Open Access Article
Open Access ArticleWhite light emission generated by two stacking patterns of a single organic molecular crystal†
Yuma
Nakagawa
 *a,
Kuon
Kinoshita
a,
Megumi
Kasuno
a,
Ryo
Nishimura
*a,
Kuon
Kinoshita
a,
Megumi
Kasuno
a,
Ryo
Nishimura
 b,
Masakazu
Morimoto
b,
Masakazu
Morimoto
 b,
Satoshi
Yokojima
b,
Satoshi
Yokojima
 *cd,
Makoto
Hatakeyama
*cd,
Makoto
Hatakeyama
 de,
Yuki
Sakamoto
de,
Yuki
Sakamoto
 d,
Shinichiro
Nakamura
df and
Kingo
Uchida
d,
Shinichiro
Nakamura
df and
Kingo
Uchida
 *ad
*ad
aDepartment of Materials Chemistry, Faculty of Science and Technology, Ryukoku University, Seta, Otsu, Shiga 520-2194, Japan. E-mail: uchida@rins.ryukoku.ac.jp
bDepartment of Chemistry and Research Center for Smart Molecules, Rikkyo University, 3-34-1 Nishi-Ikebukuro, Toshima-ku, Tokyo 171-8501, Japan
cSchool of Pharmacy, Tokyo University of Pharmacy and Life Sciences, 1432-1 Horinouchi, Hachioji, Tokyo 192-0392, Japan
dRIKEN, Cluster for Science, Technology and Innovation Hub, Nakamura Laboratory, 2-1 Hirosawa, Wako, Saitama 351-0198, Japan
eFaculty of Pharmaceutical Science, Sanyo-Onoda City University, 1-1-1 Daigakudori, Sanyo-Onoda, Yamaguchi 756-0884, Japan
fPriority Organization for Innovation and Excellence Laboratory for Data Science, Kumamoto University, 2-39-1, Kurokami, Chuo-ku, Kumamoto 860-8555, Japan
First published on 26th July 2022
Abstract
Dual emission by monomers and excimers is expected for single-molecule white light emitters. However, in the case of a system with an ideal excimer in a single conformation, it is very difficult to achieve their dual emission unless they have multiple chromophores in the molecule. Here, we report single-chromophore white light emission based on dual emission from the aggregation/crystallization of oxidative photocyclization condensates of diarylethene. These condensates form distinct π–π stacking by aggregation/crystallization and show structureless yellow emission due to the excited multimer in addition to blue monomer emission. Especially in the crystalline state, white light emission was observed at the International Commission on Illumination (CIE) 1931 coordinates of (0.31, 0.30). Experimental and theoretical studies have shown that white light emission is based on a balanced dual emission produced by two different stacking patterns having different overlapping areas in the crystal.
Introduction
Organic light-emitting molecules having a π-conjugated structure are used in a wide range of fields such as organic light-emitting diodes (OLEDs), chemical sensors, and fluorescent probes.1–3 Most of these materials exhibit strong fluorescence emission in dilute solution but weak or even quenched fluorescence, termed “aggregation-caused quenching” (ACQ), in the solid state.4 ACQ is generally produced by the formation of delocalized excitons via strong intermolecular π–π stacking interactions between molecules in the aggregated state. Therefore, various methods such as “aggregation-induced emission” (AIE)4 and “crystallization-induced emission enhancement” (CIEE)5 have been proposed to improve the fluorescence efficiency of organic light-emitting materials in the solid state. The structural feature of molecules showing AIE and CIEE is a highly twisted configuration that avoids the possibility of intermolecular π–π interaction. As a result, intramolecular motions (intramolecular rotation and vibration) are restricted and non-radioactive decay pathways are suppressed.4,6 Nonetheless, some planar conjugated molecules also exhibit the AIE property with excimer fluorescence from pairwise stacking states.6 Therefore, intermolecular π–π stacking may exhibit molecular aggregation-based emission such as excimers as well as ACQ.White organic light-emitting materials have long attracted great attention due to their fundamental importance and practical applications.7–10 White light emission is achieved by the combination of two (blue and yellow/orange) or three emitted colours (blue, green, and red). Most examples reported so far have relied on combinations of multiple components with emission colours that cover the entire visible range.11–13 Compared to these mixed dyes, single-molecule white light emitters (SMWLEs) are expected to exhibit superior performance without segregation or colour degradation as well as improved reproducibility.14–17 White light emission by a single molecule requires simultaneous dual or ternary emission.9 In the simplest case of dual emission, it can be assigned to two emission-excited states: one excited state is responsible for the blue emission, and the other contributes to the yellow/orange emission. Generally, the former is the locally excited singlet state, and the latter may be the charge transfer state,18,19 the excimer (excited dimer) state,15,16 the proton transfer state,20,21 through self-assembly,22–24 or phosphorescence from triplet excited state.14,25,26
Highly planar aromatic compounds, such as pyrene, often promote the formation of exciton interactions due to efficient intermolecular π–π stacking.27 These exciton interactions in the crystalline state are affected by the π–π stacking distance between aromatic compounds, their π–π overlap area,28,29 and the stacking manner.30,31 Such non-covalent interactions produce a broad, structureless, and red-shifted emission band that differs from that of monomer emission. In many cases, single crystals consist of a single stacking pattern. Therefore, they result in either monomer or exciton-interaction emission. In systems formed in this single conformation, it is very difficult to achieve dual emission: one from a single molecule and the other from non-covalent multiple molecules. Therefore, one solution to this problem may be to create a system of chromophores that have multiple stacking patterns in a crystal, each with different transition orbital properties. Conformational isomerism in each monomer, achieved by restricting covalent bonds at different dihedral angles, leads to different spatial arrangements of atoms in the molecule and multiple stacking patterns.32–34 Due to differences in electron distribution and interactions, the conformational isomers usually exhibit very different photophysical properties, including individual emission from each conformer. Using these properties, white light emission has been achieved by mixing multiple crystal polymorphs that exhibit different emission colors,32 doping them into a polymer matrix,33 or applying mechanical stimuli to them.34 These approaches required manipulation of two or three colour elements to produce white light emission. To our knowledge, there is no example of such a system being used to produce white light emission in the single-crystal state.
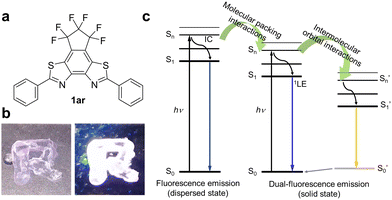
In this work, we report the fluorescence emission properties of 1ar (Fig. 1a). The fluorescent molecule 1ar shows deep-blue fluorescence in areas including the near UV region (λmax: 380–390 nm) with relatively high fluorescence quantum yields in the dispersed state such as in dilute solution. Interestingly, it shows white emission in the solid state (Fig. 1b). This white emission is caused by the mixing of two emissions with different wavelengths and lifetimes, i.e., blue and yellow (the latter is not observed in dilute solutions). In the crystal state of a single molecule, there are two conformers with different stacking patterns and different overlapped areas. These generate two electronically excited singlet states (monomer and excited multimer) with different transition orbital properties via intermolecular orbital interactions due to π–π stacking (Fig. 1c). Moreover, the packing structures are obtained through self-assembly. Since this type of self-assembly, such as recrystallization, aggregation, and solution casting methods, can be done in a straightforward manner, it is thermodynamically stable and industrially useful in practical applications.
Results and discussion
Molecular design and synthesis
There are two types of molecular aggregation: J-type and H-type.35,36 The H-type (parallel configuration with no slipping) generally shows a blue-shifted absorption band and ideally does not fluoresce. Therefore, to achieve dual emission in the aggregated state, aggregation of the J-type is desirable. Accordingly, in an effort to induce J-type aggregation (antiparallel configuration with slipping), we designed a structure of benzo[2,1-d:3,4-d]bisthiazole (BBT; planar structure) condensed with 3,3,4,4,5,5-hexafluorocyclopentene with high ring strain at the 5,6-position. In this structure, phenyl groups were introduced at both ends.1ar was formed upon UV light irradiation to diarylethene 1o in n-hexane in the presence of iodine (I2) by photocyclization and dehydrogenation reactions, as in the case of typical stilbenes37 (Fig. S1 and S2, ESI†). Stilbenes are well known to undergo photocyclization to form dihydrophenanthrenes,38 which then revert to stilbenes in the dark in a degassed solution, while in the presence of an oxidant, dihydrophenanthrenes are irreversibly converted to phenanthrenes. Such a reaction can be used to produce condensation products that allow 1o to be transformed into the condensed product of 1ar. This condensation reaction has also proceeded in other solvents (e.g., ethanol) and oxidants (e.g., tetrabutylammonium perchlorate: TBAP). As the oxidative photocyclization proceeded, the apparent hydrogen-ion concentration in the organic solvent increased and the fluorescence intensity increased significantly (Fig. S3 and S4, ESI†). These results strongly support the observation that the oxidative photocyclization reaction was in progress and that the 1ar produced by the reaction was fluorescent. The absorption band of 1ar was more blue-shifted than that of 1o because its fused ring structure cut off the conjugation length in the molecule. The purified 1ar (λmax: 335 nm and ε: 3.3 × 104 M−1 cm−1) showed deep blue emission with a maximum fluorescence wavelength of 380 nm (ΦFlu = 0.34) in n-hexane (Fig. S5, ESI†). The 1H NMR signals derived from 1o and 1ar shifted to lower fields as the reaction progressed. This is likely due to the increased concentration of H+ in the solution as the reaction progressed and the protonation of the nitrogen atoms in the thiazole rings of 1o and 1ar (Fig. S4, ESI†). In the absence of the oxidant, 1ar was not produced (Fig. S1, ESI†). The synthesis of 1ar was confirmed by 1H, 13C, and 19F NMR measurements, elemental analysis, and high-resolution mass spectrometry (HRMS) (see ESI† for details).
Photophysical properties
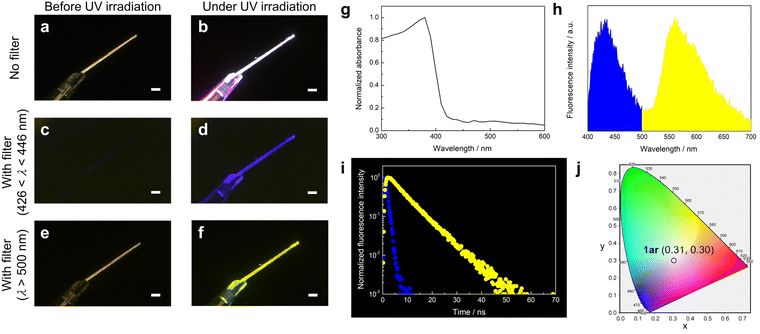
Fig. 2 shows the photophysical properties of 1ar in the crystalline state. The absorption spectra of 1ar crystals prepared by recrystallization from n-hexane are red-shifted compared to the absorption spectra in solution, suggesting a ground state interaction between the molecules (Fig. 2g). The 1ar crystals showed white fluorescence emission when irradiated with UV light (λ = 365 nm) of the absorbance wavelength (Fig. 2a and b). There are two emission bands in 1ar crystals: a blue emission band with a maximum fluorescence wavelength at 425 nm and a yellow emission band with a maximum fluorescence wavelength at 565 nm (Fig. 2h). In fact, when the optical filter (λ = 436 nm or λ > 500 nm) was attached to the objective lens of the digital microscope, blue and yellow fluorescence emissions were independently observed (Fig. 2c–f). Therefore, this white emission is produced by the combination of blue and yellow emissions. Fluorescence spectra are usually characterized as single emission bands that are independent of the excitation wavelength. If two emission bands are observed in the fluorescence spectrum in the solid state of a highly planar molecule, this can be attributed to one ground state having two excited state species due to excimer formation.15,39The lifetime of excimer emission is often longer than that of monomer emission.28,29,40,41 The blue fluorescence emission lifetime of 1ar crystal was τ = 0.81 ns (Fig. 2i: blue square). In contrast, the yellow fluorescence lifetime was τ = 6.70 ns (Fig. 2i: yellow circle), which was about eight times longer than the blue fluorescence lifetime. These results strongly suggest that the yellow emission of 1ar in the solid state is due to excimer. Excimer is categorized into two types based on the mode of its formation.41 One is called a “dynamic excimer”, in which an excited chromophore comes into close proximity to another ground state molecule and forms an excimer. The other is called a “static excimer”, in which two chromophores are conjugated by covalent or supramolecular interactions. Mainly, there are two ways to distinguish between dynamic and static excimers: analyzing excitation spectra and fluorescence lifetime decay profiles.41–43 In the case of dynamic excimer, excitation spectra are the same when monitored at monomer- and excimer-derived emission wavelengths. Excitation spectra of 1ar crystals at emission wavelengths at 425 and 565 nm were measured, and these spectra were very similar (Fig. S6, ESI†). In addition, the decay profile of the fluorescence lifetime at yellow (565 nm) emission showed an upward component of excimer formation characteristic of dynamic excimer followed by a negative slope of fluorescence decay (Fig. 2i). Therefore, the yellow emission of 1ar crystal was concluded to be due to a dynamic excimer.
The International Commission on Illumination (CIE) 1931 colour space is the most well-known defined quantitative link between physically pure colours and physiologically perceived colours in human colour vision.44 The pure white colour has CIE coordinates of (1/3, 1/3). The 1ar crystal shows CIE coordinates of (0.31, 0.30) (Fig. 2j), which is close to the value for pure white, and its fluorescence quantum yield is 0.12. The emission quantum yields of the 1ar crystals were in the middle range of those reported so far for molecules that emit white light in the solid state (Fig. S7 and Table S1, ESI†).
The emission properties that depend on the molecular conformation often change the emission colour upon mechanical stimulation.32,34,45,46 The application of molecules showing such stimulation-induced phenomena to white light-emitting devices will naturally be limited. Therefore, a promising approach would be to make molecules with no remarkable colour change even under stimulation. The powder of ground 1ar crystals also showed white light emission due to the dual emission of blue and yellow, and its CIE coordinates were (0.35, 0.33) with a fluorescence quantum yield of 0.14 (Fig. S8, ESI†). Similarly, a film prepared by the casting method from chloroform solution also showed blue and yellow dual emission, and its CIE1931 coordinates were (0.33, 0.30) with a fluorescence quantum yield of 0.18 (Fig. S9, ESI†). In addition, no phase transition of the solid state of 1ar was observed between melting point temperature (265 °C) and room temperature (Fig. S10, ESI†). Therefore, the molecular packing and white light emission of 1ar is likely to be maintained at temperatures below the melting point. In fact, white light emission was observed when 1ar in solid state was irradiated with ultraviolet light under heating at about 200 °C (Fig. S11, ESI†).
First, we investigated the polarity effect on the dual emission of 1ar by comparing the emissions in nine different solvents. The solubility of 1ar in organic solvents was not so good, and it was difficult to make highly concentrated solutions. Therefore, the effect of solvent polarity on the absorption and emission properties of 1ar was performed in dilute solution. The maximum absorption and fluorescence wavelengths of 1ar showed maximum deviations of 8 and 9 nm, respectively, and were nearly unchanged among the nine solvents (Fig. S12 and Table S2, ESI†). Then, the difference between the maximum absorption and fluorescence wavelengths (λmaxem − λmaxabs = Δλ) in these solvents was plotted against the ET(30)47 value, which is a measure of solvent polarity. The results show that 1ar has a relatively small positive correlation with ET(30). The fluorescence quantum yield of 1ar was also measured in the nine different kinds of solvents, with resulting values in the range of 0.3–0.5. These results show that the optical properties such as λmaxabs, λmaxem, and ΦFlu of 1ar have relatively small dependence on the solvent, which excludes the polarity effect on the emission of 1ar.
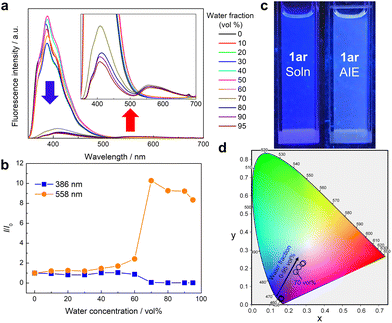
Then, to investigate the origin of the excimer emission of 1ar, absorption and fluorescence spectra were measured at different concentrations (5–100 μM) (Fig. S13, ESI†). Absorbance increased with increasing concentration of 1ar. Fluorescence intensity increased with increasing concentration from 5–20 μM of 1ar, but fluorescence intensity decreased with increasing concentration above 25 μM due to concentration quenching. The normalized fluorescence spectra and these difference spectra showed red-shifted monomer emission due to intermolecular interactions, but excimer emission was not observed. Free rotation of the phenyl group at the end of 1ar can occur in solution, which potentially creates the intermolecular distance for suppressing the excimer emission. Since 1ar has high molecular planarity, it could form intermolecular interactions such as π–π stacking upon aggregation/crystallization. Thus, we investigated the fluorescence emission characteristics of 1ar in its aggregated state. This state was recorded in ethanol/water mixtures containing different amounts of water, which acted as a poor solvent (Fig. 3). The maximum fluorescence at 1ar was not significantly changed by the addition of a small amount of water (<70 vol%) (Fig. 3a and b). When more than 70 vol% water was added, a new fluorescence with a maximum wavelength at 558 nm (yellow emission region) was observed in addition to the previous decrease in deep-blue fluorescence intensity (Fig. 3a and b). The calculated CIE coordinate of 1ar in ethanol/water mixtures approaches a white value due to the decrease in the ratio of blue to yellow emission bands as the water fraction in the solution increases (Fig. 3c and d).
Crystal structure
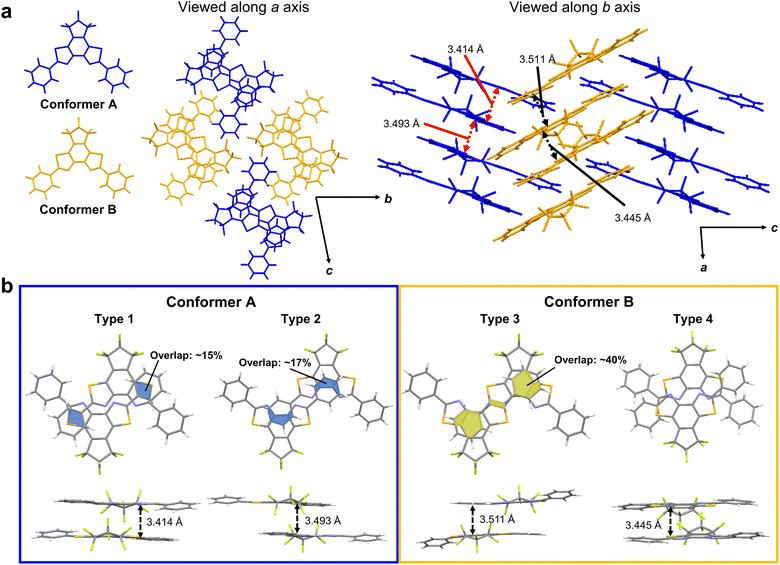
To clarify the white-light-emitting mechanism of 1ar crystal, X-ray crystallographic analysis was carried out. A rod-shaped single crystal of 1ar, suitable for X-ray crystallographic analysis, was prepared by recrystallization from n-hexane. The details of the crystal structure of 1ar are shown in Fig. S14 and Table S3 (ESI†). In the crystal, there are two crystallographically independent conformers with similar bond lengths and dihedral angles (Fig. 4 and Table S4, ESI†). They are arranged in an alternating zigzag pattern, indicating herringbone packing. Each conformer is antiparallel to the same conformer with slipping, and each of those conformers forms a column with a ladder arrangement. There are two types of stacking dimers for each conformer. These dimers are referred to Type 1 and 2 dimers for conformer A and Type 3 and Type 4 dimers for conformer B (Fig. 4b). The interplanar distances between two blue (bright yellow) conformers, which correspond to Type 1 and Type 2 (Type 3 and Type 4) dimers, are 3.414 and 3.493 (3.493 and 3.445), respectively. These interplanar distances are typical for π–π stacking molecules,48 and they strongly suggest the existence of π–π interactions.To examine the effect of these structures to the characteristics of excited states, we performed quantum chemical calculations for monomers, stacked dimers, a unit cell, two adjacent unit cells, and 8mer or 9mer as specified in Supporting Information. In our calculations, we found that the excited states spread over many molecules as the cluster size increases, that is, a redshift of the wavelength of the lowest absorption edge from 349 nm to 399 nm, even for the limited size of the 1ar cluster (9mer). Considering the further redshift with larger size together with the structural relaxation after excitation, we can assume that the fluorescence of yellow colour is due to the excited state from the many molecules. We further found that the lowest excited state has a large oscillator strength by consistently including the effect of double excitation. Thus, the computational results indicate that fluorescence occurs not only from the monomer but also from the excited state formed by multiple molecules, possibly due to the presence of multimer (for details, see Fig. S15–S20 and Tables S5–S14, ESI†).
The recent pioneering works have shown that not only the distance between the planes but also the overlap between the planes is important for π–π interaction.28,29 A π–π overlap of more than 40% could potentially cause a red-shift in emission wavelength by more than 100 nm.29 This advantageous overlap has the potential to induce excimer emission.28 In the case of conformer A, there was an overlap between the phenyl group and the BBT unit, but the overlap regions were very narrow, at less than 20%. In conformer B, there was more than twice as much overlap as in conformer A. Moreover, there is a very narrow but overlapping region between the BBT units. Therefore, the dimer of conformer B with its usefully wide overlap region is expected to provide excimer emission.
We thus attribute the blue emission to the superposition of the monomer excitation over multiple molecules of the conformer A and the yellow emission to the excimer on the conformer B.
Control of emission properties of polymer composite film
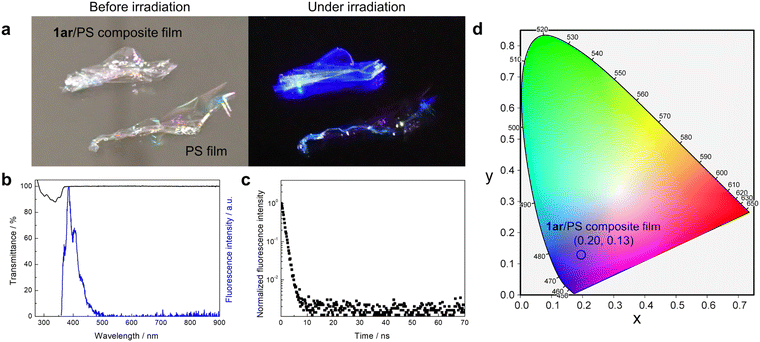
Deep-blue light emission (emission peak λ < 450 nm) as well as white light emission is very important. Deep-blue light emitting materials have great potential to function as light-converting materials as well as fluorescence-based chemical and biological sensors.49,50 However, the solid state of 1ar shows white light emission instead of deep-blue light emission due to the formation of intermolecular interactions by aggregation. Dispersion of 1ar in the polymer matrix is an effective strategy to avoid this. Here, solid films were prepared by dispersing 1ar in polystyrene (PS). The produced film is very transparent (about 100% transmittance in the entire visible region: Fig. 5a and b) under daylight conditions. Under UV light irradiation, it then shows deep-blue fluorescence emission (Fig. 5a and b). The fluorescence spectra and fluorescence quantum yield (ΦFlu = 0.34) of the prepared 1ar/PS composite film were almost the same as those of the n-hexane solution, and the fluorescence lifetime was almost the same as that of the blue emission band of 1ar in the crystalline state (Fig. 5c).To further investigate the formation of excimer, 1ar/PS composite films with increasing concentrations of 1ar were prepared (Fig. S21, ESI†). As the concentration of 1ar in the 1ar/PS composite film increased, a structureless yellow emission at 565 nm appeared at 20 wt% or more. In addition, the fluorescence lifetime of the blue and yellow emissions of these films was similar to that of 1ar crystal, and the emission colour approaches white light as the concentration of 1ar increases. The observation of such concentration-dependent emission can be attributed to excimer emission caused by the molecules packing more tightly together at higher concentrations, as in the 1ar crystal.
Conclusions
In summary, white light emission based on dual emission of oxidative photocyclization condensates of diarylethene in the solid state was reported. In dilute solutions and in PS composite films with concentrations of 10 wt% or less, the condensation product shows deep blue emission due to the absence of intermolecular orbital interactions, but in the aggregated state, it shows structureless yellow emission due to the formation of intermolecular orbital interactions in addition to the monomer-derived blue emission. Here, 1ar is SMWLE, which in the crystalline state shows white emission at CIE1931 coordinates of (0.31, 0.30). Our experimental and theoretical investigations show that white light emission is based on a balanced dual emission produced by two conformers with different stacking patterns and overlapping areas in the crystal. Interestingly, the packing structure with these two conformers, which results in white light emission, is achieved by self-assembly. Therefore, white light emission of 1ar was observed in all of the crystal, powder, and cast film. White light is used in a variety of applications, including the backlight of display, lighting, and illumination. Although inorganic materials still account for a majority of these devices, some works are starting to use organic materials. The white light emission achieved by self-assembly of 1ar has the potential to provide excellent processability in the fabrication of these devices. Thus, we believe our current results will provide important clues for the development of organic materials for applications in the future.Author contributions
Y. Nakagawa and K. Kinoshita synthesized all materials. Y. Nakagawa grew the crystals, performed all photophysical measurements and analyses, and prepared the paper. R. Nishimura and M. Morimoto performed the X-ray crystallographic analysis. Y. Nakagawa, S. Yokojima, M. Hatakeyama, and Y. Sakamoto performed the theoretical calculations. S. Yokojima and S. Nakamura supervised the theoretical calculations. M. Kasuno assisted the pH measurement. K. Uchida supervised the research. So, S. Yokojima, S. Nakamura, and K. Uchida jointly supervised this work. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to Zeon Co., Ltd, for providing octafluorocyclopentene to synthesize the diarylethene derivatives with a perfluorocyclopentene moiety. This work was supported by JSPS KAKENHI grant number 20J21342 in JSPS Fellows, CREST program grant JPMJCR17N2 of the Japan Science and Technology Agency, and the Nanotechnology Platform of the Ministry of Education, Culture, Sports, Science and Technology (MEXT) grant number JPMXP09S20NR0028. The authors express their thanks to Y. Nishikawa, M. Yamagaki, Y. Shimizu, and T. Kawai of Nara Institute of Science and Technology (NAIST) for their help in taking measurements of high-resolution mass spectra (HRMS).Notes and references
- J. H. Burroughes, D. D. C. Bradley, A. R. Brown, R. N. Marks, K. Mackay, R. H. Friend, P. L. Burns and A. B. Holmes, Nature, 1990, 347, 539–541 CrossRef CAS.
- O. Ostroverkhova, Chem. Rev., 2016, 116, 13279–13412 CrossRef CAS PubMed.
- Y. Yang, Q. Zhao, W. Feng and F. Li, Chem. Rev., 2013, 113, 192–270 CrossRef CAS PubMed.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361–5388 RSC.
- J. Tong, Y. J. Wang, Z. Wang, J. Z. Sun and B. Z. Tang, J. Phys. Chem. C, 2015, 119, 21875–21881 CrossRef CAS.
- Q. Li and Z. Li, Adv. Sci., 2017, 4, 1600484 CrossRef PubMed.
- Y. Yin, M. U. Ali, W. Xie, H. Yang and H. Meng, Mater. Chem. Front., 2019, 3, 970–1031 RSC.
- S. Mukherjee and P. Thilagar, Dyes Pigm., 2014, 110, 2–27 CrossRef CAS.
- G. M. Farinola and R. Ragni, Chem. Soc. Rev., 2011, 40, 3467–3482 RSC.
- K. T. Kamtekar, A. P. Monkman and M. R. Bryce, Adv. Mater., 2010, 22, 572–582 CrossRef CAS PubMed.
- R. W. Pridmore, Color Res. Appl., 2009, 34, 233–252 CrossRef.
- L. Simonot and M. Hébert, J. Opt. Soc. Am. A, 2014, 31, 58–66 CrossRef PubMed.
- V. K. Vishwakarma, M. R. Nagar, N. Lhouvum, J. H. Jou and A. A. Sudhakar, Adv. Opt. Mater., 2022, 10, 2200241 CrossRef CAS.
- Z. He, W. Zhao, J. W. Y. Lam, Q. Peng, H. Ma, G. Liang, Z. Shuai and B. Z. Tang, Nat. Commun., 2017, 8, 416 CrossRef PubMed.
- Q. Y. Yang and J. M. Lehn, Angew. Chem., Int. Ed., 2014, 53, 4572–4577 CrossRef CAS PubMed.
- Y. H. Chen, K. C. Tang, Y. T. Chen, J. Y. Shen, Y. S. Wu, S. H. Liu, C. S. Lee, C. H. Chen, T. Y. Lai, S. H. Tung, R. J. Jeng, W. Y. Hung, M. Jiao, C. C. Wu and P. T. Chou, Chem. Sci., 2016, 7, 3556–3563 RSC.
- Z. Xie, C. Chen, S. Xu, J. Li, Y. Zhang, S. Liu, J. Xu and Z. Chi, Angew. Chem., Int. Ed., 2015, 54, 7181–7184 CrossRef CAS PubMed.
- Z. R. Grabowski, K. Rotkiewicz and W. Rettig, Chem. Rev., 2003, 103, 3899–4032 CrossRef PubMed.
- X. H. Jin, C. Chen, C. X. Ren, L. X. Cai and J. Zhang, Chem. Commun., 2014, 50, 15878–15881 RSC.
- Y. Yang, M. Lowry, C. M. Schowalter, S. O. Fakayode, J. O. Escobedo, X. Xu, H. Zhang, T. J. Jensen, F. R. Fronczek, I. M. Warner and R. M. Strongin, J. Am. Chem. Soc., 2006, 129, 14081–14092 CrossRef PubMed.
- D. Li, W. Hu, J. Wang, Q. Zhang, X. M. Cao, X. Ma and H. Tian, Chem. Sci., 2018, 9, 5709–5715 RSC.
- S. Samanta, U. Manna and G. Das, New J. Chem., 2017, 41, 1064–1072 RSC.
- K. Pal, V. Sharma and A. L. Koner, Chem. Commun., 2017, 53, 7909–7912 RSC.
- N. Kapuria, V. Sharma, P. Kumar and A. L. Koner, J. Mater. Chem. C, 2018, 6, 11328–11335 RSC.
- Y. Wang, Q. Sun, L. Yue, J. Ma, S. Yuan, D. Liu, H. Zhang, S. Xue and W. Yang, Adv. Opt. Mater., 2021, 9, 2101075 CrossRef CAS.
- J. Ma, Y. Zhou, H. Gao, F. Zhu and G. Liang, Mater. Chem. Front., 2021, 5, 2261–2270 RSC.
- S. Kundu, B. Sk, P. Pallavi, A. Giri and A. Patra, Chem. – Eur. J., 2020, 26, 5557–5582 CrossRef CAS PubMed.
- Y. Ge, Y. Wen, H. Liu, T. Lu, Y. Yu, X. Zhang, B. Li, S. T. Zhang, W. Li and B. Yang, J. Mater. Chem. C, 2020, 8, 11830–11838 RSC.
- T. Schillmöller, R. Herbst-Irmer and D. Stalke, Adv. Opt. Mater., 2021, 9, 2001814 CrossRef.
- B. Shi, D. Nachtigallová, A. J. A. Aquino, F. B. C. Machado and H. Lischka, Phys. Chem. Chem. Phys., 2019, 21, 9077–9088 RSC.
- A. Sakai, E. Ohta, Y. Yoshimoto, M. Tanaka, Y. Matsui, K. Mizuno and H. Ikeda, Chem. – Eur. J., 2015, 21, 18128–18137 CrossRef CAS PubMed.
- Y. Zhang, Y. Miao, X. Song, Y. Gao, Z. Zhang, K. Ye and Y. Wang, J. Phys. Chem. Lett., 2017, 8, 4808–4813 CrossRef CAS PubMed.
- B. Li, Z. Li, F. Guo, J. Song, X. Jiang, Y. Wang, S. Gao, J. Wang, X. Pang, L. Zhao and Y. Zhang, ACS Appl. Mater. Interfaces, 2020, 12, 14233–14243 CrossRef CAS PubMed.
- Z. He, X. Cai, Z. Wang, D. Chen, Y. Li, H. Zhao, K. Liu, Y. Cao and S. J. Su, Sci. China: Chem., 2018, 61, 677–686 CrossRef CAS.
- N. J. Hestand and F. C. Spano, Chem. Rev., 2018, 118, 7069–7163 CrossRef CAS PubMed.
- F. Würthner, T. E. Kaiser and C. R. Saha-Möller, Angew. Chem., Int. Ed., 2011, 50, 3376–3410 CrossRef PubMed.
- D. H. Waldeck, Chem. Rev., 1991, 91, 415–436 CrossRef CAS.
- M. Irie, Chem. Rev., 2000, 100, 1685–1716 CrossRef CAS PubMed.
- Rohini, M. Baral and B. K. Kanungo, RSC Adv., 2016, 6, 108017–108027 RSC.
- M. J. Snare, P. J. Thistlethwaite and K. P. Ghiggino, J. Am. Chem. Soc., 1983, 105, 3328–3332 CrossRef CAS.
- V. Kumar, B. Sk, S. Kundu and A. Patra, J. Mater. Chem. C, 2018, 6, 12086–12094 RSC.
- F. M. Winnik, Chem. Rev., 1993, 93, 587–614 CrossRef CAS.
- S. Karuppannan and J. C. Chambron, Chem. – Asian J., 2011, 6, 964–984 CrossRef CAS.
- T. Smith and J. Guild, Trans. Opt. Soc., 1931, 33, 73 CrossRef.
- C. Wang and Z. Li, Mater. Chem. Front., 2017, 1, 2174–2194 RSC.
- J. Yang, J. Qin, P. Geng, J. Wang, M. Fang and Z. Li, Angew. Chem., 2018, 130, 14370–14374 CrossRef.
- C. Reichardt, Chem. Rev., 1994, 94, 2319–2358 CrossRef CAS.
- Y. Zhao, Y. Guo and Y. Liu, Adv. Mater., 2013, 25, 5372–5391 CrossRef CAS PubMed.
- P. Han, Z. Xu, C. Lin, D. Ma, A. Qin and B. Z. Tang, J. Mater. Chem. C, 2020, 8, 7012–7018 RSC.
- Z. Shen, X. Zhu, W. Tang, X. J. Feng, Z. Zhao and H. Lu, J. Mater. Chem. C, 2020, 8, 9401–9409 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, supplementary figures and tables. CCDC 2076557. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2ma00670g |
| This journal is © The Royal Society of Chemistry 2022 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: