9–17 CrossRef CAS.
- H. J. Kim, et al., High-performance reverse osmosis nanocomposite membranes containing the mixture of carbon nanotubes and graphene oxides, J. Mater. Chem. A, 2015, 3(13), 6798–6809 RSC.
- J. Lee, et al., A facile route to enhance the water flux of a thin-film composite reverse osmosis membrane: Incorporating thickness-controlled graphene oxide into a highly porous support layer, J. Mater. Chem. A, 2015, 3(44), 22053–22060 RSC.
- M. E. Ali, et al., Thin film composite membranes embedded with graphene oxide for water desalination, Desalination, 2016, 386, 67–76 CrossRef CAS.
- H.-R. Chae, et al., Graphene oxide-embedded thin-film composite reverse osmosis membrane with high flux, anti-biofouling, and chlorine resistance, J. Membr. Sci., 2015, 483, 128–135 CrossRef CAS.
- M. Y. Ashfaq, M. A. Al-Ghouti and N. Zouari, Functionalization of reverse osmosis membrane with graphene oxide to reduce both membrane scaling and biofouling, Carbon, 2020, 166, 374–387 CrossRef CAS.
- F. Perreault, M. E. Tousley and M. Elimelech, Thin-film composite polyamide membranes functionalized with biocidal graphene oxide nanosheets, Environ. Sci. Technol. Lett., 2014, 1(1), 71–76 CrossRef CAS.
- S. Bakhsh, Fullerene, Carbon Nanotubes and Graphene: A comprehen-sive review, Karbala Int. J. Mod. Sci., 2023, 9(3), 1 Search PubMed.
- L. Ye, et al., Thin film composite nanofiltration membrane with tannic acid-Fe (III) complexes functionalized CNTs interlayer toward energy efficient remediation of groundwater, Desalination, 2023, 552, 116438 CrossRef CAS.
- M. A. Ahmed and A. A. Mohamed, The use of chitosan-based composites for environmental remediation: A review, Int. J. Biol. Macromol., 2023, 242, 124787 CrossRef CAS PubMed.
- R. Das, et al., Carbon nanotube membranes for water purification: A bright future in water desalination, Desalination, 2014, 336, 97–109 CrossRef CAS.
- H. Zhao, et al., Improving the performance of polyamide reverse osmosis membrane by incorporation of modified multi-walled carbon nanotubes, J. Membr. Sci., 2014, 450, 249–256 CrossRef CAS.
- Y. Manawi, et al., Can carbon-based nanomaterials revolutionize membrane fabrication for water treatment and desalination?, Desalination, 2016, 391, 69–88 CrossRef CAS.
- H. J. Kim, et al., The improvement of antibiofouling properties of a reverse osmosis membrane by oxidized CNTs, RSC Adv., 2014, 4(62), 32802–32810 RSC.
- A. Alberdi, et al., Tribological behavior of nanocomposite coatings based on fullerene-like structures, Vacuum, 2011, 85(12), 1087–1092 CrossRef CAS.
- P. Daraei, et al., Enhancing antifouling capability of PES membrane via mixing with various types of polymer modified multi-walled carbon nanotube, J. Membr. Sci., 2013, 444, 184–191 CrossRef CAS.
- H. J. Kim, et al., High-performance reverse osmosis CNT/polyamide nanocomposite membrane by controlled interfacial interactions, ACS Appl. Mater. Interfaces, 2014, 6(4), 2819–2829 CrossRef CAS PubMed.
- Y. Baek, et al., Evaluation of carbon nanotube-polyamide thin-film nanocomposite reverse osmosis membrane: Surface properties, performance characteristics and fouling behavior, J. Ind. Eng. Chem., 2017, 56, 327–334 CrossRef CAS.
- J. L. Fajardo-Diaz, et al., Antifouling performance of spiral wound type module made of carbon nanotubes/polyamide composite RO membrane for seawater desalination, Desalination, 2022, 523, 115445 CrossRef CAS.
- Y. Takizawa, et al., Antiorganic fouling and low-protein adhesion on reverse-osmosis membranes made of carbon nanotubes and polyamide nanocomposite, ACS Appl. Mater. Interfaces, 2017, 9(37), 32192–32201 CrossRef CAS PubMed.
- S. Inukai, et al., High-performance multi-functional reverse osmosis membranes obtained by carbon nanotube· polyamide nanocomposite, Sci. Rep., 2015, 5(1), 13562 CrossRef PubMed.
- J. Li, W. Ye and C. Chen, Removal of toxic/radioactive metal ions by metal-organic framework-based materials, in Interface Science and Technology, Elsevier, 2019, pp. 217–279 Search PubMed.
- S. K. Elsaidi, et al., Flexibility in metal–organic frameworks: a fundamental understanding, Coord. Chem. Rev., 2018, 358, 125–152 CrossRef CAS.
- Y. Liu, et al., Probing the correlations between the defects in metal–organic frameworks and their catalytic activity by an epoxide ring-opening reaction, Chem. Commun., 2016, 52(50), 7806–7809 RSC.
- C. J. Doonan and C. J. Sumby, Metal–organic framework catalysis, CrystEngComm, 2017, 19(29), 4044–4048 RSC.
- B. Seoane, et al., Multi-scale crystal engineering of metal organic frameworks, Coord. Chem. Rev., 2016, 307, 147–187 CrossRef CAS.
- Q. Yuan and G. Zhu, A review on metal organic frameworks (MOFs) modified membrane for remediation of water pollution, Environ. Eng. Res., 2021, 26(3), 190435 Search PubMed.
- B. L. Bonnett, et al., PCN-222 metal–organic framework nanoparticles with tunable pore size for nanocomposite reverse osmosis membranes, ACS Appl. Mater. Interfaces, 2020, 12(13), 15765–15773 CrossRef CAS PubMed.
- Y. Xu, et al., Highly and stably water permeable thin film nanocomposite membranes doped with MIL-101 (Cr) nanoparticles for reverse osmosis application, Materials, 2016, 9(11), 870 CrossRef PubMed.
- T. H. Lee, et al., ZIF-8 particle size effects on reverse osmosis performance of polyamide thin-film nanocomposite membranes: Importance of particle deposition, J. Membr. Sci., 2019, 570, 23–33 CrossRef.
- H. M. Park, K. Y. Jee and Y. T. Lee, Preparation and characterization of a thin-film composite reverse osmosis membrane using a polysulfone membrane including metal-organic frameworks, J. Membr. Sci., 2017, 541, 510–518 CrossRef CAS.
- I. Jahan, et al., Water adsorption characterization of bivalent metal doped HKUST-1, Therm. Sci. Eng. Prog., 2022, 35, 101453 CrossRef CAS.
- L. Y. Ng, et al., Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review, Desalination, 2013, 308, 15–33 CrossRef CAS.
- M. Adel, M. A. Ahmed and A. A. Mohamed, Effective removal of indigo carmine dye from wastewaters by adsorption onto mesoporous magnesium ferrite nanoparticles, Environ. Nanotechnol., Monit. Manage., 2021, 16, 100550 CAS.
- B.-H. Jeong, et al., Interfacial polymerization of thin film nanocomposites: a new concept for reverse osmosis membranes, J. Membr. Sci., 2007, 294(1–2), 1–7 CrossRef CAS.
- M. Ben-Sasson, et al., In situ formation of silver nanoparticles on thin-film composite reverse osmosis membranes for biofouling mitigation, Water Res., 2014, 62, 260–270 CrossRef CAS PubMed.
- A. S. Al-Hobaib, et al., Characterization and evaluation of reverse osmosis membranes modified with Ag 2 O nanoparticles to improve performance, Nanoscale Res. Lett., 2015, 10, 1–13 CrossRef CAS PubMed.
- M. Sun, et al., Ambient temperature NO2 removal by adsorption on transition metal ion-exchanged chabazite zeolites, Results Eng., 2023, 18, 101134 CrossRef CAS.
- J. Li, et al., Regulation of the Si/Al ratios and Al distributions of zeolites and its impact on properties, Chem. Sci., 2023, 14, 1935–1959 RSC.
- Y. Liang, et al., Catalytic oxidation performance and ion-exchange of Ti-MWW zeolite membrane with dual organic template agents and potassium carbonate, J. Catal., 2023, 417, 432–444 CrossRef CAS.
- R. Mahdavi Far, et al., A review of zeolite materials used in membranes for water purification: History, applications, challenges and future trends, J. Chem. Technol. Biotechnol., 2022, 97(3), 575–596 CrossRef CAS.
- B. Lv, et al., Effective adsorption of methylene blue from aqueous solution by coal gangue-based zeolite granules in a fluidized bed: Fluidization characteristics and continuous adsorption, Powder Technol., 2022, 408, 117764 CrossRef CAS.
- J. Xu, et al., The morphology of fully-aromatic polyamide separation layer and its relationship with separation performance of TFC membranes, J. Membr. Sci., 2017, 541, 174–188 CrossRef CAS.
- M. L. Lind, et al., Tailoring the structure of thin film nanocomposite membranes to achieve seawater RO membrane performance, Environ. Sci. Technol., 2010, 44(21), 8230–8235 CrossRef CAS PubMed.
- Y. Liu and X. Chen, High permeability and salt rejection reverse osmosis by a zeolite nano-membrane, Phys. Chem. Chem. Phys., 2013, 15(18), 6817–6824 RSC.
- M. Fathizadeh, A. Aroujalian and A. Raisi, Effect of added NaX nano-zeolite into polyamide as a top thin layer of membrane on water flux and salt rejection in a reverse osmosis process, J. Membr. Sci., 2011, 375(1–2), 88–95 CrossRef CAS.
- H. Dong, et al., High-flux reverse osmosis membranes incorporated with NaY zeolite nanoparticles for brackish water desalination, J. Membr. Sci., 2015, 476, 373–383 CrossRef CAS.
- N. Sun, et al., Hydrophilic mesoporous silica materials for highly specific enrichment of N-linked glycopeptide, Anal. Chem., 2017, 89(3), 1764–1771 CrossRef CAS PubMed.
- D. Wang, et al., Recent advances of ordered mesoporous silica materials for solid-phase extraction, J. Chromatogr. A, 2022, 1675, 463157 CrossRef CAS PubMed.
- X. Zhang, et al., Facile incorporation of aggregation-induced emission materials into mesoporous silica nanoparticles for intracellular imaging and cancer therapy, ACS Appl. Mater. Interfaces, 2013, 5(6), 1943–1947 CrossRef CAS PubMed.
- M. Shafiee, et al., Synthesis of Pore-Size-Tunable Mesoporous Silica Nanoparticles by Simultaneous Sol-Gel and Radical Polymerization to Enhance Silibinin Dissolution, Iran. J. Med. Sci., 2021, 46(6), 475 Search PubMed.
- R. Takahashi, et al., High surface-area silica with controlled pore size prepared from nanocomposite of silica and citric acid, J. Phys. Chem. B, 2000, 104(51), 12184–12191 CrossRef CAS.
- S. Wijaya, M. Duke and J. D. da Costa, Carbonised template silica membranes for desalination, Desalination, 2009, 236(1–3), 291–298 CrossRef CAS.
- M. B. M. Y. Ang, et al., Embedding hollow silica nanoparticles of varying shapes and dimensions in nanofiltration membranes for optimal performance, J. Membr. Sci., 2020, 611, 118333 CrossRef.
- M. Tian, et al., Synthesis and characterization of thin film nanocomposite forward osmosis membranes supported by silica nanoparticle incorporated nanofibrous substrate, Desalination, 2017, 401, 142–150 CrossRef CAS.
- J.-W. Zhang, et al., Preparation of silicon nitride hollow fibre membrane for desalination, Mater. Lett., 2012, 68, 457–459 CrossRef CAS.
- H. Abadikhah, et al., Amino functionalized silica nanoparticles incorporated thin film nanocomposite membrane with suppressed aggregation and high desalination performance, Polymer, 2018, 154, 200–209 CrossRef CAS.
- G. M. Urper-Bayram, et al., Comparative impact of SiO2 and TiO2 nanofillers on the performance of thin-film nanocomposite membranes, J. Appl. Polym. Sci., 2020, 137(44), 49382 CrossRef CAS.
- D. Zhang, et al., Development of robust and high-performance polysilsesquioxane reverse osmosis membranes modified by SiO2 nanoparticles for water desalination, Sep. Purif. Technol., 2022, 296, 121421 CrossRef CAS.
- S. H. Kim, et al., Design of TiO2 nanoparticle self-assembled aromatic polyamide thin-film-composite (TFC) membrane as an approach to solve biofouling problem, J. Membr. Sci., 2003, 211(1), 157–165 CrossRef CAS.
- M. A. Ahmed and A. A. Mohamed, Advances in ultrasound-assisted synthesis of photocatalysts and sonophotocatalytic processes: A review, iScience, 2023, 27(1), 108583 CrossRef PubMed.
- R. Zhang, et al., Antifouling membranes for sustainable water purification: strategies and mechanisms, Chem. Soc. Rev., 2016, 45(21), 5888–5924 RSC.
- R. A. Damodar, S.-J. You and H.-H. Chou, Study the self cleaning, antibacterial and photocatalytic properties of TiO2 entrapped PVDF membranes, J. Hazard Mater., 2009, 172(2–3), 1321–1328 CrossRef CAS PubMed.
- Y. Guo, et al., Contemporary antibiofouling modifications of reverse osmosis membranes: State-of-the-art insights on mechanisms and strategies, Chem. Eng. J., 2022, 429, 132400 CrossRef CAS.
- A. M. A. El-Aassar, Improvement of reverse osmosis performance of polyamide thin-film composite membranes using TiO2 nanoparticles, Desalination Water Treat., 2015, 55(11), 2939–2950 CAS.
- B. Khorshidi, et al., Robust fabrication of thin film polyamide-TiO2 nanocomposite membranes with enhanced thermal stability and anti-biofouling propensity, Sci. Rep., 2018, 8(1), 784 CrossRef PubMed.
- J. Schneider, et al., Understanding TiO2 photocatalysis: mechanisms and materials, Chem. Rev., 2014, 114(19), 9919–9986 CrossRef CAS PubMed.
- Y. Yu, et al., Purifying water with silver nanoparticles (AgNPs)-incorporated membranes: Recent advancements and critical challenges, Water Res., 2022, 118901 CrossRef CAS PubMed.
- T. C. Dakal, et al., Mechanistic basis of antimicrobial actions of silver nanoparticles, Front. Microbiol., 2016, 7, 1831 Search PubMed.
- S.-H. Park, et al., Direct incorporation of silver nanoparticles onto thin-film composite membranes via arc plasma deposition for enhanced antibacterial and permeation performance, J. Membr. Sci., 2016, 513, 226–235 CrossRef CAS.
- M. A. Ahmed and A. A. Mohamed, The use of chitosan-based composites for environmental remediation: A review, Int. J. Biol. Macromol., 2023, 124787 CrossRef CAS PubMed.
- M. R. Khan, et al., Schottky barrier and surface plasmonic resonance phenomena towards the photocatalytic reaction: study of their mechanisms to enhance photocatalytic activity, Catal. Sci. Technol., 2015, 5(5), 2522–2531 RSC.
- A. Amirjani and D. F. Haghshenas, Ag nanostructures as the surface plasmon resonance (SPR)- based sensors: a mechanistic study with an emphasis on heavy metallic ions detection, Sensor. Actuator. B Chem., 2018, 273, 1768–1779 CrossRef CAS.
- J. Nikkola, et al., Surface modification of thin film composite RO membrane for enhanced anti-biofouling performance, J. Membr. Sci., 2013, 444, 192–200 CrossRef CAS.
- H. Karkhanechi, et al., Improvement of antibiofouling performance of a reverse osmosis membrane through biocide release and adhesion resistance, Sep. Purif. Technol., 2013, 105, 106–113 CrossRef CAS.
- I. Sawada, et al., Development of a hydrophilic polymer membrane containing silver nanoparticles with both organic antifouling and antibacterial properties, J. Membr. Sci., 2012, 387, 1–6 CrossRef.
- J. Yin, et al., Attachment of silver nanoparticles (AgNPs) onto thin-film composite (TFC) membranes through covalent bonding to reduce membrane biofouling, J. Membr. Sci., 2013, 441, 73–82 CrossRef CAS.
- Z. Yang, et al., Hydrophilic silver nanoparticles induce selective nanochannels in thin film nanocomposite polyamide membranes, Environ. Sci. Technol., 2019, 53(9), 5301–5308 CrossRef CAS PubMed.
- I. Karmal, et al., Structural and morphological characterization of scale deposits on the reverse osmosis membranes: case of brackish water demineralization station in Morocco, Groundw. Sustain. Dev., 2020, 11, 100483 CrossRef.
- M. A. Ahmed, M. A. Ahmed and A. A. Mohamed, Removal of 4-nitrophenol and indigo carmine dye from wastewaters by magnetic copper ferrite nanoparticles: Kinetic, thermodynamic and mechanistic insights, J. Saudi Chem. Soc., 2023, 27(6), 101748 CrossRef CAS.
- M. A. Ahmed, M. A. Ahmed and A. A. Mohamed, Fabrication of NiO/g-C3N4 Z-scheme heterojunction for enhanced photocatalytic degradation of methylene blue dye, Opt. Mater., 2024, 151, 115339 CrossRef CAS.
- L. Reimer, Scanning electron microscopy: physics of image formation and microanalysis, Meas. Sci. Technol., 2000, 11(12), 1826 CrossRef.
- S. S. Manickam and J. R. McCutcheon, Understanding mass transfer through asymmetric membranes during forward osmosis: A historical perspective and critical review on measuring structural parameter with semi-empirical models and characterization approaches, Desalination, 2017, 421, 110–126 CrossRef CAS.
- Q. Song, et al., Mechanism insights into the role of the support mineralization layer toward ultrathin polyamide nanofilms for ultrafast molecular separation, J. Mater. Chem. A, 2021, 9(46), 26159–26171 RSC.
- S. Habib and S. T. Weinman, A review on the synthesis of fully aromatic polyamide reverse osmosis membranes, Desalination, 2021, 502, 114939 CrossRef CAS.
- K. Zarshenas, et al., Atomic scale manipulation of sublayer with functional TiO2 nanofilm toward high-performance reverse osmosis membrane, Desalination, 2020, 480, 114342 CrossRef CAS.
- B. Rodríguez, et al., Antibiofouling thin-film composite membranes (TFC) by in situ formation of Cu-(m-phenylenediamine) oligomer complex, J. Mater. Sci., 2018, 53(9), 6325–6338 CrossRef.
- L. Liu, et al., Effect of MCM-48 nanoparticles on the performance of thin film nanocomposite membranes for reverse osmosis application, Desalination, 2016, 394, 72–82 CrossRef CAS.
- M. A. Ahmed and A. A. Mohamed, A systematic review of layered double hydroxide-based materials for environmental remediation of heavy metals and dye pollutants, Inorg. Chem. Commun., 2023, 148, 110325 CrossRef CAS.
- G. L. Jadav and P. S. Singh, Synthesis of novel silica-polyamide nanocomposite membrane with enhanced properties, J. Membr. Sci., 2009, 328(1–2), 257–267 CrossRef CAS.
- O. W. Guirguis and M. T. Moselhey, Thermal and structural studies of poly (vinyl alcohol) and hydroxypropyl cellulose blends, Nat. Sci., 2012, 4, 57–67 CAS.
- R. Shami, et al., Synergistic effect of GO/ZnO loading on the performance of cellulose acetate/chitosan blended reverse osmosis membranes for NOM rejection, Heliyon, 2023, 9(3), e13736 CrossRef CAS PubMed.
- T. H. Lee, et al., Facile preparation of polyamide thin-film nanocomposite membranes using spray-assisted nanofiller predeposition, Ind. Eng. Chem. Res., 2019, 58(10), 4248–4256 CrossRef CAS.
- Z. C. Ng, W. J. Lau and A. F. Ismail, GO/PVA-integrated TFN RO membrane: Exploring the effect of orientation switching between PA and GO/PVA and evaluating the GO loading impact, Desalination, 2020, 496, 114538 CrossRef CAS.
- B. Rajaeian, et al., Fabrication and characterization of polyamide thin film nanocomposite (TFN) nanofiltration membrane impregnated with TiO2 nanoparticles, Desalination, 2013, 313, 176–188 CrossRef CAS.
- F. Asempour, et al., Chlorine-resistant TFN RO membranes containing modified poly (amidoamine) dendrimer-functionalized halloysite nanotubes, J. Membr. Sci., 2021, 623, 119039 CrossRef CAS.
- I. Tavakol, et al., Synthesis of novel thin film composite (TFC) forward osmosis (FO) membranes incorporated with carboxylated carbon nanofibers (CNFs), J. Environ. Chem. Eng., 2020, 8(6), 104614 CrossRef CAS.
- S. Karki and P. G. Ingole, Development of polymer-based new high performance thin-film nanocomposite nanofiltration membranes by vapor phase interfacial polymerization for the removal of heavy metal ions, Chem. Eng. J., 2022, 446, 137303 CrossRef CAS.
- D. L. Zhao, et al., Emerging thin-film nanocomposite (TFN) membranes for reverse osmosis: A review, Water Res., 2020, 173, 115557 CrossRef CAS PubMed.
- Z. Yang, et al., A critical review on thin-film nanocomposite membranes with interlayered structure: mechanisms, recent developments, and environmental applications, Environ. Sci. Technol., 2020, 54(24), 15563–15583 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |