Open Access Article
Open Access ArticleSilver incorporated SeTe nanoparticles with enhanced photothermal and photodynamic properties for synergistic effects on anti-bacterial activity and wound healing†
Irfan Ullah‡
 a,
Shahin Shah Khan‡a,
Waqar Ahmada,
Luo Liua,
Ahmed Radyb,
Badr Aldahmashb,
Changyuan Yu
a,
Shahin Shah Khan‡a,
Waqar Ahmada,
Luo Liua,
Ahmed Radyb,
Badr Aldahmashb,
Changyuan Yu *a and
Yushu Wang*c
*a and
Yushu Wang*c
aCollege of Life Science and Technology, Beijing University of Chemical Technology, No. 15 East Road of North Third Ring Road, Chao Yang District, Beijing 100029, China. E-mail: yucy@mail.buct.edu.cn
bDepartment of Zoology, College of Science, King Saud University, P. O. Box 2455, Riyadh 11451, Saudi Arabia
cSchool of Pharmaceutical Sciences, Southern Medical University, No. 1023, South Shatai Road, Guangzhou, 510515, P. R. China. E-mail: wysmjeda@gmail.com
First published on 12th June 2024
Abstract
Bacteria invade the host's immune system, thereby inducing serious infections. Current treatments for bacterial infections mostly rely on single modalities, which cannot completely inhibit bacteria. This study evaluates the therapeutic potential of SeTe–Ag NPs, designed with excellent photo responsiveness, with a particular focus on their dual-action antibacterial effect and wound healing properties. SeTe–Ag NPs exhibited promising synergistic antibacterial effects due to their superior photothermal and photodynamic properties. The investigation records substantial zones of inhibition of bacteria, demonstrating potent antibacterial effect. Furthermore, upon the irradiation of near-infrared (NIR) light, SeTe–Ag NPs exhibit remarkable antibiofilm and wound-healing capabilities. Overall, this study shows the applications of NIR-active SeTe–Ag NPs, which serve as a versatile platform for biomedical applications.
1. Introduction
The emergence of a large number of pathogenic bacteria resistant to antibiotics poses a serious concern for human health.1–4 Furthermore, the formation of bacterial biofilms hinders drug penetration, necessitating elevated drug dosages, which can lead to adverse effects from antibiotic overdosing.5 Therefore, a safe and effective treatment strategy is highly desired for the treatment of drug-resistant pathogens.Photothermal therapy (PTT) and photodynamic therapy (PDT) rely on the absorption of light by photosensitizers to generate heat or reactive oxygen species (ROS) for disinfection and biofilm destruction.6–11 However, when used independently, these methods often necessitate higher temperatures or dosages. For instance, PTT typically requires temperatures exceeding 60 °C, which can adversely affect normal tissues.8,12 Similarly, PDT can be hindered by the hypoxic conditions within biofilms, necessitating higher dosages of photosensitizers.13–16 Hence, combining different antibacterial mechanisms in one system can reduce side effects and enhance antibacterial efficacy.17–20
The antibacterial activity of Ag NPs depends on their size. Smaller-sized NPs show better activities as compared to larger-sized NPs.21,22 Ag NPs can reduce the duration of the wound inflammation stage and promote tissue repair and regeneration.23–25 Furthermore, selenium (Se) and tellurium (Te) NPs have demonstrated microbicidal activities.26 The modification of Te NPs enhances biocompatibility and confirms their therapeutic effectiveness in biological applications. Excellent nanoenzyme activity was shown by Te nanorods, which enhanced the phototherapeutic effect.27 Bimetallic SeTe alloy NPs exhibit distinctive characteristics, exceptional photostability, and good absorbance due to their energy bandgaps, which allow them to absorb light in the visible and near-infrared regions.28 Therefore, the combination of SeTe NPs and Ag NPs can exhibit synergistic effects against pathogenic bacteria and for wound healing.
Herein, we developed composite SeTe–Ag NPs that exhibit combined photothermal and photodynamic activity as dual-mode therapy against bacterial infection. Such a synergistic effect greatly improves antibacterial effect (Fig. 1). These NPs have photothermal and photodynamic effects, which can not only eradicate Gram-positive Staphylococcus aureus (S. aureus) and Gram-negative Escherichia coli (E. coli) by their synergetic effect but can also eradicate biofilm-associated pathogens. Furthermore, SeTe–Ag NPs also promote wound healing. Overall, SeTe–Ag NPs serve as a versatile platform to treat bacterial infections.
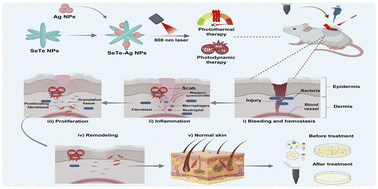 | ||
| Fig. 1 Schematic illustration showing the synthesis of SeTe–Ag NPs and their antibacterial activity and wound healing mechanism using photothermal and photodynamic therapy. | ||
2. Materials and methods
2.1. Materials and bacterial strains
All chemicals used in this work were purchased from Sigma-Aldrich, St. Louis, MO, USA., except telluric acid (H6TeO6), sodium selenite (Na2SeO3), ascorbic acid, hydrazine, cetyltrimethylammonium bromide (CTAB), 2,2,6,6-tetramethylpiperidine (TEMP), 5,5-dimethyl-1-pyrroline-N-oxide (DMPO), 4,6-diamidino-2-phenylindole (DAPI), 2,7-dichlorofluorescein diacetate (DCFH-DA). Ag NPs (CAS: 7440-22-4, 60–120 nm) were acquired from Shanghai Yien Chemical Technology Co., Ltd. Bacteria strains S. aureus (ATCC6538) and E. coli (ATCC8739) were obtained from China General Microbiological Culture Collection Center, Chinese Academy of Sciences.2.2. Synthesis of SeTe NPs and SeTe–Ag NPs
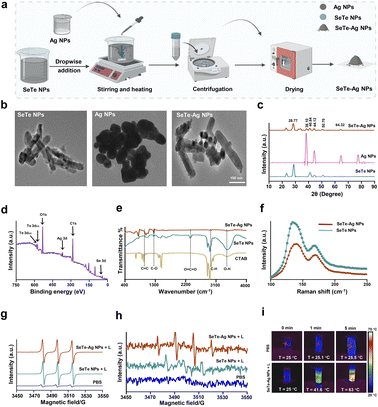
SeTe NPs were synthesized by mixing two different reducing agents, i.e., hydrazine and ascorbic acid. Briefly, sodium selenite (20 mM) and telluric acid (20 mM) were separately prepared in the presence of CTAB (2 mg mL−1), and the final volume of the solution was raised to 100 mL. This was followed by sonication for 30 min and constant stirring in an oil bath at 250 rpm for 3 h at 95 °C. Subsequently, a mixture of two reducing agents [ascorbic acid (1 g) and hydrazine (500 μL)] prepared in 10 mL of dH2O was added slowly to the above reaction and maintained for 30 min at 95 °C. Finally, a change in color appeared abruptly, from colorless to deep grey. This was followed by collection and purification of the product using centrifugation and drying overnight at 60 °C. Afterward, CTAB-mediated SeTe NPs were dissolved in dH2O (0.3 g of the NPs in 30 mL) with sonication for 30 min. Subsequently, Ag NPs (2 mM in 10 mL) were mixed with sonication for 30 min and dropwise added to the as-synthesized SeTe NPs solution. The solution was stirred vigorously at 75 °C for 2 h, followed by adding 3 mL of hydrazine to reduce the free materials in the solution. Finally, SeTe–Ag NPs were collected and purified using centrifugation at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 minutes with 50% ethanol three times and dried overnight at 60 °C in an incubator (Fig. 2a).
000 rpm for 10 minutes with 50% ethanol three times and dried overnight at 60 °C in an incubator (Fig. 2a).
2.3. In vitro anti-bacteria and antibiofilm study
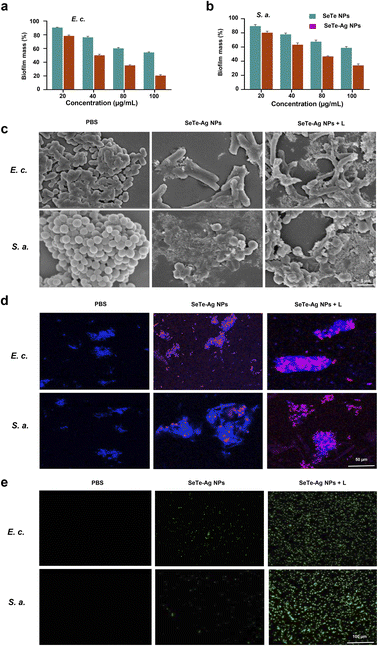
The bactericidal activities of SeTe and SeTe–Ag NPs were analyzed using S. aureus and E. coli bacterial strains. Luria–Bertani (LB) broth medium was used for bacteria culture, and SeTe NPs and SeTe–Ag NPs were added in different concentrations, i.e., 14, 28, 56, and 112 μg mL−1. The bacteria and NPs mixture were irradiated with an 808 nm laser (1 W cm−2) for 5 min. Then, the mixture was incubated for 2 h at 37 °C, followed by plating 100 μL aliquot and culturing overnight at 37 °C. To find the growth curve pattern of bacteria under the influence of NPs, bacteria with an OD of 0.4 were treated with different concentrations of NPs and cultured for 10 h, and OD600 was recorded every hour.29 Furthermore, for antibiofilm assay, bacterial culture of OD600, 0.025, was added to 96 well plate and grown for 24 h at 37 °C. When the biofilms were formed on the walls, the media was removed by gently inverting the plate, and PBS was used to wash the biofilm and remove the planktonic cells. Afterward, different concentrations of SeTe NPs and SeTe–Ag NPs prepared in PBS (20, 40, 80, and 100 μg mL−1) were added to the wells and incubated, while taking PBS treated biofilm as negative control. Then, methanol was added to fix the biofilm for 15 min and treated for 15 min with crystal violet (0.5%) prepared in PBS. Sterile PBS was used to wash the biofilm, and 33% acetic acid (v/v) was used to dissolve the crystal violet with gentle shaking for 10 min. To quantify the biofilm, the microplate reader was used to check the absorbance at 590 nm.302.4. Living/dead bacteria fluorescence detection
The live/dead assay was used to visualize bacterial cells. A 1.5 mL of bacterial cell culture was collected and washed with PBS, followed by treatment with 56 μg mL−1, SeTe NPs, and SeTe–Ag NPs, and irradiated with an 808 nm laser with a power of 1 W cm−2 for 10 minutes. After culturing the mixture for 1 h, fluorescence dye DAPI and propidium iodide (PI) were added, incubated for 15 min, and washed 3 times with PBS. For sample observation, an inverted fluorescent microscope (Leica DMI, 4000B, Danaher, Duesseldorf, Germany) was used to take photographs.2.5. Intracellular ROS detection of bacteria
An intracellular ROS dye DCFH-DA (10 μM) was added to 20 mL of 0.9% NaCl solution and co-incubated for 30 min with 106 CFU mL−1 of bacteria. Afterward, the solution was centrifuged for 5 min at 8000 rpm to remove the combined DCFH-DA. The bacterial cell treatment was performed as follows: PBS, PBS + L, SeTe–Ag NPs, SeTe–Ag NPs + L. The treatment with NIR laser (808 nm, 1 W cm−2) lasted for 5 min and incubated for 4 h at 37 °C. The intensity of the fluorescence was recorded with a fluorometer, observed, and photographed using a confocal laser scanning microscope (CLSM).2.6. SEM characterization of bacteria
The overnight-grown bacterial cells were harvested, washed three times, and incubated for 1 h with 56 μg mL−1 of SeTe NPs and SeTe–Ag NPs for SEM analysis. All the samples were collected after irradiation with NIR laser for 10 min, centrifuged at 5000 rpm, and washed with PBS. This was followed by fixing the cells overnight with glutaraldehyde solution (2.5%) at 4 °C and subsequently dehydrated with different concentrations of ethanol. The samples were photographed using a scanning electron microscope (SEM, Hitachi SU, 8080, Tokyo, Japan).2.7. Biocompatibility evaluation
The biocompatibility of SeTe–Ag NPs was assessed using a hemolysis test. Briefly, fresh mouse blood was collected, centrifuged for 15 min at 1500 rpm to concentrate the red blood cells, and washed three times with normal saline. The concentrated red blood cells were diluted to 5% and treated with PBS, water, and different concentrations of SeTe NPs and SeTe–Ag NPs, i.e., 25, 50, and 100 μg mL−1, incubated at 37 °C for 3 h, followed by centrifugation at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min. The supernatant was collected, and absorbance was recorded at 540 nm. The rate of hemolysis of SeTe–Ag NPs was calculated using PBS and water-treated groups as control.
000 rpm for 5 min. The supernatant was collected, and absorbance was recorded at 540 nm. The rate of hemolysis of SeTe–Ag NPs was calculated using PBS and water-treated groups as control.
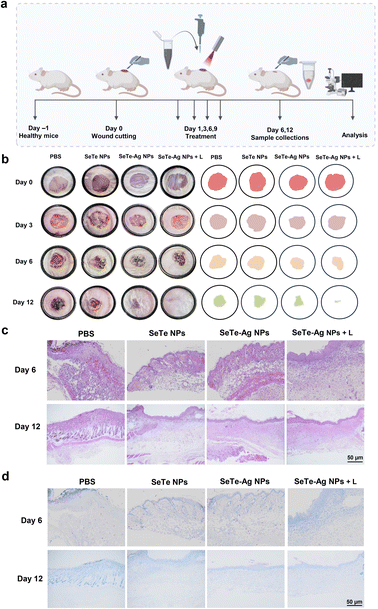
2.8. Promotion of wound healing in vivo
All animal experiments reported in this study were conducted in accordance with the guidelines (ZYZY202209005S) and were assessed and approved by the Institutional Animal Care and Use Committee of Sino Research (Beijing) Biotechnology Co., Ltd. (China).In vivo tests on wound healing were carried out on female BALB/c mice (20 ± 2 g, 5–6 weeks). All mice were subjected to skin wound creation using the reported method.12 For the aseptic server, dorsal hairs were removed using 7% sodium sulfide and then disinfected using 75% ethanol. A round wound with a diameter of 8 mm was made on the back of each mouse using a hole puncher and infected with 10 μL of bacteria with a 10−6 dilution factor. After 24 h, 20 μL of the synthesized NPs were added to the wounds of the corresponding groups. The synthesized NPs were applied every second day. The images of the wounds of each mouse were photographed with a camera. The wound size was calculated using ImageJ software. The wound healing rate (%) was calculated using the formula:
3. Results and discussions
3.1. Characterization of SeTe–Ag NPs
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.31 The band at 1045.21 cm−1 belongs to the C–O–C stretching vibration of the alkoxy group in SeTe NPs, where a slight shifting occurs to 1085 cm−1 in SeTe–Ag NPs, thus suggesting the presence of Ag NPs on the surface of CTAB mediated SeTe NPs.32 The IR band at 2337.30 cm−1 corresponds to the CO2 vibration line in SeTe NPs, while in SeTe–Ag NPs, it shifted to 2339 cm−1, a very negligible peak. The symmetrical and asymmetrical stretching vibrations of the methylene group of aliphatic compounds were responsible for the two prominent bands at 2959 cm−1 and 2840 cm−1, respectively.33
O.31 The band at 1045.21 cm−1 belongs to the C–O–C stretching vibration of the alkoxy group in SeTe NPs, where a slight shifting occurs to 1085 cm−1 in SeTe–Ag NPs, thus suggesting the presence of Ag NPs on the surface of CTAB mediated SeTe NPs.32 The IR band at 2337.30 cm−1 corresponds to the CO2 vibration line in SeTe NPs, while in SeTe–Ag NPs, it shifted to 2339 cm−1, a very negligible peak. The symmetrical and asymmetrical stretching vibrations of the methylene group of aliphatic compounds were responsible for the two prominent bands at 2959 cm−1 and 2840 cm−1, respectively.333.2. Photodynamic and photothermal analysis
3.3. Enhanced antibacterial and antibiofilm activity
3.4. Antibacterial mechanism
There is a close association of oxidative stress with the bactericidal process of Ag NPs.43 ROS is involved in pathological and physiological processes, including apoptosis.44 It is reported that the activity of Ag NPs with a size below 10 nm is because of the nanoparticles themselves. In fact, the small-sized Ag NPs adhere to the bacterial surface, altering the permeability and entering inside the bacteria, causing damage to various targets. However, for large-size NPs, the silver ion release is the main mechanism. Various studies reported that silver ions kill bacteria by up-regulating ROS levels.45,46 Therefore, the change in ROS level by SeTe NPs, SeTe–Ag NPs, and SeTe–Ag NPs + L was evaluated, as shown in Fig. 3e, and the corresponding fluorescence activities are mentioned in Fig. S7.† The green fluorescence increased gradually in the order of SeTe NPs, SeTe–Ag NPs, and SeTe–Ag NPs + L, showing an increase in ROS production. Furthermore, these results confirm inducing hydroxyl radicals by SeTe–Ag NPs + L. Due to the production of basal level of ROS as a respiratory product by cells, the Ag (0) can be oxidized to Ag+, which further increases the production of ROS to damage the subcellular components.47 The induced oxidative stress of the cell is likely responsible for bacterial apoptosis, and the higher level of ROS can affect the function and activity of the bacterial biomacromolecules. From these results, it is clear that the bactericidal effect of SeTe–Ag NPs was due to the induced production of ROS.3.5. In vivo antibacterial activity and wound healing
4. Conclusions
In this study, hybrid NPs were synthesized using the one-pot synthesis method for combating both the Gram-positive and Gram-negative bacterial strains. The results reveal that appropriately combining the NPs with NIR laser enhances the therapeutic efficacy compared to the individual components of the NPs. In comparison with the bactericidal efficiency of Ag NPs alone, the composite NPs showed a pronounced and combinational bactericidal effect. Furthermore, the synthesized SeTe–Ag NPs exhibited higher stability and good biocompatibility. The mechanism of activity exploration of the combination bactericidal strategy shows that the designed nanomaterial disturbs cell integrity, generating ROS and damaging the cell membrane of bacteria. In vivo studies showed improved wound healing in mice. Overall, synergistic activities using dual or multiple nanomaterials could significantly reduce the dosage required for traditional antibiotics, reduce toxicity effects, and screen effective candidates for the treatment of drug-resistant bacterial infections.Author contributions
Irfan Ullah: experimental work, writing the original draft. Shahin Shah Khan: conceptualization, experimental work, data analysis, and reviewing the draft. Waqar Ahmad: formal analysis, Liu Luo: conceptualization, draft modification. Ahmed Rady: conceptualization, Badr Aldahmash: conceptualization, Changyuan Yu: conceptualization, supervision, and reviewing the draft. Yushu Wang: conceptualization, reviewing the draft.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support of the Natural Science Foundation of China project (52103084), National High Level Hospital Clinical Research Funding (XK2023-13), Scientific and Technological Research Project of Xinjiang Production and Construction Corps (grant number 2022AB022), China Postdoctoral Science Foundation (2021M701591) and Research Supporting Project No. (RSP2024R214) King Saud University, Riyadh, Saudi Arabia.References
- C. J. Murray, K. S. Ikuta, F. Sharara, L. Swetschinski, G. R. Aguilar, A. Gray, C. Han, C. Bisignano, P. Rao and E. Wool, Lancet, 2022, 399, 629–655 CrossRef CAS PubMed.
- Z. Wang, B. Koirala, Y. Hernandez, M. Zimmerman, S. Park, D. S. Perlin and S. F. Brady, Nature, 2022, 601, 606–611 CrossRef CAS PubMed.
- Y. Yu, F. Bu, H. Zhou, Y. Wang, J. Cui, X. Wang, G. Nie and H. Xiao, Mater. Chem. Front., 2020, 4, 1930–1953 RSC.
- J. Zhang, H. Guo, M. Liu, K. Tang, S. Li, Q. Fang, H. Du, X. Zhou, X. Lin and Y. Yang, Exploration, 2023, 20230087 Search PubMed.
- M. Berditsch, S. Afonin, J. Reuster, H. Lux, K. Schkolin, O. Babii, D. S. Radchenko, I. Abdullah, N. William and V. Middel, Sci. Rep., 2019, 9, 17938 CrossRef PubMed.
- Y. Wang, H. Yao, Y. Zu and W. Yin, RSC Adv., 2022, 12, 8862–8877 RSC.
- Y. Zhao, P. He, J. Yao, M. Li, B. Wang, L. Han, Z. Huang, C. Guo, J. Bai and F. Xue, Biomaterials, 2023, 301, 122237 CrossRef CAS PubMed.
- H. Zhou, D. Tang, X. Kang, H. Yuan, Y. Yu, X. Xiong, N. Wu, F. Chen, X. Wang and H. Xiao, Adv. Sci., 2022, 9, 2200732 CrossRef CAS PubMed.
- Y. Yu, D. Tang, C. Liu, Q. Zhang, L. Tang, Y. Lu and H. Xiao, Adv. Mater., 2022, 34, 2105976 CrossRef CAS PubMed.
- L. Wang, Y. Yu, D. Wei, L. Zhang, X. Zhang, G. Zhang, D. Ding, H. Xiao and D. Zhang, Adv. Mater., 2021, 33, 2100599 CrossRef CAS PubMed.
- M. Shen, Y. Wang, T. Bing, Y. Tang, X. Liu and Y. Yu, Adv. Funct. Mater., 2023, 33, 2307013 CrossRef CAS.
- H. L. Zhou, D. S. Tang, X. X. Kang, H. T. Yuan, Y. J. Yu, X. L. Xiong, N. E. Wu, F. Z. Chen, X. Wang, H. H. Xiao and D. S. Zhou, Adv. Sci., 2022, 9, 2200732 CrossRef CAS PubMed.
- H. X. Zhang, Y. Zou, K. Y. Lu, Y. Wu, Y. C. Lin, J. J. Cheng, C. X. Liu, H. Chen, Y. X. Zhang and Q. Yu, J. Mater. Sci. Technol., 2024, 169, 209–219 CrossRef CAS.
- T. Du, Z. Xiao, G. Zhang, L. Wei, J. Cao, Z. Zhang, X. Li, Z. Song, W. Wang and J. Liu, Acta Biomater., 2023, 161, 112–133 CrossRef CAS PubMed.
- M. Qi, X. Ren, W. Li, Y. Sun, X. Sun, C. Li, S. Yu, L. Xu, Y. Zhou and S. Song, Nano Today, 2022, 43, 101447 CrossRef CAS.
- H. He, L. Du, H. Xue, J. Wu and X. Shuai, Acta Biomater., 2022, 149, 297–306 CrossRef CAS PubMed.
- J. Lv, Y. Qi, Y. Tian, G. Wang, L. Shi, G. Ning and J. Ye, Biomater. Sci., 2022, 10, 3747–3756 RSC.
- Y. Zhang, X.-F. Qu, C.-L. Zhu, H.-J. Yang, C.-H. Lu, W.-L. Wang, Y. Pang, C. Yang, L.-J. Chen and X.-F. Li, ACS Appl. Bio Mater., 2021, 4, 4821–4832 CrossRef CAS PubMed.
- G. Xiong, D. Huang, L. Lu, X. Luo, Y. Wang, S. Liu, M. Chen, S. Yu, M. Kappen and C. You, Small Methods, 2022, 6, 2200379 CrossRef CAS PubMed.
- B. Yu, Y. Wang, T. Bing, Y. Tang, J. Huang, H. Xiao, C. Liu and Y. Yu, Adv. Mater., 2024, 36, 2310456 CrossRef CAS PubMed.
- Y. Zhao, Z. Zhang, Z. Pan and Y. Liu, Exploration, 2021, 1, 20210089 CrossRef PubMed.
- S. Li, Y. Yang, S. Wang, Y. Gao, Z. Song, L. Chen and Z. Chen, Exploration, 2022, 2, 20210223 CrossRef CAS PubMed.
- S. Skanda, P. Bharadwaj, V. D. Darshan, V. Sivaramakrishnan and B. Vijayakumar, J. Microbiol. Methods, 2022, 199, 106517 CrossRef CAS PubMed.
- S. Wu, A. Li, X. Zhao, C. Zhang, B. Yu, N. Zhao and F.-J. Xu, ACS Appl. Mater. Interfaces, 2019, 11, 17177–17183 CrossRef CAS PubMed.
- J. Ma and C. Wu, Exploration, 2022, 2, 20210083 CrossRef CAS PubMed.
- W. Pan, C. Liu, Y. Li, Y. Yang, W. Li, C. Feng and L. Li, Bioact. Mater., 2022, 13, 96–104 CAS.
- S. Kang, Y.-G. Gil, D.-H. Min and H. Jang, ACS Nano, 2020, 14, 4383–4394 CrossRef CAS PubMed.
- L. D. Geoffrion and G. Guisbiers, Nanoscale Adv., 2021, 3, 4254–4270 RSC.
- S. S. Khan, I. Ullah, S. Zada, A. Ahmad, W. Ahmad, H. Xu, S. Ullah and L. Liu, Materials, 2022, 15, 4813 CrossRef CAS PubMed.
- Y. Yu, Y. Zhang, Y. Cheng, Y. Wang, Z. Chen, H. Sun, X. Wei, Z. Ma, J. Li and Y. Bai, Bioact. Mater., 2022, 13, 269–285 CAS.
- N. Alfryyan, M. G. Kordy, M. Abdel-Gabbar, H. A. Soliman and M. Shaban, Sci. Rep., 2022, 12, 12495 CrossRef PubMed.
- S. Ullah, A. Ahmad, F. Subhan, A. Jan, M. Raza, A. U. Khan, A.-U. Rahman, U. A. Khan, M. Tariq and Q. Yuan, J. Photochem. Photobiol., B, 2018, 183, 342–348 CrossRef CAS PubMed.
- A. Negash, S. Mohammed, H. D. Weldekirstos, A. D. Ambaye and M. Gashu, Sci. Rep., 2023, 13, 22234 CrossRef CAS PubMed.
- G. Vasiliev, A.-L. Kubo, H. Vija, A. Kahru, D. Bondar, Y. Karpichev and O. Bondarenko, Sci. Rep., 2023, 13, 9202 CrossRef CAS PubMed.
- S. Zada, W. Dai, Z. Kai, H. Lu, X. Meng, Y. Zhang, Y. Cheng, F. Yan, P. Fu and X. Zhang, Angew. Chem., Int. Ed., 2020, 59, 6601–6606 CrossRef CAS PubMed.
- Z. Liu, Z. Liu, Z. Zhao, D. Li, P. Zhang, Y. Zhang, X. Liu, X. Ding and Y. Xu, Nanomaterials, 2022, 12, 2469 CrossRef CAS PubMed.
- O. Tarawneh, H. Abu Mahfouz, L. Hamadneh, A. A. Deeb, I. Al-Sheikh, W. Alwahsh and A. Fadhil Abed, Sci. Rep., 2022, 12, 3900 CrossRef CAS PubMed.
- D. P. Linklater, V. A. Baulin, X. Le Guével, J. B. Fleury, E. Hanssen, T. H. P. Nguyen, S. Juodkazis, G. Bryant, R. J. Crawford and P. Stoodley, Adv. Mater., 2020, 32, 2005679 CrossRef PubMed.
- Y. Yu, R. Tian, Y. Zhao, X. Qin, L. Hu, J. J. Zou, Y. W. Yang and J. Tian, Adv. Healthcare Mater., 2023, 12, 2201651 CrossRef CAS PubMed.
- Y. Yu, D. Wei, T. Bing, Y. Wang, C. Liu and H. Xiao, Adv. Mater., 2024 DOI:10.1002/adma.202402452.
- Z. Li, S. Lu, W. Liu, T. Dai, J. Ke, X. Li, R. Li, Y. Zhang, Z. Chen and X. Chen, Angew. Chem., Int. Ed., 2021, 60, 19201–19206 CrossRef CAS PubMed.
- Z. Liu, X. Zhao, B. Yu, N. Zhao, C. Zhang and F.-J. Xu, ACS Nano, 2021, 15, 7482–7490 CrossRef CAS PubMed.
- G. Fang, W. Li, X. Shen, J. M. Perez-Aguilar, Y. Chong, X. Gao, Z. Chai, C. Chen, C. Ge and R. Zhou, Nat. Commun., 2018, 9, 129 CrossRef PubMed.
- X. Bi, Q. Bai, M. Liang, D. Yang, S. Li, L. Wang, J. Liu, W. W. Yu, N. Sui and Z. Zhu, Small, 2022, 18, 2104160 CrossRef CAS PubMed.
- P.-P. Li, Y. Zhang, C. Wang, S.-J. Wang, W.-Q. Yan, D.-X. Xiao, J. Kang, D.-Z. Yang, H.-X. Wu and A. Dong, Rare Met., 2023, 1–17 Search PubMed.
- N. Tripathi and M. K. Goshisht, ACS Appl. Bio Mater., 2022, 5, 1391–1463 CrossRef CAS PubMed.
- E. M. Mateo and M. Jiménez, Antibiotics, 2022, 11, 1205 CrossRef CAS PubMed.
- A. Chahardoli, F. Qalekhani, Y. Shokoohinia and A. Fattahi, Bull. Mater. Sci., 2022, 45, 88 CrossRef CAS.
- D. Wei, Y. Yu, X. Zhang, Y. Wang, H. Chen, Y. Zhao, F. Wang, G. Rong, W. Wang and X. Kang, ACS Nano, 2020, 14, 16984–16996 CrossRef CAS PubMed.
- H. Wang, Z. Xu, Q. Li and J. Wu, Eng. Regen., 2021, 2, 137–153 Search PubMed.
- P. Dam, M. Celik, M. Ustun, S. Saha, C. Saha, E. A. Kacar, S. Kugu, E. N. Karagulle, S. Tasoglu and F. Buyukserin, RSC Adv., 2023, 13, 21345–21364 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra01343c |
| ‡ I. Ulah and S. S. Khan contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |