Open Access Article
Open Access ArticleGlowing discoveries: Schiff base-cyanostilbene probes illuminating metal ions and biological entities
Afrin
A
,
Chinna Ayya
Swamy P
 * and
Angel
Rose
* and
Angel
Rose
Main Group Organometallics Optoelectronic Materials and Catalysis Lab, Department of Chemistry, National Institute of Technology, Calicut, 673601, India. E-mail: swamy@nitc.ac.in
First published on 12th August 2024
Abstract
Schiff bases featuring cyanostilbene units have emerged as versatile and highly effective probes for the selective detection of various metal ions as well as biologically important species. This review comprehensively highlights recent advances in the development and application of the probes, which exhibit remarkable Aggregation-Induced Emission (AIE), Twisted Intramolecular Charge Transfer (TICT), and Excited-State Intramolecular Proton Transfer (ESIPT) properties. These unique structural characteristics facilitate their potential applications in the detection of biologically important metal ions such as Zn2+, Fe3+, Cu2+, Hg2+ and Co2+ ions with high sensitivity and selectivity. Furthermore, these probes have demonstrated significant potential in the recognition of vital biological species, including arginine, hydrazine and hypochlorite (ClO−). The present review discusses the underlying detection mechanisms, emphasizing the role of the Schiff base and cyanostilbene moieties for the selective detection of particular biologically important entities. Moreover, this discussion highlights the practical applications, problems, and future directions in this fast-growing field, emphasizing the vital importance of these probes in both analytical chemistry and bioassays.
Introduction
Detecting metal ions in environmental and biological systems is crucial due to their significant roles in health and disease.1 Essential metals such as iron, copper, and zinc are vital for numerous biochemical processes, yet their imbalance can lead to toxicity and various health issues.2 In contrast, heavy metals such as lead, mercury, and cadmium are toxic even at low concentrations.3 Therefore, developing probes that are both effective and highly sensitive for metal ion detection is essential for monitoring and managing these ions. Among the various chemical sensors developed, Schiff bases stand out due to their imine (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) functional group.4–11 These compounds are known for their structural versatility, ease of synthesis, and strong affinity for binding with metal ions, making them excellent candidates for sensor development.12 Schiff bases are synthesized through the condensation of primary amines with carbonyl compounds and can be modified to enhance their electronic properties through different substitutions. Their applications are vast, including roles in catalysis,13–15 medicinal chemistry,16,17 and metal ion detection.18–20 The ability to tailor the Schiff bases to form complex structures enhances their sensitivity and binding properties towards specific metal ions, making them vital tools in this field.
N) functional group.4–11 These compounds are known for their structural versatility, ease of synthesis, and strong affinity for binding with metal ions, making them excellent candidates for sensor development.12 Schiff bases are synthesized through the condensation of primary amines with carbonyl compounds and can be modified to enhance their electronic properties through different substitutions. Their applications are vast, including roles in catalysis,13–15 medicinal chemistry,16,17 and metal ion detection.18–20 The ability to tailor the Schiff bases to form complex structures enhances their sensitivity and binding properties towards specific metal ions, making them vital tools in this field.
Recently, researchers have focused on incorporating cyanostilbene units into Schiff bases for metal ion detection, garnering considerable attention. Cyanostilbene derivatives are noted for their significant photophysical properties, such as twisted intramolecular charge transfer (TICT)21–23 and aggregation-induced emission (AIE).24–31 These properties greatly enhance the sensitivity and selectivity of probes, making them highly effective for metal ion detection. TICT facilitates fluorescence changes when metal ions bind, while AIE helps to prevent the common issue of fluorescence quenching in the aggregated state. Combining Schiff bases with cyanostilbene units results in highly responsive probes that exhibit noticeable fluorescence changes upon interacting with specific metal ions. The cyanostilbene moiety, featuring a conjugated system and an electron-withdrawing cyano group, improves the probe's sensitivity and selectivity toward metal ions. The mechanism of metal ion detection, typically involves coordination between the metal ions and the nitrogen or oxygen atoms in the Schiff base. This interaction leads to changes in the electronic properties of the cyanostilbene unit, causing observable fluorescence changes. These changes can be quantified to determine the presence and concentration of metal ions, making these probes highly valuable for analytical and biological applications.
This review provides an extensive overview of recent progress in the development of Schiff bases integrated with cyanostilbene units as fluorescent probes for selective and sensitive detection of biologically important metal ions. The studies collectively highlight the advancements and potential of these hybrid compounds, demonstrating their high sensitivity and selectivity. We begin by examining the molecular design strategies used to create Schiff base-cyanostilbene compounds. Key approaches include modifying Schiff base structures to improve metal ion binding and integrating cyanostilbene moieties to exploit their distinctive photophysical properties. The resulting structural diversity and tunability of these compounds are essential for optimizing their performance as metal ion sensors. Additionally, the review investigates into the practical applications of these probes, showcasing their effectiveness in detecting various metal ions such as Zn2+, Cu2+, Fe3+, and Hg2+, along with other significant species like hydrazine, ClO−, arginine. These examples underscore the versatility and practical utility of Schiff base-cyanostilbene probes in environmental and biological contexts. We also address the challenges faced in developing and applying these probes, including issues of stability, selectivity in complex environments, and the necessity for real-time and on-site detection capabilities. The review concludes with a forward-looking perspective, suggesting potential enhancements and innovations to further improve the performance and expand the applicability of Schiff base-cyanostilbene probes.
The versatility of Schiff base-based probes in metal ion detection: synthesis, tunability, and applications
Schiff bases, first synthesized by Hugo Schiff in 1864, are characterized by the presence of an azomethine group (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–).32 These compounds have garnered significant attention in various fields, particularly in the development of probes for metal ion detection.33–35 The versatility of Schiff base-based probes stems from their facile synthesis, structural flexibility, tunable properties, and strong coordination ability with metal ions, making them indispensable in analytical chemistry. One of the primary advantages of Schiff bases is their ease of synthesis, involving the condensation reaction between a primary amine and carbonyl compounds (usually aldehydes or ketones) to form an imine linkage (–C
N–).32 These compounds have garnered significant attention in various fields, particularly in the development of probes for metal ion detection.33–35 The versatility of Schiff base-based probes stems from their facile synthesis, structural flexibility, tunable properties, and strong coordination ability with metal ions, making them indispensable in analytical chemistry. One of the primary advantages of Schiff bases is their ease of synthesis, involving the condensation reaction between a primary amine and carbonyl compounds (usually aldehydes or ketones) to form an imine linkage (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) (Fig. 1). This reaction generally takes place under mild conditions, often with the aid of a dehydrating agent to remove the water produced during the process. This simple method allows for the rapid generation of a wide range of Schiff bases with diverse functional groups, enabling the fine-tuning of their chemical and physical properties. The azomethine group in Schiff bases is particularly effective as a ligand for coordinating with metal ions. The nitrogen atom in the imine linkage, along with other donor atoms such as oxygen or sulfur in the Schiff base structure, can form stable complexes with different metal ions, enhancing the probe's sensitivity and allowing for the detection of metal ions at very low concentrations. Furthermore, the formation of these complexes often leads to notable changes in the probe's optical properties, such as shifts in absorption or emission spectra, which can be easily tracked using spectroscopic techniques. The structural versatility of Schiff bases is crucial in designing probes for specific metal ions, as even minor structural modifications can greatly influence their selectivity and sensitivity. Schiff bases are highly adaptable in their photophysical and chemical properties. By altering the substituents on the amine or carbonyl components, researchers can adjust the electronic characteristics of the azomethine bond, affecting the probe's fluorescence, colorimetric response, and binding affinity towards metal ions. This adaptability is crucial for creating highly selective probes capable of differentiating between various metal ions in complex environments, which is particularly valuable in environmental monitoring, clinical diagnostics, and industrial applications.
N–) (Fig. 1). This reaction generally takes place under mild conditions, often with the aid of a dehydrating agent to remove the water produced during the process. This simple method allows for the rapid generation of a wide range of Schiff bases with diverse functional groups, enabling the fine-tuning of their chemical and physical properties. The azomethine group in Schiff bases is particularly effective as a ligand for coordinating with metal ions. The nitrogen atom in the imine linkage, along with other donor atoms such as oxygen or sulfur in the Schiff base structure, can form stable complexes with different metal ions, enhancing the probe's sensitivity and allowing for the detection of metal ions at very low concentrations. Furthermore, the formation of these complexes often leads to notable changes in the probe's optical properties, such as shifts in absorption or emission spectra, which can be easily tracked using spectroscopic techniques. The structural versatility of Schiff bases is crucial in designing probes for specific metal ions, as even minor structural modifications can greatly influence their selectivity and sensitivity. Schiff bases are highly adaptable in their photophysical and chemical properties. By altering the substituents on the amine or carbonyl components, researchers can adjust the electronic characteristics of the azomethine bond, affecting the probe's fluorescence, colorimetric response, and binding affinity towards metal ions. This adaptability is crucial for creating highly selective probes capable of differentiating between various metal ions in complex environments, which is particularly valuable in environmental monitoring, clinical diagnostics, and industrial applications.
Cyanostilbene derivatives: enhancing optoelectronics through unique properties
Compounds featuring cyanostilbene units have become significant components in the field of organic materials and optoelectronics, primarily due to their unique photophysical properties and versatile applications.36 These compounds, characterized by a distinctive twisted structure with a cyano group attached to a stilbene core, exhibit remarkable features that make them valuable in various areas. One of the most distinguished characteristics of cyanostilbene units is their exceptional photophysical properties. They exhibit strong fluorescence and high quantum yields in the solid state, making them excellent candidates for optoelectronic devices and fluorescence-based applications.37 Cyanostilbene units attached to donor moieties such as carbazole,38–40 triphenylamine,41,42 tetraphenylethene,43,44 and so on are particularly notable for their significant solvatochromism, where their emission color changes with the polarity of the solvent. This phenomenon is utilized in designing molecular sensors that can detect environmental changes by altering their fluorescence in response to different stimuli. The structural versatility of cyanostilbene units allows for extensive modifications, enabling the design of molecules with fine-tunable properties. The stilbene core can be functionalized with various substituents, altering the electronic and steric properties of the molecule. This design flexibility is crucial for developing new materials with specific characteristics, such as improved stability, solubility, or enhanced interaction with other molecules or surfaces. The ability to fine-tune their photophysical properties through chemical modifications further enhances the utility of cyanostilbene in various applications. In summary, cyanostilbene units' unique combination of strong fluorescence, high quantum yields, solvatochromism, and structural versatility makes them vital in the development of advanced materials for optoelectronic and sensing applications.45,46Advancements on Schiff-base with cyanostilbene conjugates
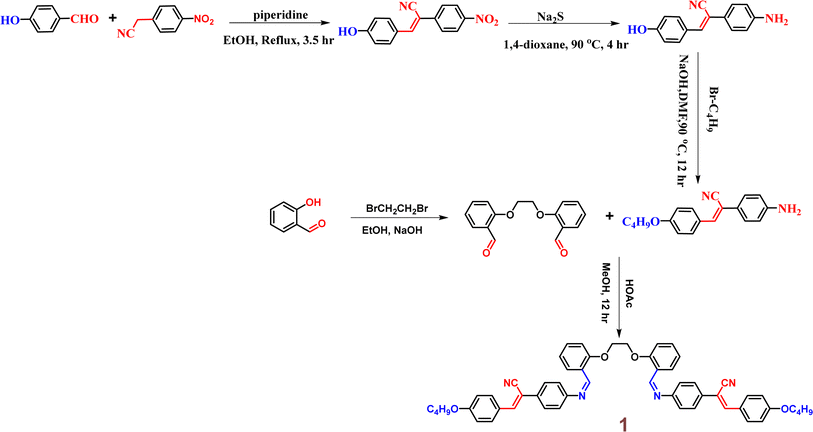
In 2014, Zhang et al. were the first to discover a novel Schiff base incorporating a cyanostilbene unit probe 1, for the selective detection of Hg2+ ions with aggregation-induced emission enhancement (AIEE) properties in THF/H2O mixtures (Fig. 2).47 The probe 1 was developed by following simple synthetic pathway as depicted in the Scheme 1. This tweezer-shaped 1 detects Hg2+ ions through a fluorescence turn-on response due to a configuration change and/or the formation of intermolecular aggregates. The selectivity of 1 towards Hg2+ ions were also confirmed by the UV-vis as well as fluorescence titration studies (Fig. 2). The binding ability of 1 with Hg2+ ions were further confirmed by 1H NMR spectroscopy. The Job's plot analysis revealed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry between 1 and Hg2+ ions. The association constant (K) and detection limit estimated by the Benesi–Hilderbrand equation as 1.5 × 105 M−1 and 2.4 × 10−5 M, respectively. The competitive binding studies in the presence of various metal ions showed no significant changes, thereby emphasizing the high selectivity of 1 towards Hg2+ ions even in the presence of other metal ions.
1 binding stoichiometry between 1 and Hg2+ ions. The association constant (K) and detection limit estimated by the Benesi–Hilderbrand equation as 1.5 × 105 M−1 and 2.4 × 10−5 M, respectively. The competitive binding studies in the presence of various metal ions showed no significant changes, thereby emphasizing the high selectivity of 1 towards Hg2+ ions even in the presence of other metal ions.
 | ||
| Fig. 2 (a) Emission spectra of probe 1 in THF–H2O mixtures; (b) change in the emission spectra of 1 upon increasing the concentrations of Hg2+ ions in THF; (c) absorption spectra of 1 upon increasing concentrations Hg2+ ions in THF. Reproduced with permission from ref. 47. Copyright 2014 Elsevier. | ||
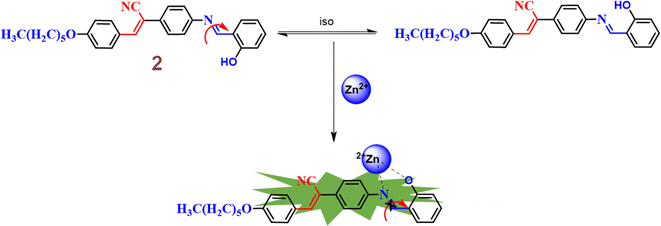
In the same year, the research group subsequently designed and synthesized a novel Schiff-base featuring an α-cyanostilbene unit 2, aimed at the selective detection of Zn2+ ions under physiological conditions.48 The sensing studies strongly suggested that the probe 2 effectively distinguishes Zn2+ ions from Cd2+ and Hg2+ ions (Fig. 4). The Job's plot demonstrated a binding ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for 2 and Zn2+ ions. The association constant and detection limit of 2 with Zn2+ were determined using the Benesi–Hilderbrand equation and calculated to be 2.317 × 105 M−1 and 0.1 μM, respectively. The fluorescence turn-on sensing mechanism of probe 2 for Zn2+ ions is primarily attributed to the restriction of C
1 for 2 and Zn2+ ions. The association constant and detection limit of 2 with Zn2+ were determined using the Benesi–Hilderbrand equation and calculated to be 2.317 × 105 M−1 and 0.1 μM, respectively. The fluorescence turn-on sensing mechanism of probe 2 for Zn2+ ions is primarily attributed to the restriction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization, which results in a chelation-enhanced fluorescence (CHEF) effect (Fig. 3). Upon binding with Zn2+ ions, the C
N isomerization, which results in a chelation-enhanced fluorescence (CHEF) effect (Fig. 3). Upon binding with Zn2+ ions, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond's rotational freedom is significantly reduced, leading to an enhanced fluorescence signal. This restriction prevents non-radiative decay pathways, allowing for a more efficient radiative emission, thus intensifying the fluorescence. The CHEF effect is a common phenomenon in metal ion sensing, where the coordination of the metal ion stabilizes the molecular structure and enhances the fluorescent properties of the sensor. Further, the probe 2 demonstrated a strong response towards the Zn2+ ions over a pH range of 6–12. In contrast, at pH 1 to 5, the fluorescence intensity was weak and gradually decreased with lower pH levels. This results strongly suggests that the sensing behavior of 2 towards Zn2+ ions is effective in alkaline, neutral, and mildly acidic conditions.
N bond's rotational freedom is significantly reduced, leading to an enhanced fluorescence signal. This restriction prevents non-radiative decay pathways, allowing for a more efficient radiative emission, thus intensifying the fluorescence. The CHEF effect is a common phenomenon in metal ion sensing, where the coordination of the metal ion stabilizes the molecular structure and enhances the fluorescent properties of the sensor. Further, the probe 2 demonstrated a strong response towards the Zn2+ ions over a pH range of 6–12. In contrast, at pH 1 to 5, the fluorescence intensity was weak and gradually decreased with lower pH levels. This results strongly suggests that the sensing behavior of 2 towards Zn2+ ions is effective in alkaline, neutral, and mildly acidic conditions.
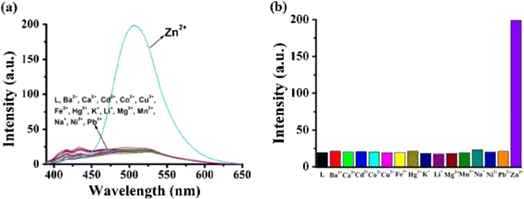 | ||
| Fig. 4 (a) Emission spectra of probe 2 alone and with different metal ions. (b) Fluorescent intensity of 2 at 509 nm with different metal ions. Reproduced with permission from ref. 48. Copyright 2014 Springer. | ||
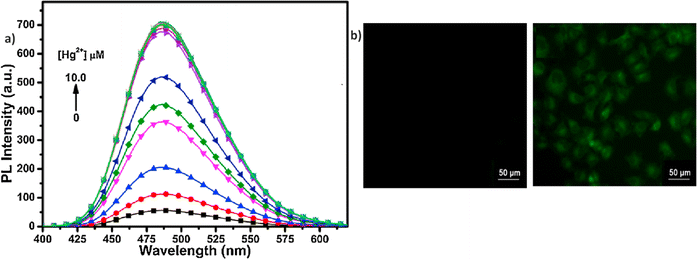
In 2015, authors combined a coumarin unit with a cyanostilbene-containing Schiff base to design and synthesize probe 3 (Fig. 5), which exhibits selective detection of Hg2+ ions over other competitive metal ions.49 The probe 3 exhibited excellent AIEE ability due to the synergistic effect of intramolecular planarization of the α-cyanostilbene unit and the formation of excimers between molecular dimers. The nitrogen and oxygen atoms in the structure provide binding sites for Hg2+ coordination, leading to the aggregation of probe 3 and resulting in a fluorescence turn-on response (Fig. 6). The coordination with Hg2+ ions induces molecular aggregation, which restricts intramolecular rotations and vibrations, thereby enhancing the fluorescence emission through the AIEE mechanism. This probe has proven to be effective for detecting Hg2+ in both aqueous environments and in vivo biological systems (Fig. 6). In 2016, the same team designed another AIE-active probe, probe 4 (Fig. 5), featuring a linear conjugated bis-Schiff base for the selective detection of Hg2+ ions (Fig. 7).50 The unique color shifts and fluorescence emission of Hg2+ ions in THF and THF/H2O mixtures provided a quick and simple method for discriminating Hg2+ ions using the naked eye or a UV lamp. Probe 4 exhibited a low limit of detection of 3.4 × 10−9 M in THF and 2.4 × 10−7 M in THF/H2O, demonstrating high sensitivity and selectivity for Hg2+ ions through a fluorescence turn-on response. Moreover, probe 4 shows potential for the detection of Hg2+ ions in living cells as well as in THF and THF/H2O, showcasing its versatility and practical application in various environments (Fig. 7).
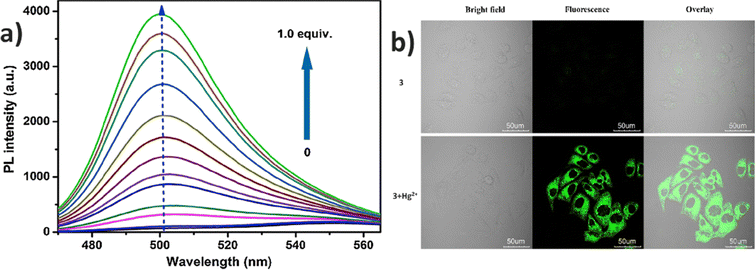 | ||
| Fig. 6 (a) Emission spectra of probe 3 with increasing concentrations of Hg2+ ions in a DMSO/H2O (fw = 20%) mixture; (b) fluorescence images of HeLa cells treated with 3 and Hg2+ ions. Reproduced with permission from ref. 49. Copyright 2015 Elsevier. | ||
 | ||
| Fig. 7 (a) Emission spectra of probe 4 with increasing concentrations of Hg2+ ions in a DMSO/H2O (fw = 20%) mixture; (b) fluorescence images of HeLa cells treated with 4 and Hg2+ ions. Reproduced with permission from ref. 50. Copyright 2016 Elsevier. | ||
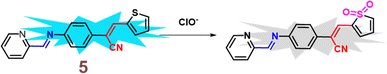
In 2021, Yang et al. developed a highly sensitive fluorescent receptor, probe 5, designed for the detection of ClO− ions in aqueous media, which based on a thiophene-cyanostilbene conjugated Schiff base.51 Probe 5 exhibited strong fluorescence quantum yields as prepared and demonstrated selective detection of ClO− ions, even in the presence of other competing species, with a fluorescence turn-off response (Fig. 9). The limit of detection (LOD) for ClO− was determined to be 3.2 × 10−8 M. The sensing mechanism was validated using FT-IR spectroscopy, fluorescence Job's plot, 1H NMR spectroscopy, and mass spectrometry. The study revealed that ClO− oxidizes the sulfur in the thiophene unit of probe 5 (Fig. 8). This oxidation reaction significantly alters the electronic properties of the thiophene unit, leading to a detectable change in the fluorescence signal of the probe. The specific interaction between ClO− and the sulfur atom in the thiophene ring provides a selective and sensitive mechanism for detecting ClO− ions, the probe 5 was successfully applied to detect ClO− in real samples and for living-cell imaging, highlighting its potential for in vitro assays and environmental monitoring of ClO− ions (Fig. 9). This study underscores the versatility and efficacy of probe 5 in practical applications, offering a valuable tool for both environmental detection and biological research.
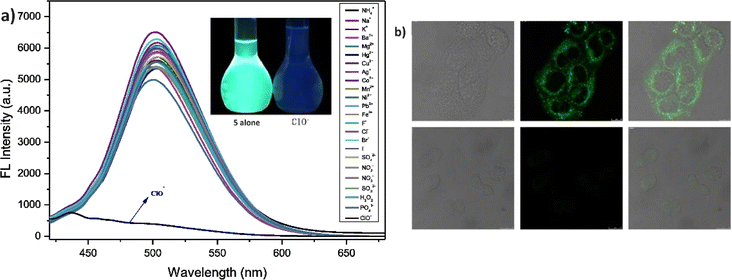 | ||
| Fig. 9 (a) Emission spectra of probe 5 with increasing concentrations of ClO− ions in a THF/H2O mixture; (b) confocal fluorescence images of MCF-7 cells treated with probe 5 and probe 5 with ClO− ions MCF-7 cells with probe 5 alone (top) MCF-7 cells with 5 + ClO− (bottom). Reproduced with permission from ref. 51. Copyright 2021 Elsevier. | ||
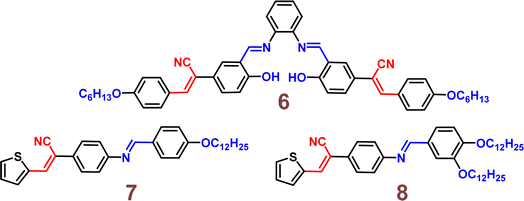
The following year, the same research group developed another biphenyl-cyanostilbene conjugate Schiff base dual sensor (probe 6) for the simultaneous detection of Cu2+ and Zn2+ ions.52 This probe features long-wavelength fluorescence in the 550–750 nm range in aqueous media, with a fluorescence turn-off response for Cu2+ ions and a ratiometric response for Zn2+ ions (Fig. 10). Additionally, probe 6 exhibited an excellent selective colorimetric response towards Zn2+ ions, changing from red to yellow. The detection of Cu2+ and Zn2+ ions were achieved without mutual interference in their coexistence system, facilitated by the presence of ATP. The detection limits for Cu2+ and Zn2+ ions were determined to be 2.3 × 10−7 M and 1.8 × 10−6 M, respectively. Furthermore, probe 6 demonstrated strong bioimaging performance and in situ sensing capabilities for Cu2+ and Zn2+ ions in living cells, indicating its potential for detecting these ions in both in vitro tests and in vivo environments (Fig. 11). This dual sensor offers a valuable tool for the simultaneous monitoring of these metal ions in complex biological and environmental samples, showcasing its versatility and efficacy in practical applications.
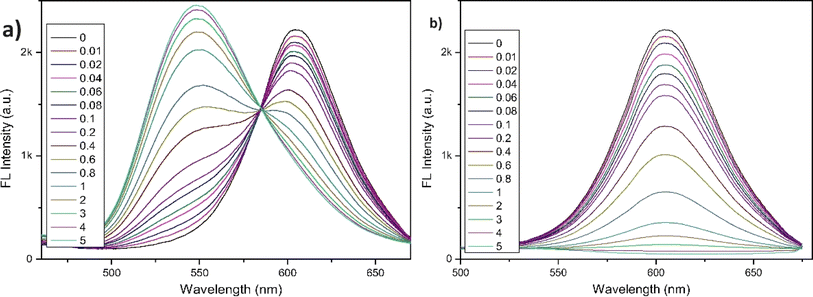 | ||
| Fig. 10 (a) Fluorescence spectra of probe 6 with different concentrations of Zn2+ in THF/H2O (b) fluorescence spectra of probe 6 with different concentrations of Cu2+ ions in THF/H2O. Reproduced with permission from ref. 52. Copyright 2022 Elsevier. | ||
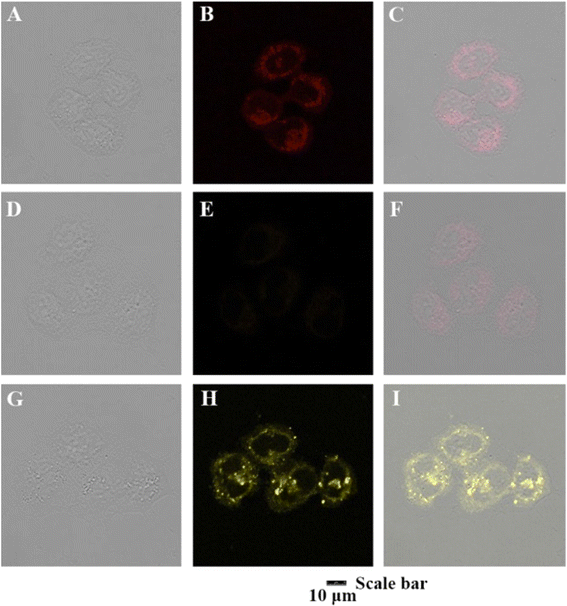 | ||
| Fig. 11 Confocal fluorescence images for the living MCF-7 cells in the presence of probe 6, probe 6+ Cu2+ and probe 6 + Zn2+ (1.0 × 10−5 M, each), respectively. (A)–(C) Cells with probe 6; (D)–(F) cells with probe 6 + Cu2+; (G)–(I) cells with probe 6 + Zn2+.Reproduced with permission from ref. 52. Copyright 2022 Elsevier. | ||
They have developed two novel fluorescent liquid crystals based on thiophene-vinyl nitrile Schiff-base derivatives, 7 and 8, which differ in the number of alkyl chains (Fig. 12).53 Both probes exhibited smectic phases, with phase transition temperatures ranging from 91.5 to 130.6 °C for 7 and from 71.3 to 120.2 °C for 8. They displayed excellent fluorescence in the aggregated states of the mesophase and solid film, with absolute fluorescence quantum yields of 0.64 and 0.76, respectively, in the solid film. The numerous alkyl chains on these derivatives contributed to their low mesophase transition temperatures and high fluorescence. This study presents the first example of thiophene-containing AIE fluorescent liquid crystals.
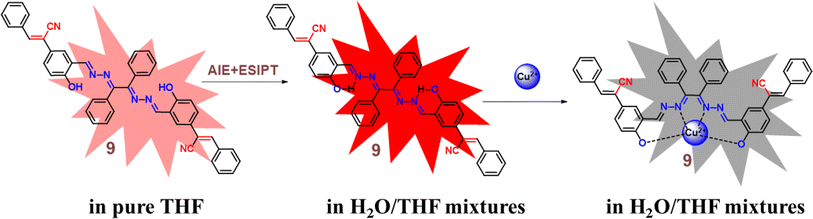
Zhu and co-workers designed a new Schiff base probe 9 composed of salicylaldehyde-analogue α-cyanostilbene and benzophenone hydrazone which emits red fluorescence in 2022 (Fig. 13).54 The enhanced red emission for probe 9 is due to the combined effects of ESIPT and AIE. ESIPT facilitates a proton transfer in the excited state, resulting in red-shifted emission, while AIE restricts molecular motions in the aggregated state, enhancing fluorescence. Together, these effects lead to a significantly intensified red emission. In the THF/H2O system, the probe's highly sensitive and the selective chelating ability towards Cu2+ ions were demonstrated. The probe 9 senses Cu2+ ions with a fluorescence turn-off behavior with detection value of 2.34 × 10−8 M (Fig. 14). The probe 9 has been effectively applied to detect Cu2+ ions in ambient water samples. The fluorophore-infused silica gel test strip was employed for the spot observation of Cu2+ under UV light, suggesting the receptors feasible use for visible Cu2+ ions sensing (Fig. 14).
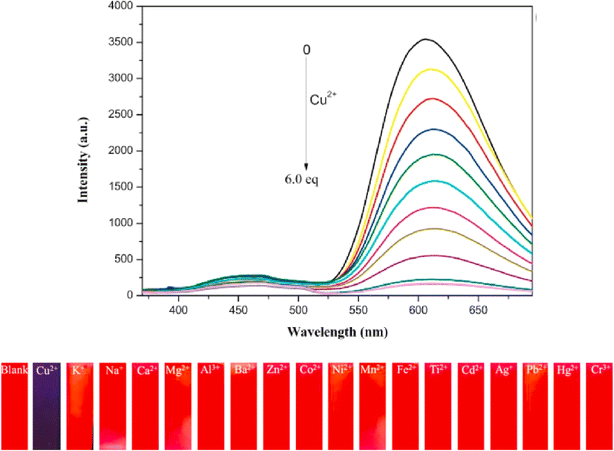 | ||
Fig. 14 Emission spectra of probe 9 upon increasing concentrations of Cu2+ ions in THF/H2O mixtures (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, pH = 7.4, λex = 350 nm) (top) and photographs of the silica gel testing strips for detecting various ions including under UV 365 nm illumination (bottom). Reproduced with permission from ref. 54. Copyright 2022 Elsevier. 9, pH = 7.4, λex = 350 nm) (top) and photographs of the silica gel testing strips for detecting various ions including under UV 365 nm illumination (bottom). Reproduced with permission from ref. 54. Copyright 2022 Elsevier. | ||
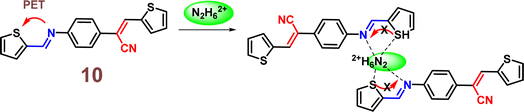
Yang et al. introduced a first fluorescence sensor for hydrazine ion based on thiophene-cyanodistyrene Schiff-base (10) in the year 2022.55 The probe 10 demonstrated weak fluorescence at 450–550 nm in aqueous media as a result of the strong PET effect, which attenuates the AIE effect. However, by interfering with the PET effect (Fig. 15), it demonstrated a high fluorescence turn-on response and selective sensing capabilities for Hg2+ and N2H62+ (Fig. 16). Notably, the presence of Cl− covered the fluorescence response to Hg2+, allowing for the extremely selective detection of N2H62+ alone with 10. The detection limit of 10 for N2H62+ ions was established to be 1.05 × 10−7 M. The binding ratio of 10 with N2H62+ ions were calculated to be 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 which was verified by Job's plot, MS spectrum, 1H NMR spectrum, FT-IR spectrum, and theoretical computations. The experiment conducted using the probe 10 in tap water and Minjiang River water demonstrated significant potential for sensing N2H62+in real water environments.
1 which was verified by Job's plot, MS spectrum, 1H NMR spectrum, FT-IR spectrum, and theoretical computations. The experiment conducted using the probe 10 in tap water and Minjiang River water demonstrated significant potential for sensing N2H62+in real water environments.
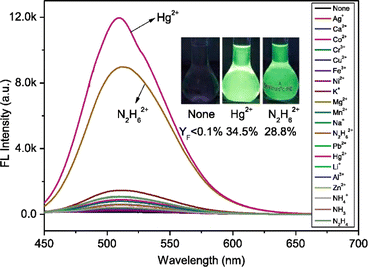 | ||
| Fig. 16 Fluorescence spectra of probe 10 with different cations. Reproduced with permission from ref. 55. Copyright 2022 Elsevier. | ||
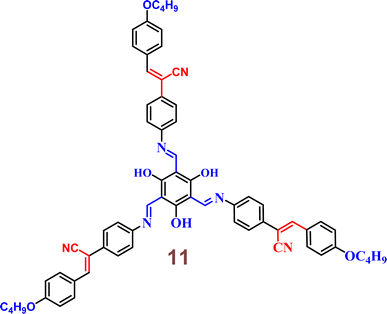
In the year 2022, Yang and colleagues discovered a novel star-shaped Schiff base-cyanostilbene probe 11 which founds application in white light-emitting diodes (w-LEDs) (Fig. 17).56 The photophysical properties and solvent effects of 11 were studied and further analyzed using DFT. LEDs incorporating 11 were fabricated, resulting in the emission of white light. The findings indicate that the probe 11 exhibits high thermal stability and favorable fluorescence emission in both solution and solid states, demonstrating dual-state luminescence. The solid state of the probe 11 shows promises for use in w-LEDs because of its high thermal stability and beneficial fluorescence emission.
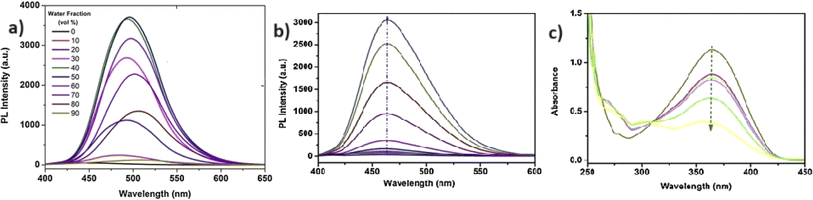
In 2022, Zhu et al. synthesized a salicylaldehyde-functionalized Schiff base with an α-cyanostilbene unit (probe 12) for the selective detection of Co2+ ions.57 Probe 12 exhibited exceptional AIE and ESIPT emission properties in solution, aggregation, and solid states. The probe 12 displayed distinct fluorescence with varying morphologies when crystallized in pure ethanol or mixtures of ethanol and water (1/2, v/v). For Co2+ ion sensing, probe 12 demonstrated clear spectro-photometric fluorescence quenching of its strong green fluorescence in a THF/H2O mixture (Fig. 18). The limit of detection for Co2+ ions was determined to be 0.41 × 10−8 M, with a binding ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Additionally, probe 12 proved effective for sensing Co2+ ions in real water samples and on silica gel testing strips, showcasing its practical application in environmental monitoring.
1. Additionally, probe 12 proved effective for sensing Co2+ ions in real water samples and on silica gel testing strips, showcasing its practical application in environmental monitoring.
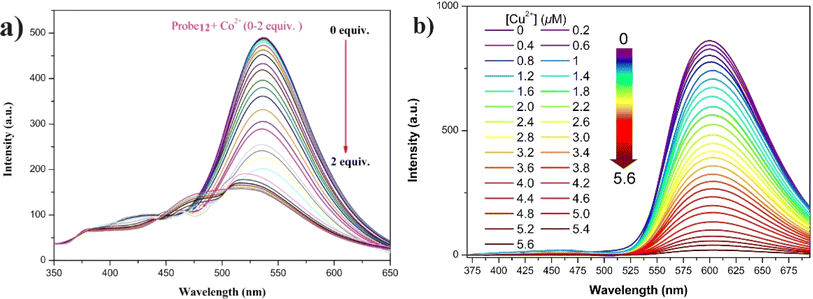 | ||
| Fig. 18 (a) Emission spectra of probe 12 upon increasing concentrations of Co2+ ions in THF/H2O mixtures (fw = 60%, pH = 7.4, λex = 330 nm). (b) Emission spectra of probe 13 with increasing Cu2+ ions concentration in THF/H2O (fw = 90%, pH = 7.4, λex = 350 nm). Reproduced with permission from ref. 57. Copyright 2023 Springer. | ||
In 2023, the same research group developed a new sensor, probe 13, specifically tailored for the selective detection of Cu2+ ions.58 This innovative probe incorporates a benzophenone hydrazone unit, replacing the phenyl unit found in its predecessor, probe 12. Probe 13 emitted red fluorescence at 602 nm, attributed to the combined effects of ESIPT and AIE. In the THF/H2O system, probe 13 demonstrated highly sensitive and selective chelating ability towards Cu2+ ions, evidenced by the observable fading of red fluorescence with a detection limit of 2.34 × 10−8 M (Fig. 18). Job's plot analysis suggested a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding ratio of probe 13 with Cu2+ ions. Furthermore, probe 13 was effectively applied to detect Cu2+ ions in ambient water samples. Utilizing probe-permeated silica gel test strips allowed for spot observation of Cu2+ under UV light, indicating its practical potential for visible Cu2+ sensing.
1 binding ratio of probe 13 with Cu2+ ions. Furthermore, probe 13 was effectively applied to detect Cu2+ ions in ambient water samples. Utilizing probe-permeated silica gel test strips allowed for spot observation of Cu2+ under UV light, indicating its practical potential for visible Cu2+ sensing.
Later in the same year, probe 14 was designed, differing from probe 12 by the addition of a dimethylamino group (Fig. 19).59 Harnessing the synergistic mechanisms of AIE, ESIPT, and ICT, probe 14 exhibited red fluorescence emission at 627 nm in THF/H2O mixtures (Fig. 20). This probe 14 found effectiveness in various applications, particularly demonstrated through TLC-based test strips loaded with probe 14. These strips exhibited a reversible fluorescence response to amine/acid vapors and a sensitive, selective fluorescence response to Cu2+ ions (Fig. 20). Fluorescence titration experiments conducted between probe 14 and Cu2+ in THF/H2O mixtures revealed a detection limit of 1.18 × 10−7 M and a binding constant of 1.59 × 105. Job's plot experiments and HR-MS analysis confirmed a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry between probe 14 and Cu2+. This probe enabled real-time assessment of Cu2+ in actual water samples, providing valuable insights into the development of long-wavelength emissive luminogens based on α-cyanostilbene.
1 binding stoichiometry between probe 14 and Cu2+. This probe enabled real-time assessment of Cu2+ in actual water samples, providing valuable insights into the development of long-wavelength emissive luminogens based on α-cyanostilbene.
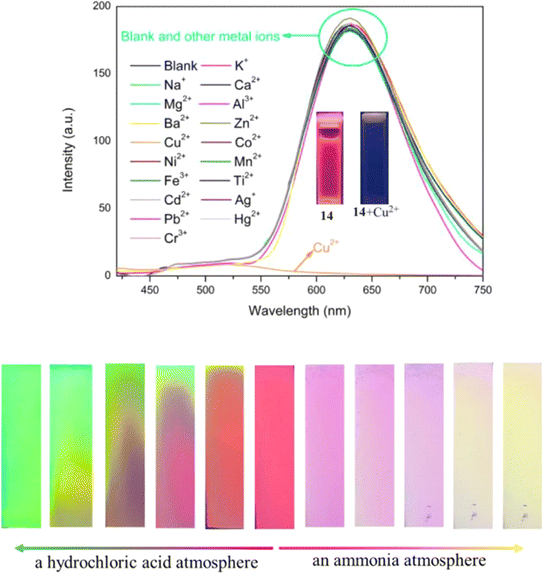 | ||
| Fig. 20 Emission spectra changes of probe 14 in the presence of various metal cations (in THF/H2O system (fw = 95%, pH = 7.4, λex = 400 nm)) (top); fluorescence color responses of the TLC-based test strips loaded with probe 14 after exposure to HCl and NH3 vapors for different times (bottom). Reproduced with permission from ref. 59. Copyright 2023 Elsevier. | ||
Yang and co-workers synthesized a fluorescence sensor for arginine based on bis-cyanodistyrene Schiff-base probe (15) in 2024.60 This probe 15 showed high fluorescence in aqueous media and selective sensing property for arginine with a limit of detection of 1.34 × 10−7 M. The multiple intermolecular hydrogen bonds provided an explanation for the binding mechanism. The selective sensing ability of 15 for arginine was successfully demonstrated on test paper and in sample analyses of Minjiang River water and adult urine. The probe 15 was further employed in living-cell imaging experiments, showcasing its excellent fluorescence imaging properties and strong detection capabilities for arginine in a cellular environment (Fig. 21). This research provides an effective method for real-time and in situ sensing of arginine in complex environments and living organisms, offering significant potential for practical applications in biochemical and medical fields.
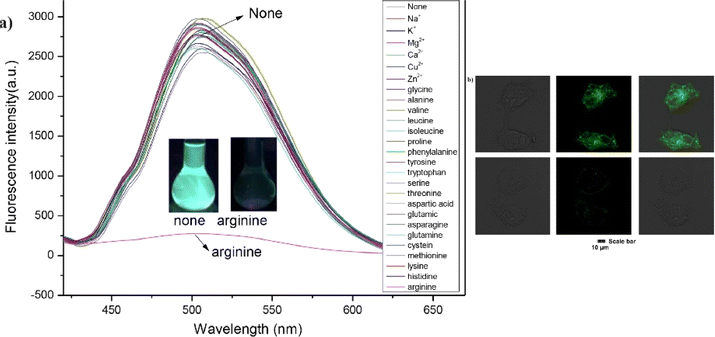 | ||
| Fig. 21 (a) Fluorescence spectra of probe 15 in the presence of various metal ions and amino acids THF/H2O system (b) fluorescence images of MCF-7 cells with probe 15 with arginine. Reproduced with permission from ref. 60. Copyright 2024 Elsevier. | ||
Very recently, in 2024, Zhu and colleagues developed a new Schiff base designated as 16, which incorporates an electron-donating dimethylamino unit, building upon the design of probe 13.61 This probe 16 senses Fe3+ ions with a decrease in fluorescence intensity and exceptional AIE in THF/H2O mixtures (Fig. 22). The limit of detection and the binding constant (Ka) values calculated for 16 towards Fe3+ ions were 5.50 × 10−8 M and 1.69 × 105 M, respectively. In addition, the reversible fluorescence response to amine/acid vapor of 16 was tested using TLC-based test strip. The real-world application of 16 was tested in natural water sample analysis.
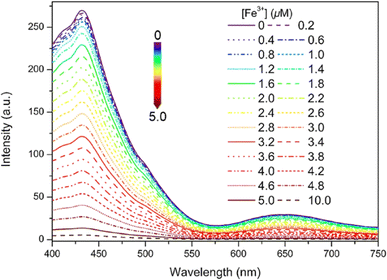 | ||
| Fig. 22 Emission spectra of probe 16 (10 μM) with various concentration of Fe3+ ions in THF/water mixtures (fw = 90%, pH = 7.4, λex = 380 nm). Reproduced with permission from ref. 61. Copyright 2024 Elsevier. | ||
Mechanistic insights into photophysical properties
Understanding the underlying mechanisms affecting the photophysical properties of the probes is essential for a comprehensive evaluation of their performance. This section summarizes the three key mechanisms: AIE, TICT, and ESIPT, each of which plays a critical role in the behavior of these probes.By integrating these mechanistic insights AIE, TICT, and ESIPT into our understanding of the probes' behavior, we gain a comprehensive view of how these processes influence their photophysical properties. This in-depth analysis not only explains the observed phenomena but also provides a foundation for further refinement and application of the probes in detecting metal ions and other sensing tasks.
Conclusion and future outlook
The detection of metal ions is a crucial task across various domains, including environmental monitoring, biomedical diagnostics, and industrial processes. Schiff bases incorporating cyanostilbene units have emerged as promising probes for this purpose due to their remarkable photophysical properties, structural versatility, and the ability to undergo significant fluorescence changes in the presence of metal ions. This review has provided a comprehensive overview of the synthesis, structural characteristics, and sensing mechanisms of Schiff base-cyanostilbene probes, highlighting their efficacy and potential applications. The incorporation of the cyanostilbene unit into Schiff bases has been shown to enhance the sensitivity and selectivity of these probes towards metal ions. The electron-withdrawing nature of the cyano group in cyanostilbene plays a pivotal role in modulating the electronic properties of the probe, which, in turn, affects its fluorescence response. This unique combination allows for the development of highly responsive and tunable sensors capable of detecting a wide range of metal ions at low concentrations. The studies reviewed have demonstrated that Schiff base-cyanostilbene probes exhibit excellent selectivity for specific metal ions, such as Fe3+, Cu2+, Co2+, Zn2+, Hg2+ and other important species including ClO−, arginine, hydrazine and with excellent AIE, TICT characteristics. The coordination interactions with the nitrogen and oxygen atoms of the Schiff base framework led to distinct changes in fluorescence, which can be easily monitored and quantified. Furthermore, the structural diversity of Schiff bases allows for the design of probes with tailored properties to meet specific sensing requirements.Despite these advancements, several challenges and opportunities remain for future research. A key goal is to further enhance the selectivity and sensitivity of these probes. Modifications to the Schiff base and cyanostilbene structures could enable the detection of a broader range of metal ions at even lower concentrations. Additionally, the development of probes capable of real-time and in situ monitoring in complex and dynamic environments such as live tissues and flowing water systems will significantly expand their practical applications. In particular, Schiff base-cyanostilbene probes have shown considerable promise in detecting metal ions in environmental samples, particularly water sources. These probes exhibit high sensitivity and selectivity for harmful metal ions like Hg2+, Cu2+, and Fe3+, making them valuable tools for monitoring water quality. However, real-world environmental samples often contain a complex mixture of substances that can interfere with probe performance. Matrix effects, such as the presence of organic matter, competing ions, and varying pH levels, can compromise the accuracy and reliability of the probes. Therefore, to overcome these challenges, further optimization is necessary to enhance the selectivity and effectiveness of these probes under diverse environmental conditions. In the field of clinical diagnostics, Schiff base-cyanostilbene probes hold potential for detecting metal ions in biological samples, aiding in diagnosing diseases associated with metal ion imbalances, such as Wilson's disease or iron overload disorders. Despite this potential, the complex biological environment poses significant challenges. Interactions with proteins, lipids, and other biomolecules can lead to false positives or negatives. Therefore, developing probes with enhanced selectivity for target metal ions, even in the presence of complex biological matrices, is crucial for successful clinical applications. Further research should focus on designing probes that can function accurately in the challenging conditions of biological systems. Looking forward, several research directions hold promise for advancing the field of Schiff base-cyanostilbene probes. One exciting area is the development of multifunctional probes capable of simultaneous detection of multiple metal ions, which would allow for comprehensive analysis with a single sensor. Improving probe stability under real-world conditions is also crucial. Research should explore composite materials or hybrid systems to enhance operational stability and effectiveness, ensuring accurate functioning under harsh conditions such as high temperatures, pressures, and varying pH levels. Techniques like encapsulation or protective coatings could also be investigated to prevent probe degradation. Moreover, optimizing probe design for rapid response times and high sensitivity in dynamic conditions will facilitate continuous monitoring of metal ion levels. Advanced spectroscopic and computational studies will further aid in understanding the fundamental mechanisms of fluorescence changes, leading to more effective sensor designs. Integrating these probes into portable and user-friendly devices will be essential for field applications and clinical diagnostics, making them more accessible for routine monitoring and diagnostic purposes.
In conclusion, Schiff base-cyanostilbene probes represent a highly promising class of sensors for metal ion detection. By addressing current challenges and exploring new research directions, these probes can be further refined and expanded. Future efforts should focus on translating these advancements into practical, reliable sensors for widespread use in environmental monitoring, biomedical diagnostics, and industrial applications. The continued development of Schiff base-cyanostilbene probes holds great promise for enhancing our ability to detect and quantify metal ions, ultimately contributing to improved environmental protection, healthcare, and industrial processes.
Abbreviations
| AIE | Aggregation-induced emission |
| TICT | Twisted intramolecular charge transfer |
| ESIPT | Excited-state intramolecular proton transfer |
| PET | Photoinduced electron transfer |
| ICT | Intramolecular charge transfer |
| CHEF | Chelation induced enhanced fluorescence |
| FRET | Fluorescence resonance energy transfer |
| nm | Nanometer |
| nM | Nanomolar |
| μM | Micromolar |
| NMR | Nuclear magnetic resonance |
| HRMS | High-resolution mass spectrometry |
| FT-IR | Fourier transform infrared |
| XRD | X-ray diffraction |
| LOD | Limit of detection |
| B–H | Benesi–Hildebrand |
| DFT | Density functional theory |
| TD-DFT | Time-dependent density-functional theory |
| ESI-MS | Electrospray ionisation mass spectrometry |
| WHO | World health organisation |
| EDTA | Ethylene diamine tetraacetate |
| AIEE | Aggregation-induced emission enhancement |
| DMF | Dimethylformamide |
| PPi | pyrophosphate |
| UV/PL | UV-vis absorption/photoluminescence |
| PXRD | Powder X-ray diffraction |
| THF | Tetrahydrofuran |
| ppb | Parts per billion |
| ppm | Parts per million |
| ns | Nanoseconds |
| DMSO | Dimethylsulfoxide |
| ACN | Acetonitrile |
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
Afrin A.-conceptualization and design of the review, conducted the comprehensive literature search, analyzed and summarized the relevant research, and wrote the initial draft of the manuscript, revised the manuscript for important intellectual content. Chinna Ayya Swamy P.-conceptualization, critical review, editing and finalization. Angel Rose contributed to the preliminary data.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
CAS P. thanks, SERB/SRG/2021/002112 for funding and National Institute of Technology for support. Ms. A. A. thanks the National Institute of Technology, Calicut, for the GATE-JRF fellowship.References
- X. Zheng, W. Cheng, C. Ji, J. Zhang and M. Yin, Rev. Anal. Chem., 2020, 39, 231–246 CrossRef CAS.
- K. P. Carter, A. M. Young and A. E. Palmer, Chem. Rev., 2014, 114, 4564–4601 CrossRef CAS PubMed.
- Y. Shen, C. Nie, Y. Wei, Z. Zheng, Z.-L. Xu and P. Xiang, Coord. Chem. Rev., 2022, 469, 214676 CrossRef CAS.
- M. Patitapaban, B. Rubi, B. Vinita, P. D. Pragyan, K. S. Suban and R. J. Bigyan, Trends Environ. Anal. Chem., 2022, 34, e00166 CrossRef.
- L. B. Asnake, Gaurav, M. Irshad, K. M. Asho, S. A. Jatinder, K. Vanish and K. Ki-Hyun, TrAC, Trends Anal. Chem., 2019, 116, 74–91 CrossRef.
- K. G. Manoj, K. P. Goutam and T. Neetu, Mater. Adv., 2022, 3, 2612–2669 RSC.
- M. J. Hafiz, B. Madeeha, W. H. Farah, S. A. Muhammad and S. Nabila, Crit. Rev. Anal. Chem., 2022, 52, 463–480 CrossRef PubMed.
- K. Sikandar, C. Xiaojing, A. Albandary, S. A. Esam, A. A Fahad, M. I. Munjed and A. Shujat, J. Environ. Chem. Eng., 2021, 9, 106381 CrossRef.
- U. Duraisamy and V. Inbaraj, J. Fluoresc., 2020, 30, 1203–1223 CrossRef PubMed.
- A. Md Zafer, Alimuddin and A. K. Salman, J. Fluoresc., 2023, 33, 1241–1272 CrossRef PubMed.
- J. Wang, Q. Meng, Y. Yang, S. Zhong, R. Zhang, Y. Fang, Y. Gao and X. Cui, ACS Sens., 2022, 7, 2521–2536 CrossRef CAS PubMed.
- A. Afrin, A. Jayaraj, M. S. Gayathri and P. C. A. Swamy, Sens. Diagn., 2023, 2, 988–1076 RSC.
- K. Juyal, A. Pathak, M. Panwar, S. C. Thakuri, O. Prakash, A. Agrwal and V. Nand, J. Organomet. Chem., 2023, 122825 CrossRef.
- A. T. Latha and P. C. A. Swamy, J. Org. Chem., 2024, 89(12), 8376–8384 CrossRef CAS PubMed.
- A. T. Latha and P. C. A. Swamy, Chem.–Eur. J., 2024, 1841 Search PubMed.
- D. Karati, S. Mukherjee and S. Roy, Med. Chem., 2023, 19, 960–985 CrossRef CAS PubMed.
- S. Kaushik, S. K. Paliwal, M. R. Iyer and V. M. Patil, Med. Chem. Res., 2023, 32, 1063–1076 CrossRef CAS PubMed.
- A. Afrin and P. C. A. Swamy, Coord. Chem. Rev., 2023, 494, 215327 CrossRef CAS.
- A. Jayaraj, M. S. Gayathri, G. Sivaraman and P. C. A. Swamy P, J. Photochem. Photobiol., B, 2022, 226, 112371 CrossRef CAS PubMed.
- M. K. Goshisht, G. K. Patra and N. Tripathi, Mater. Adv., 2022, 3, 2612–2669 RSC.
- S. Sasaki, G. P. C. Drummen and G. Konishi, J. Mater. Chem. C, 2016, 4, 2731–2743 RSC.
- E. Zohry, M. Ahmed, E. A. Orabi, M. Karlsson and B. Zietz, J. Phys. Chem. A, 2021, 14, 1252885–1252894 Search PubMed.
- J. Jovaišaitė, P. Baronas, G. Jonusauskas, D. Gudeika, A. Gruodis, J. V. Gražulevičius and S. Juršėnas, Phys. Chem. Chem. Phys., 2023, 25(3), 2411–2419 RSC.
- L. Zhu and Y. Zhao, J. Mater. Chem. C, 2013, 1, 1059–1065 RSC.
- M. Martínez-Abadía, R. Giménez and M. B. Ros, Adv. Mater., 2018, 30, 1704161 CrossRef PubMed.
- B. Kumari and S. Kanvah, Stilbene Stilbene Shining Bright: α-Cyanostilbenes as Functional Organic Materials, 2019 Search PubMed.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740–1741 RSC.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 21, 11718–11940 CrossRef PubMed.
- Y. D. Tu, J. Zhang, Y. Zhang, H. H. Y. Sung, L. Liu, R. T. K. Kwok, J. W. Y. Lam, I. D. Williams, H. Yan and B. Z. Tang, J. Am. Chem. Soc., 2021, 143, 11820–11821 CrossRef PubMed.
- Z. Wang, Y. Zhou, R. Xu, Y. Xu, D. Dang, Q. Shen, L. Meng and B. Z. Tang, Coord. Chem. Rev., 2022, 451, 214279 CrossRef CAS.
- Y. Sun, Z. Lei and H. Ma, J. Mater. Chem. C, 2022, 10, 14834–14867 RSC.
- H. Schiff, J. Am. Chem. Soc., 1864, 3, 343–349 Search PubMed.
- A. L. Berhanu, Gaurav, I. Mohiuddin, A. Malik, A. Singh, V. Kumar and H. Kim, Trends Anal. Chem., 2019, 116, 74–91 CrossRef CAS.
- S. Khan, X. Chen, A. Almahri, E. S. Allehyani, F. A. Alhumaydhi, M. M. Ibrahim and S. Ali, J. Environ. Chem. Eng., 2021, 9, 106381 CrossRef CAS.
- A. Kumar, M. Saini, B. Mohan and M. Kamboj, Microchem. J., 2022, 181, 107798 CrossRef CAS.
- P. Mahalingavelar and S. Kanvah, Phys. Chem. Chem. Phys., 2022, 24, 23049–23075 RSC.
- Y. H. Chu, C. Y. Zeng, Z. Cao, B. M. Shao, D. H. Li, M. Sun and W. Y. Fang, Opt. Mater., 2023, 139, 113767 CrossRef CAS.
- A. Afrin and P. C. A. Swamy, J. Mater. Chem. C, 2024, 12, 1923–1944 RSC.
- A. Afrin and P. C. A. Swamy, J. Org. Chem., 2024, 89, 7946–7961 CrossRef CAS PubMed.
- A. Afrin and P. C. A. Swamy, New J. Chem., 2023, 47, 18919–18932 RSC.
- T. Stoerkler, G. Ulrich, A. D. Laurent, D. Jacquemin and J. Massue, J. Org. Chem., 2023, 88, 9225–9236 CrossRef CAS PubMed.
- T. Yu, P. Theato, H. Yao, H. Liu, Y. Di, Z. Sun and S. Guan, Chem. Eng., 2023, 451, 138441 CrossRef CAS.
- X. Yi, X. Liu, Y. Liang, T. Gao, X. Hu and Y. Xiao, J. Solid State Chem., 2024, 334, 124688 CrossRef CAS.
- Q. Shi, Y. Ni, L. Yang, L. Kong, P. Gu, C. Wang, Q. Zhang, H. Zhou and J. Yang, J. Mater. Chem. B, 2023, 11, 6859–6867 RSC.
- R. Dahiwadkar, A. Murugan, D. Johnson, R. Chakraborty, V. Thiruvenkatam and S. Kanvah, J. Photochem. Photobiol., C, 2023, 434, 114227 CrossRef CAS.
- D. Dey, P. Giri, N. Sepay, A. Husain and M. K. Panda, J. Photochem. Photobiol., A, 2023, 437, 114480 CrossRef CAS.
- G. Zhang, A. Ding, Y. Zhang, L. Yang, L. Kong, X. Zhang, X. Tao, Y. Tian and J. Yang, Sens. Actuators, B, 2014, 202, 209–216 CrossRef CAS.
- A. Ding, F. Tang, T. Wang, X. Tao and J. Yang, J. Chem. Sci., 2015, 127, 375–382 CrossRef CAS.
- G. Zhang, X. Zhang, Y. Zhang, H. Wang, L. Kong, Y. Tian, X. Tao, H. Bi and J. Yang, Sens. Actuators, B, 2015, 221, 730–739 CrossRef CAS.
- W. Fang, G. Zhang, J. Chen, L. Kong, L. Yang, H. Bi and J. Yang, Sens. Actuators, B, 2016, 229, 338–346 CrossRef CAS.
- H. Guo, J. Lin, L. Zheng and F. Yang, Spectrochim. Acta, Part A, 2021, 256, 119744 CrossRef CAS PubMed.
- B. Zha, S. Fang, H. Chen, H. Guo and F. Yang, Spectrochim. Acta, Part A, 2022, 269, 120765 CrossRef CAS PubMed.
- Z. Yang, X. Huang, J. Cheng, H. Guo and F. Yang, Liq. Cryst., 2023, 50, 2521–2528 CrossRef CAS.
- M. Zhu, M. Zhong, M. Chen, S. Huang, Y. Li and F. Cao, Opt. Mater., 2022, 125, 112059 CrossRef CAS.
- S. Huang, L. Zheng, S. Zheng, H. Guo and F. Yang, J. Photochem. Photobiol., A, 2022, 427, 113851 CrossRef CAS.
- W. Fang, Z. Cao, Q. Liu, Y. Chu, H. Zhu, W. Zhou and J. Yang, Results Opt., 2022, 7, 100228 CrossRef.
- M. Chen, W. Huang, Y. Li, Y. Chen, D. Ji and M. Zhu, Methods Appl. Fluoresc., 2022, 11, 015002 CrossRef PubMed.
- M. Chen, M. Zhong, S. Huang, Y. Chen, F. Cao, H. Hu, W. Huang, D. Ji and M. Zhu, Inorg. Chem. Commun., 2023, 152, 110640 CrossRef CAS.
- M. Chen, Y. Chen, M. Zhong, D. Xie, C. Wang, X. Ren, S. Huang, J. Xu and M. Zhu, J. Fluoresc., 2024, 34, 1075–1090 CrossRef CAS PubMed.
- H. Zhou, X. Huang, S. Zheng, H. Guo and F. Yang, J. Mol. Struct., 2024, 1305, 137798 CrossRef CAS.
- M. Chen, W. Chen, Q. Zhu, L. Yang, X. Zhang, D. Xie, J. Chen, Y. Wu, Y. Zhu and M. Zhu, J. Fluoresc., 2024, 1–12 Search PubMed.
- B. Sun, L. Liu, W. Liu, F. Meng and Q. Huang, J. Lumin., 2020, 223, 117203 CrossRef CAS.
- U. Duraisamy, P. Jerome, N. Vijay and T. H. Oh, J. Lumin., 2023, 120350 Search PubMed.
| This journal is © The Royal Society of Chemistry 2024 |