Open Access Article
Open Access ArticleAdvances in biopolymeric active films incorporated with emulsified lipophilic compounds: a review
Ilyes Dammak ab,
Carla Giovana Lucianob,
Luis Jaime Pérez-Córdoba
ab,
Carla Giovana Lucianob,
Luis Jaime Pérez-Córdoba b,
Maria Lúcia Monteiroa,
Carlos Adam Conte-Junior
b,
Maria Lúcia Monteiroa,
Carlos Adam Conte-Junior *a and
Paulo José do Amaral Sobralbc
*a and
Paulo José do Amaral Sobralbc
aGraduate Program in Food Science (PPGCAL), Institute of Chemistry (IQ), Federal University of Rio de Janeiro (UFRJ), Cidade Universitária, Avenida Athos da Silveira Ramos, no. 149, Bloco A, 5° andar, sala 534 e 545, Rio de Janeiro, RJ, 21941-909, Brazil. E-mail: conte@iq.ufrj.br; Tel: +55-21-3938-7825
bDepartment of Food Engineering, FZEA, University of São Paulo (USP), Pirassununga, SP, Brazil
cFood Research Center (FoRC), University of São Paulo (USP), São Paulo (SP), Brazil
First published on 19th August 2021
Abstract
The attention towards active films has increased due to consumer demand for high-quality foods without chemical additives. Active biopolymer-based films have shown great potential for active films by impacting food safety, acting as the carriers of various natural antioxidant and antimicrobial compounds, and decreasing environmental pollution from petrol-derived packaging materials. However, there is a wide range of challenges concerning the different characteristics of biopolymers and plasticizers, often hygroscopic/hydrophilic, compared to numerous lipophilic bioactive compounds. Therefore, recent studies have focused on applying oil-in-water emulsion-based systems to enhance the lipophilic bioactive compounds' dispersibility into the film matrix, improving their performance. It is worth emphasizing that resulting complex systems give rise to new challenges such as (i) dispersion technology of the bioactive compounds with minimum adverse effects on its bioactivities, (ii) interactions between different components of the active films, giving rise to new physicochemical properties, and (iii) the change of the diffusion properties of bioactive compounds into the active films, resulting in different release properties. These challenges are profound and critically discussed in this review, as well as the encapsulation techniques employed in preparing emulsions loaded with lipophilic bioactive compounds for the active film development. An outlook of future directions in the research, development, and application of these active films are given.
Introduction
Food packaging aims to contain food in a cost-effective way that satisfies industry requirements and consumer desires, maintains food safety, and minimizes environmental impact.1 This concept is valid, including a passive packaging system. Nevertheless, a more or less recent concept is that of active packaging, which contains deliberately incorporated components intended to release (controlled) or absorb substances into or from the packaged food or the environment surrounding the food.2 In principle, this technique can imply the reduction of additives into packaged foods. Active packages can have several activities, such as oxygen and ethylene scavenging, carbon dioxide emitting, and antimicrobial and antioxidant activities, among others.3–6One specific kind of active packaging is this one based on active films, defined as a carrier system for active ingredients. The development of active films is a relatively new concept in food technology. It is based on the incorporation of bioactive compounds inside the film matrices.6,7 Besides the traditional packaging functions,1 active films provide different functions that do not exist in passive films as an antioxidant3–5 and/or antimicrobial protection,8 among others, depending on the incorporated active compounds into films. Despite their excellent benefits, biopolymeric active films lack mechanical characteristics and are highly sensitive to moisture, which represents the main limitations for their commercial use.9 The main biopolymers used in the production of active films are polysaccharides, as starch, chitosan, pectin, cellulose derivatives and different gums10 and proteins, especially gelatin, gluten, zein, soy proteins, milk proteins, among others.11,12
Despite many advantages, these films are still produced on laboratory scale due to the problems previously cited. Therefore, it is essential to overcome these difficulties for scaling up the production to industrial scale, and making these films commercially successful.13 More fundamental concepts or potential applications of active films can be found in several reviews2,3,14,15 or books.14,16–19
Several lipophilic bioactive compounds (i.e., essential oils, carotenoids, α-tocopherol, flavonoids, aromas) have shown suitable biological activities, such as antioxidants and/or antimicrobial activities, and have even been applied in biopolymeric films.5,20–26 Examples of active commercial agents for active food packaging can be found in the excellent review by Vilela et al.6
Numerous research studies on the application of these lipophilic bioactive compounds in the development of active films have been performed. However, the physical and chemical stability of the incorporated lipophilic bioactive compounds into the biopolymeric matrices was not fulfilled for the long-term.27 Indeed, it is highly challenging to disperse them uniformly in a hydrophilic matrix due to its hydrophobic characteristic.28,29 Usually, water is the solvent used in biopolymer-based films.
Additionally, some bioactive compounds with antioxidant and/or antimicrobial activities, i.e., essential oils (EO), have a high vapor pressure, causing evaporation over time and a rapid decrease of its concentration in the film matrix, principally during drying of the film-forming solution, thus affecting the sensory properties of the foodstuffs, which can be unacceptable.30–32
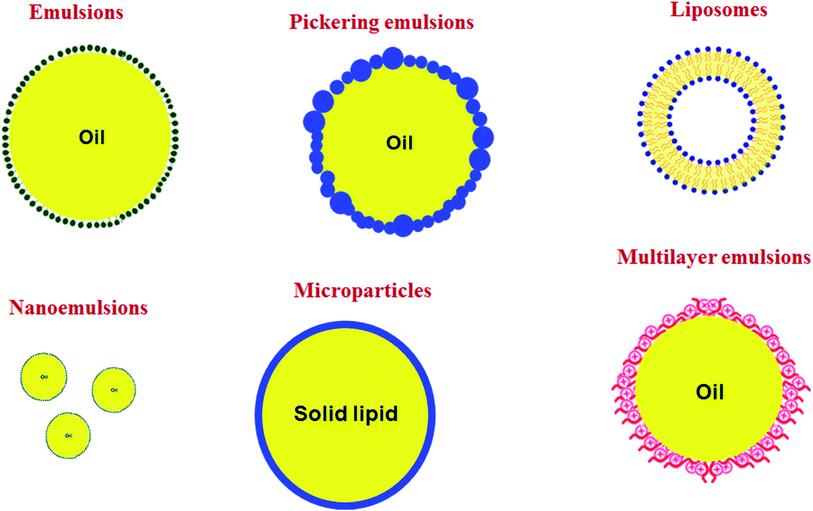
Recently, many research works aim to overcome these difficulties through the use of oil-in-water (O/W) emulsions-based delivery systems allowing enhanced dispersibility into a film matrix, therefore producing stable active films. Moreover, these emulsion-based systems have a proven role in protecting the chemical stability of encapsulated bioactive compounds.33–35 Micro/nanoemulsions, liposomes, solid-lipid micro/nanoparticles, and Pickering emulsions are examples of many emulsion-based systems often used to encapsulate lipophilic bioactive compounds for the food industry.
In this context, this review aims at summarizing the recent contribution to the literature regarding the development of active films incorporated with emulsion-based systems. The first part of this review gives a survey of the principal concepts for the fabrication of emulsions loaded with lipophilic bioactive compounds. The second part of this review is ongoing research on the resulting physicochemical properties of the recently produced active films in the literature. In addition, this review will summarize examples of the recent applications of active films for food packaging applications. In the last part, it will provide insights into the future trends in this relevant area.
O/W emulsions as carriers of lipophilic bioactive compounds
Emulsions production
An emulsion is a colloidal system that consists of two or more immiscible phases, where one of the phases was dispersed (dispersed or non-continuous phase) as fine droplets in the other phase (dispersing or continuous phase).36 The O/W (oil-in-water) emulsion is the most used for active films production because water is its main solvent.16 Thus, the formation of an emulsion is always thermodynamically unfavorable because of the increase in the interfacial area after emulsification. Using an adequate emulsifier, the interfacial tension is decreased, and these systems are therefore thermodynamically stable. This type of thermodynamically stable system is usually referred to as a microemulsion to distinguish it from thermodynamically unstable (macro)emulsions.37 Commonly, these emulsions have a mean droplet size of approximately 1 μm. If it has a droplet with a mean size lower than 100 nm, it is a so-called nanoemulsion.38Emulsifiers usually stabilize the emulsions, but the so-called “Pickering emulsions” can also be stabilized by solid particles.39 The most common emulsifiers used in food emulsions are small-molecule surfactants (e.g., monoglycerides, polyglycerol esters of fatty acids, sorbitan monostearate, polyoxyethylene sorbitan monostearate), phospholipids (e.g., lecithin), proteins (e.g., gelatin, sodium caseinate, whey protein, egg protein) and polysaccharides (e.g., pectin, arabic gome, modified starches, modified celluloses), and are usually amphiphilic molecules that absorb to the surface of freshly formed droplets during homogenization, forming a protective membrane that prevents the droplets from coming close enough together to aggregate.37 Information on natural emulsifiers can be found in the review by Dammak et al.38
Depending on the energy consumption, high-energy or low-energy homogenization methods can be used to produce emulsions.38,40,41 Low-energy methods consist of mixing the oil-water-emulsifier with a spontaneous formation of droplets.42,43 These methods are of importance from an economic viewpoint, and as a potential carrier to protect labile molecules that are eventually sensitive to some processing stress.44 In these methods, the physicochemical behavior of the emulsifiers primarily controls the production and stability of the emulsion. Thus, the selection of the emulsifier or combinations of emulsifiers is of paramount importance.45,46
High-energy methods employing mechanical apparatuses to create a strong homogenization force that splits up the oil droplets into smaller ones include the high-pressure valve homogenizer, microfluidizer, and sonication methods.47,48 Evidently, the quality (droplet size distribution) is a function of the quantity of applied energy during homogenization, but it can also be affected by the nature of the components (i.e., emulsifiers).49 High-pressure homogenization generally produces an emulsion with submicron droplets. Therefore, it is generally used for producing emulsions in the food industry.50,51 A high-speed homogenizer, such as an ultraturrax, was able to produce nanoemulsions when used at very high (ca. 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm) speed.52 However, when it was used with a lower speed (ca. 15
000 rpm) speed.52 However, when it was used with a lower speed (ca. 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 rpm), it produced only a sub-micrometric emulsion.53
500 rpm), it produced only a sub-micrometric emulsion.53
Emulsions stability
It is essential to produce a stable emulsion for getting persistent characteristics, i.e., without phase separation during its shelf life.54 Usually, the emulsion stability is linked to the droplet size distribution, zeta-potential, emulsifier characteristics, and adsorption dynamic mechanism of biopolymers in the oil–water interface. First, the mean droplet size in an emulsion is influenced by the effectiveness of an emulsifier to quickly adsorb to the droplet surfaces during homogenization, decreasing the surface tension of the system.55 The zeta-potential is the effective surface potential of a droplet suspended in a medium, which takes into account that charged species in the surrounding medium may adsorb to the surface of the droplet and alter its net charge.37 Emulsifiers can change the density of the droplets by creating a dense layer around them, reducing the difference in density between the droplets and the surrounding medium, thereby avoiding destabilization.37,56,57 Furthermore, the dynamic interfacial adsorption of biopolymers at the oil–water interface is linked to the evolution of interfacial tension with time.58 Readers interested in the instability mechanisms of nanoemulsions are advised to read the excellent review by Karthik et al.59Usually, the stability (phase separation, creaming) of emulsions is studied visually over a long period of storage, or by monitoring the evolution of the droplet size distribution, zeta-potential, and/or interfacial tension during storage under controlled conditions using appropriate apparatuses.24,35,58,60 Nevertheless, the most used apparatus to study the stability of nanoemulsions by monitoring the phase separation front is the Turbiscan, a vertical scan analyzer whose reading head is composed of a pulsed near-IR light source (λ = 850 nm) and two synchronous detectors (transmission and backscattering), which can detect the change of the droplet size of the nanoemulsions due to coalescence and/or flocculation phenomena, and the gravitational separation of the phases by sedimentation or creaming processes, as a function of the sample height into a cylindrical glass tube.61
The characterization of physical stability can also be monitored using the LUMiSizer® analytical centrifugation test (LUMiSizer®, L.U.M. GmbH, Berlin, Germany), which is considerably faster and more precise than the other analytical techniques based on Earth's gravity creaming.50 In brief, this novel instrument allows the measurement of the intensity of the transmitted lights (near-infrared light 865 nm or blue light 470 nm) across emulsions under centrifugation. The transmittances are displayed as a function of the axial position, equal to the distance from the center of rotation (transmission profiles). The shapes and kinetics of the transmission profiles contain information on the rates of the destabilization processes, as well as the evaluation of droplet–droplet interactions (Fig. 1), allowing the understanding of destabilization phenomena, such as flocculation, coalescence, creaming, gravitational separation, and Ostwald ripening.58,62
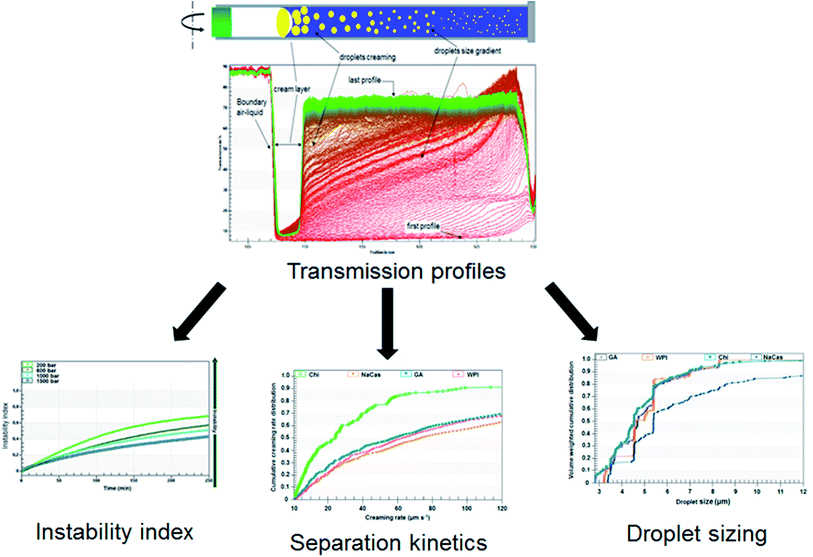 | ||
| Fig. 1 Schema of accelerated stability evaluation of emulsions using LUMiSizer® analytical centrifugation test.54,55 | ||
It will be worth noting that the effect of the temperature (5–60 °C) on the separation kinetics can be analyzed using the LUMiSizer®. The separation kinetics under the Earth's gravity of the emulsions can also be estimated by the LUMiSizer® analyzer using the front-tracking method.57 Indeed, different experiments at various relative centrifugation forces (RCF) should be carried out for the emulsion sample, and the resulting separation kinetics at each RCF should be plotted versus the applied RCF.
Dammak and Sobral62 performed a LUMiSizer® analytical centrifugation test for selecting the appropriate biopolymer emulsifiers. They observed that despite the similarity of the registered droplet sizes between the different tested biopolymer emulsifiers, differences were noted regarding the cumulative creaming rate distributions. It was attributed to differences in the stabilization mechanism with each biopolymer emulsifier. The results revealed that modified chitosan (deacetylation degree ≥ 75%) reduced the creaming rate compared to the other tested biopolymers.
These authors62 tested the stability of chitosan-stabilized emulsions at three different temperatures (20, 40, and 60 °C) using the LUMiSizer®. The creaming rate at different running temperatures can be quantitatively characterized, giving a mean creaming rate. The quantitative analysis obtained by using the LUMiSizer® gave a better understanding level of the significant factors that influence the formulation and the stabilization of emulsions, thus promoting the advancement of the emulsion-based system progress. Moreover, the knowledge of the emulsion stability in high temperature can be important because the emulsion can be added to the film-forming solution at temperatures above the room temperature. Furthermore, if it is not stable in that condition, the emulsion structure can be lost during film processing, and the bioactive compounds will not be well-dispersed into the film.
Structured emulsion-based systems
Emulsion-based systems can be produced with different structured designs (Fig. 2).63 The emulsion's ingredients and processing parameters need to be wisely selected for each type of bioactive compounds, taking into account its molecular and physicochemical properties, i.e., oil solubility, water-solubility, oil–water partition coefficient, melting point, viscosity, polarity, and chemical/biochemical stability. Some examples of these emulsion-based systems are shown in Table 1, and their properties are briefly described as follows.| Technique | Dispersed phase | Continuous phase | Emulsifier | Bioactive compounds | Droplet size distribution (nm) | Polydispersity index (—) | Viscosity (mPa s) | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Essential oil.b Medium chain triglyceride. | ||||||||
| Rotor–stator (Ultra-Turrax) + Microfluidizer | Thymol-EOa, lemongrass-EO or sage-EO (1% v/v) | Sodium alginate solution (3% w/v) | Tween 80 (3% v/v) | Thymol-EO, lemongrass-EO, sage-EO | 20–190 | 0.52–0.65 | 452–616 | Acevedo-Fani et al.107 |
| Rotor/stator (Ultra-Turrax) | Cinnamaldehyde (2% w/w) | Ultrapure water | Tween 80 (1.5% w/w) | Cinnamaldehyde | 20–500 | 0.22–0.30 | — | Otoni et al.70 |
| Servodyne mixer | Carvacrol (0.67% w/w) | Isolated soy protein solution (10% w/w) | Tween 60 (0.5% w/w) Acetema (1% w/w) | Carvacrol | 117 | 0.41 | — | Otoni et al.108 |
| Servodyne mixer | Cinnamaldehyde | Isolated soy protein solution (10% w/w) | Tween 60 (0.5% w/w) Acetem (1% w/w) | Cinnamaldehyde | 112 | 0.57 | — | Otoni et al.108 |
| Ultrasonicator | Clove bud-EO | Double-distilled water | Tween 80 (3% w/w) | Clove bud-EO | 250.4 | 0.16 | — | Otoni et al.70 |
| Ultrasonicator | Oregano-EO | Double-distilled water | Tween 80 (3% w/w) | Oregano-EO | 180.6 | 0.22 | — | Otoni et al.108 |
| Spontaneous emulsification (SE) | MCTb (75% w/w) + thymol-EO (25% w/w) | Milli-Q ultrapure water | Tween 80 (10% w/w) | Thymol-EO | 54.1 | 0.2 | — | Robledo et al.111 |
| Ultrasonic emulsification (US) | MCT (75% w/w) + thymol (25% w/w) | Milli-Q ultrapure water | Tween 80 (10% w/w) | Thymol | 132.4 | 0.26 | — | Robledo et al.111 |
| SE + US | MCT (75% w/w) + thymol (25% w/w) | Milli-Q ultrapure water | Tween 80 (10% w/w) | Thymol | 144.3–131.8 | 0.20–0.26 | — | Robledo et al.111 |
| Ultrasonic emulsification | (10% w/w) Thymus daenensis-EO (cultivated and wild) | (10% w/w) tween 80 in water + (0.5% w/w) lecithin as co-emulsifier | (10% w/w) tween 80 + (0.5% w/w) lecithin | Thymus daenensis-EO | 223.5–274.7 | 0.05–0.07 | — | Moghimi et al.115 |
| Ultrasonic emulsification | Ginger-EO | Distilled water | Tween 80 | Ginger-EO | 20–100 | 0.22 | — | Noori et al.132 |
| Ultra-turrax/microfluidizer | Mixture of Oregano-EO and tween 80 | Sodium alginate and mandarin fiber water | Tween 80 (2.5% w/w) + sodium alginate (2.0% w/w) + mandarin fiber (0.5% w/w) | Oregano-EO | 169–337 | — | 265–366 | Artiga-Artigas et al.133 |
| Ultra-turrax/high pressure homogenization | Carvacrol-EO and sunflower oil | Distilled water | Tween 20 + glycerol monooleate/whey protein isolates | Carvacrol-EO | 60–165 | 0.24–0.42 | — | Tastan et al.134 |
| Ultra-turrax/ultrasonic homogenizer | Nettle-EO | Distilled water + tween 40 (20% w/w) + glycerol plasticizer (15% w/w) + jujube gum | Tween 40 (20% w/w) + glycerol (15% w/w) and jujube gum | Nettle-EO | 63.1–240.1 | 0.21–0.59 | 1.53–2.27 | Gharibzahedi and Mohammadnabi135 |
| Spontaneous emulsification | Cinnamaldehyde, MCT, and tween 80 | Distilled water | Tween 80 (7.5% w/w) | Cinnamaldehyde | — | — | — | Chen et al.110 |
| Ultrasonic emulsification | Eucalyptus oil | Water | Tween 80 + tween 20 | Eucalyptus oil | 9.4 | 0.12 | — | Sugumar et al.136 |
| Ultra-turrax | 0.8 g L−1 of span 80 and 2 g L−1 of α-tocopherol | 10 g L−1 of glycerol, 4.2 g L−1 of tween® 80 | Span 80 (0.8 g L−1) + 4.2 g L−1 of tween 80 | α-Tocopherol | 190 | 0.12 | — | Zambrano-Zaragoza et al.137 |
| Thin-film hydration and sonication method | Nanoliposomes/Nettle extract | Distilled water | Lecithin | Nettle (Urtica dioica L.) extra | 122–136 | 0.26–0.28 | — | Haghju et al.112 |
| Ultra-turrax/ultrasonic/rotary evaporator | Sunflower oil and ethyl acetate | (2.5% w/w) sodium caseinate and (2% w/w) glycerol | (2.5% w/w) NaCas | TiO2 | 40–60 | — | — | Montes-de-Oca-Avalos et al.116 |
| Emulsion-ionic gelation technique | Citrus-EO | Chitosan solution (1% w/v) | Tween 80 (0.3% v/v) | Citrus-EO | 269–428 | 0.35–0.65 | — | Wu et al.138 |
| Film hydration method | Chloroform | Phosphate buffer | Soybean lecithin | Nisin | 140.4–516.1 | 0.14–0.27 | — | Boelter and Brandelli113 |
Nanoemulsions
Nanoemulsions are emulsions with a very fine droplet size, below 100 nm,64,65 or below 500 nm, according to some authors.49,66 The small droplet size in the nanoemulsion has two significant consequences: (i) enhancing the dynamic stability, and (ii) the ability to improve the bioactivity of the lipophilic compounds by increasing its specific surface area.67Recent studies have shown an enhancement of the antimicrobial activity in nanoemulsions encapsulating EO,68,69 possibly due to their specific physicochemical and functional properties compared to conventional emulsions. Espitia et al.41 published a well-detailed review presenting the surfactants (including naturally occurring proteins and carbohydrates), dispersants, and oil-soluble functional compounds used for designing food-grade nanoemulsions intended for packaging applications. Furthermore, Dammak et al.38 published a review on the production of nanoemulsions using emulsifiers from natural sources.
Several authors produced active films by incorporating nanoemulsions prepared via ultrasounds or high-speed homogenizations.8,21,70–74 Dammak and Sobral50 have used the two-step technique to prepare O/W nanoemulsions loaded with routine-loaded O/W nanoemulsions (Fig. 3). The first-step emulsification with a rotor–stator homogenizer was used to prepare a coarse O/W emulsion. After microfluidization of the coarse O/W, a fine emulsion was produced at operating pressures of 100 MPa. This nanoemulsion was used to produce gelatin-based films with high antioxidant activity.51 Tonyali et al.75 prepared pullulan-based films activated by incorporation of nanoemulsions encapsulating thymol, cinnamaldehyde, and eugenol, and produced using a two-stage valve homogenizer at 50 MPa for three passes. Other examples of films produced with nanoemulsions are shown in Table 2. The nanoemulsion allows a good dispersion of the bioactive compound into the biopolymer matrix due to the low dimension of the oil droplets.
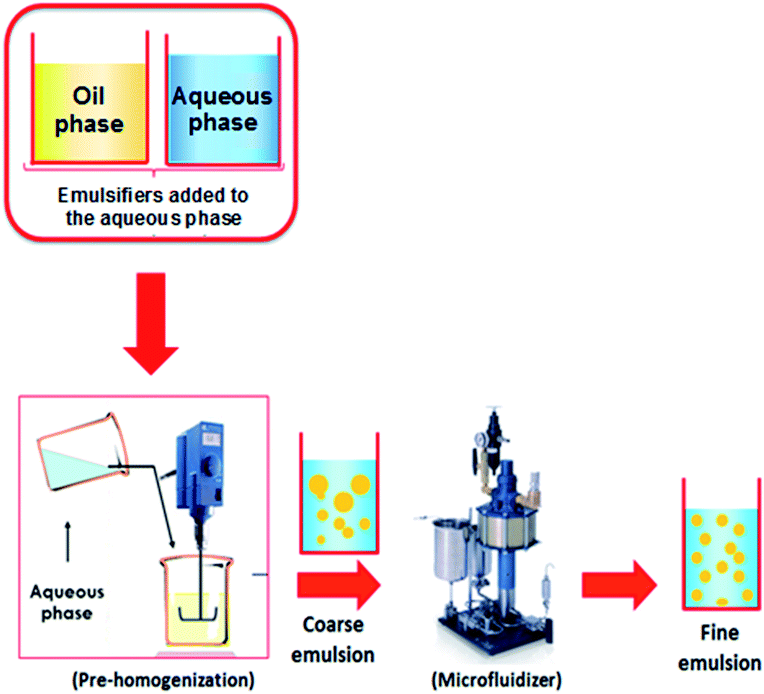 | ||
| Fig. 3 Nanoemulsions elaboration using two-step emulsification method.46 | ||
| Film matrix | Bioactive compound | Emulsion system | Functional properties | Food application and storage conditions | Ref. |
|---|---|---|---|---|---|
| a FFS: film forming solution. | |||||
| Gelatin, gelatin–chitosan and gelatin–sodium caseinate films | α-Tocopherol, cinnamaldehyde and garlic essential oil (2.5% w/w) | Nanoemulsions | Effective antioxidant activity as ABTS radical scavenger | — | Pérez-Córdoba and sobral72 |
| Basil seed gum films | Zataria multiflora essential oil (1, 2 and 3% w/w) | Nanoemulsions | Antibacterial effect against E. coli and B. cereus | — | Hashemi Gahruie et al.139 |
| Soluble soybean polysaccharide coating | Cinnamon essential oil (0.6 and 0.8% v/v) | Nanoemulsions | Antimicrobial effect against S. aureus, Streptococcus pyogenes E. coli, P. aeruginosa and Salmonella typhi. Also, inhibition the increase of total aerobic viable count, and yeast and mold growth in meat. Antioxidant activity observed using DPPH radical scavenger method | Meat refrigerated (4 °C for 8 days) | Ghani et al.140 |
| Hydroxypropyl methylcellulose films | Thymus daenensis EO (0.5, 1, 1.25 and 2.5% w/v) | Nanoemulsions | Antibacterial effect against E. coli, S. typhi, Shigella dysenetriae, Shigella flexneri, S. aureus, Staphylococcus epidermidis, Bacillus subtilis, Enterococcus faecalis, and the clinical strains Klebsiella peneumoniae, Acinetobacter baumannii, Enterococcus faecium, and methicillin-resistant Staphylococcus aureus. Antifungal effect against Candida albicans | — | Moghimi et al.115 |
| Sodium caseinate coating | Ginger (Zingiber officinale) essential oil (3 and 6% w/w) | Emulsions and nanoemulsions | Antibacterial effect against S. Typhimurium and L. monocytogenes, as well as useful in slowing down the psychrophilic bacteria, and mold and yeast. Slight antioxidant activity as DPPH radical scavenger | Chicken breast fillets (4 °C for 12 days) | Noori et al.132 |
| Quinoa protein/chitosan coating | Thymol (110 ppm) | Nanoemulsions | Antifungal effect against molds and yeast, and inhibition of inoculated Botrytis cinerea growth | Cherry tomatoes (25 °C for 7 days) | Robledo et al.111 |
| Chitosan and chitosan/quinoa protein films | Thymol (0.1% w/v) | Chitosan-tripolyphosphate nanoparticles | Potent effect on microbial viability, achieving growth inhibition of L. innocua, S. aureus, S. typhimurium, S. typhimurium, Enterobacter aerogenes, P. aeruginosa, and E. coli | — | Caro et al.141 |
| Thermoplastic corn starch sachets | Chitosan oligomer (0.3 g mL−1) | Direct incorporation in the polymeric matrix | Inhibitory effect against molds and yeasts growth | Strawberries, ricotta, and flavored bread (25 °C for 7 days) | Castillo et al.142 |
| Poly(vinyl alcohol) films | Tea polyphenols (0.5–4% w/w) | Direct incorporation in the polymeric matrix | Antimicrobial effect against E. coli and S. aureus and antioxidant activity as DPPH radical scavenger | — | Chenwei et al.143 |
| Polyhydroxybutyrate/polycaprolactone films | Nisin (50–4000 IU per cm3) | Direct incorporation in the polymeric matrix | Bacteriostatic inhibition effect over inoculated Lactobacillus plantarum | Cooked ham thermo-sealed under vacuum (5 °C for 28 days) | Correa et al.144 |
| Gelatin coating | Citric acid (0.5% and 1.0% w/w) | Direct incorporation in the polymeric matrix | Low microbial population growth (total bacterial) at the end of the storage, and had greater stability to lipid oxidation during the entire period, represented by TBARS values reduction | Ground beef (4 °C for 5 days) | Battisti et al.145 |
| Agar films | Protein hydrolysate or clove EOs (0.5% w/w) | Directly emulsified with the FFSa | Growth inhibition of total aerobic mesophiles, lactic acid bacteria, Pseudomonas spp. and H2S-producing microorganisms, as well as efficient to fish freshness in the last stages of chilled storage, since reduced the TVB-N/100 g sample values | Flounder (Paralichthys orbignyanus) fillets (5 °C for 15 days) | Rocha et al.146 |
| Oxidized corn starch-gelatin blend films | Ethyl lauroyl alginate (1.3% w/w) | Direct incorporation | Antibacterial effect contra Listeria innocua in marinated salmon. Enhancement of antimicrobial effectiveness against the total viable count | Vacuum packaged marinated salmon (5 °C for 45 days) | Moreno et al.147 |
| Low-density polyethylene films | Rosemary and cinnamon EOs (1 and 2% w/w) | Directly emulsified in the polymeric matrix | Reduction of total viable, Enterobacteriaceae, H2S producing bacteria, and psychrotrophic bacteria counts. Low volatile basic nitrogen (TVB-N) contents and thiobarbituric acid reactive substances (TBARS) values indicating inhibition of lipid oxidation | Pacific white shrimp (4 °C for 10 days) | Dong et al.148 |
| Soy protein isolate films | Clove EO (0.5% w/w) | Directly emulsified with the FFS | Antimicrobial effectivity to inhibit Pseudomonas spp., lactic bacteria, H2S-producer microorganisms, and Enterobacteriaceae growth, and antioxidant activity over time evaluated by the TVBN content and TBA index | Bluefin tuna (Thunnus thynnus) fillets (2 °C for 17 days) | Echeverría et al.149 |
| Chitosan/gelatine blend films | Silver ions (0.05% and 0.1% w/w) | Nanoparticles | Antifungal effect against mold and yeast | Red grape (Mimusops elengi) (4 °C for 25 days) | Kumar et al.150 |
| Low density polyethylene films | Copper ions (0.5, 1, 1.5, 2, 2.5 and 3.0% w/w) | Nanoparticles | Antimicrobial effect averse to E. coli and S. aureus, as well as reduction of total viable count cells growth | Peda (Indian sweet dairy product) (25 °C for two-days) | Lomate et al.151 |
| Gelatin or casein-based films | Nisin (0.5, 1.0 and 1.5 mg mL−1) | Liposomes | Antimicrobial effect against Bacillus cereus, Clostridium perfringens, and L. monocytogenes | — | Boelter and Brandelli113 |
| Chitosan coating | Satureja khuzestanica EO (1% v/v) | Nanoliposomes | Prolonged and consistent antimicrobial activity or retardation of microbial growth (total viable count, pseudomonas, and lactic acid bacteria) on meat pieces during storage, as well as inhibition of lipid oxidation showing antioxidant activity measured by TBARS assay | Lamb meat (4 °C for 20 days) | Pabast et al.152 |
| Gelatin-chitosan blend films | α-Tocopherol and garlic EO (5% w/w biopolymer) | Nanoemulsions | Reducing the initially inoculated population of L. monocytogenes and P. aeruginosa and high protective effect against aerobic mesophiles and psychrotrophic bacteria, total coliforms, and lactic acid bacteria, as well as reduction of lipid oxidation | Sliced mortadella sausage (6 °C for 7 days) | Pérez-Córdoba153 |
Solid-lipid microparticles
The physical properties of emulsions can also be monitored by using a lipid phase with a high melting temperature, therefore forming solid droplets following the emulsion preparation.76,77 Initially, the dispersed and continuous phases are heated to a temperature above the melting point of the oil phase. The ingredients are homogenized in the presence of an emulsifier. At this moment, the emulsion is chilled to promote the crystallization of the lipid droplets.This process has to be carried out carefully to avoid the expulsion of encapsulated bioactive compounds from the oil phase, and to avoid particle aggregation. When it is well done, the utilization of solid–lipid microparticles may improve the chemical stability of encapsulated bioactive compounds during storage, as well as controlling their release rate to the foodstuffs.78 This material has not been used to activate films.
Multilayer and double emulsions
The functional properties of an emulsion can be tailored after it has been prepared by adsorbing electrically charged polymers onto the oil droplet surfaces to form nanolaminated interfaces.79 This process is usually accomplished via a layer-by-layer electrostatic deposition approach. The emulsion is initially formed using a charged emulsifier so that it contains lipid droplets with a positive or negative charge. A solution of oppositely charged polymers is then mixed with the emulsions, which promotes the absorption of the polymers to the droplet surfaces through electrostatic attraction. This process can sometimes be repeated to form layers of different thickness, charge, rheology, chemical reactivity, and digestibility.80Multilayer emulsions (O/W) have been successfully tested in the encapsulation of b-carotene,81 resveratrol,82 ω-3 fatty acids,83 curcumin84 and α-lipoic acid,85 among others. Nevertheless, this kind of emulsion has not been used in the active film's technology. Moreover, double emulsions (W/O/W) encapsulating Pitanga (Eugenia uniflora L.) leaf hydroethanolic extract has been incorporated successfully into gelatin and/or chitosan-based films presenting antioxidant and antimicrobial activities.86,87
Liposomes
Liposomes are lipid vesicles artificially produced with one or more phospholipid bilayers to entrap water-soluble compounds within it. Therefore, liposomes can carry both hydrophilic and lipophilic bioactive compounds at once. Several studies investigated the encapsulation of lipophilic compounds, i.e., α-tocopherol,88 EO,89,90 lipophilic peptides,91 polyphenols,92 L-carnosine93 and carotenoids.94 In the food industry, it is essential to deeply explore the most critical factors that regulate the properties of liposomes before starting on a large scale.The liposome production at industrial levels is still rarely discussed in the scientific food literature. Moreover, the furthermost organic solvents that are usually used for liposomes preparation are toxic for food applications. Consequently, one first approach to applying liposomes in foods is to attempt to develop organic solvent-free methods that can be scaled up in the future.95 According to a recent literature review, few papers were published on the development of active films charged with liposomes (Table 2).
Pickering emulsions
Pickering emulsions are emulsions physically stabilized by solid colloidal nanoparticles. The particular advantage of the Pickering stabilization is the high stability of the emulsion with adsorbed solid nanoparticles at the oil–water interface, creating a stable and robust interfacial layer96,97 as an eggshell.98 The nanoparticles' layer provides high protection to oil droplets versus destabilizations mainly via steric stabilization.21Moreover, the Pickering emulsion provides another advantage through the capacity of nanoparticles to response to a trigger from the environment, allowing for the control of the release by applying temperature-, salt-, or pH stimulus.99–101 This functionality could lead to the potential development of active films, i.e., the trigger stimulates release of bioactive compounds from the film matrices to the foodstuffs.
Dammak and Sobral102 developed a different encapsulation approach through chitosan nanoparticles as an emulsifier for the preparation of O/W Pickering emulsions (Fig. 4). These authors outlined the importance to investigate the release kinetics in this system, and to attempt to test other lipophilic bioactive compounds. This emulsion was used to produce gelatin-based active films having oil droplets into the film matrix with the same dimension of that into the fresh emulsion, meaning that this emulsion was stable under film processing conditions.21 Moreover, Almasi et al.74 produced pectin-based films loaded with a Pickering emulsion or a nanoemulsion containing Marjoram (Origanum majorana L.) essential oil (MEO). They concluded that the active film provided unique features by the addition of the MEO-loaded Pickering emulsion as compared to the MEO-loaded nanoemulsion.
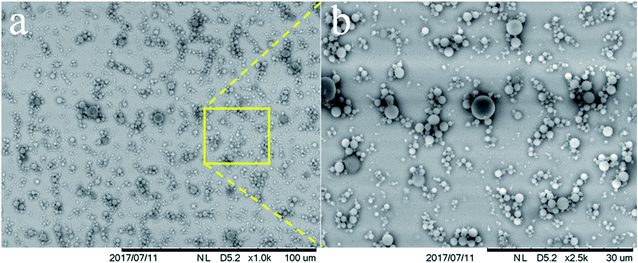 | ||
| Fig. 4 (a) SEM image of Pickering emulsion droplets stabilized with chitosan particles. (b) A magnified area of the small rectangle shown in (a).92 | ||
Physical properties of active films incorporated with emulsions
The addition of the previously described emulsion-based systems to the film-forming solution (FFS) allows the production of the material (macro-, micro-, or nanocomposite) with modified physicochemical and functional properties.4,103,104 Moreover, this addition can affect the rheological and visco-elastic properties of FFS, which must be monitored by stationary and/or dynamic (oscillatory) tests.50,57,105 This is important because the film production using the casting technique consists of properly drying the FFS onto a support. Thus, to apply FFS by simply pouring it onto the support (flowing by gravity), it is extremely important to use a FFS of low viscosity. However, the production of films using a spreading technique needs a more viscous FFS.105,106 Moreover, with a high viscosity FFS, emulsion droplets will be more protected against aggregation provoked by drag forces by water migration during FFS drying.86Usually, these films are produced in the laboratory scale, consisting of the preparation of a FFS by dispersing the biopolymer in warm or hot water, followed by addition of a plasticizer, and then the addition of the emulsion encapsulating the bioactive compound. This FFS is then spread on a convenient support and dried, usually at mild temperature, to produce a film. For more details on the preparation of active films incorporated with an emulsion, the reader is invited to consult other papers.21,24,52,70,107
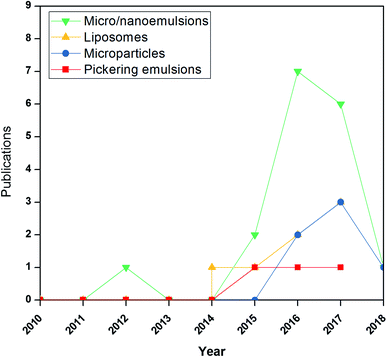
Studies on previously discussed emulsion-based systems for active films are relatively rare. Nevertheless, they show a growing research field. A search on Scopus using “micro/nanoemulsions, liposomes, micro/nanoparticle, Pickering, active films” as the title led to 20 results, of which 17 were published in 2014 or later (https://www.scopus.com/search/form.uri?display=basic) (Fig. 5). This surveillance can emphasize the new research area not yet investigated.
Table 2 summarizes some of the most recent studies on a range of bioactive compounds that have been encapsulated within emulsion-based systems, before being incorporated into film-forming solutions. The choice of emulsion-based system is a critical task that controls many resulting physicochemical and functional properties of active films. In principle, the nanoencapsulated active compounds throughout nanoemulsions or liposomes can provide a higher area-to-volume ratio with fine droplets, enhancing the antimicrobial/antioxidant activities of the films.30,103
Mechanical properties
Mechanical properties of the active films determine their performance during utilization and handling: tensile strength, related to the resistance to tension forces, and elongation at break, related to the film stretching capacity.107 Furthermore, the addition of oil droplets of EO in the polymeric matrix tends to produce weaker and stretchable films because of the decrease in the cohesion forces inside the structure.28 Some physical properties (water vapor barrier and mechanical properties) of active films incorporated with emulsions are presented in Table 3.| Film matrix | Water vapor permeability (g m−1 s−1 Pa−1) × 10−10 | Thickness (μm) | Tensile strength (MPa) | Young's modulus (MPa) | Elongation at break (%) | Puncture force (N) | Ref. |
|---|---|---|---|---|---|---|---|
| a Molecular weight. | |||||||
| Alginate thyme oil | 2.2 | 46 | 6.5 | 3.2 | — | 8.2 | Acevedo-Fani et al.107 |
| Alginate lemongrass oil | 2.1 | 42 | 4.8 | 7.8 | — | 8.5 | Acevedo-Fani et al.107 |
| Alginate sage oil | 1.9 | 38 | 5 | 4 | — | 9.5 | Acevedo-Fani et al.107 |
| Low methyl ester pectin | 2.2 | — | 6 | 125 | 139.3 | — | Otoni et al.108 |
| High methyl ester pectin | 2.7 | — | 7.62 | 74.42 | 169.6 | — | Otoni et al.108 |
| Isolated soy protein | 2.8 | 112 | — | — | — | — | Otoni et al.109 |
| Isolated soy protein | 2.8 | 111 | — | — | — | — | Otoni et al.109 |
| Methylcellulose | — | — | 6.1 | 56.79 | 34.08 | — | Otoni et al.108 |
| Methylcellulose | — | — | 7.6 | 72.94 | 54.77 | — | Otoni et al.108 |
| Quinoa protein/chitosan | 4.6 | 112 | 2.9 | — | 97.7 | — | Robledo et al.111 |
| Hydroxypropyl methyl cellulose | — | 239.2–233.4 | 19.3–22.6 | 64–62.5 | 9.02–14.2 | — | Moghimi et al.115 |
| Sodium alginate and mandarin fiber | 8.4–10 | — | — | — | — | — | Artiga-Artigas et al.133 |
| Chitosan (MWa = 278 kDa) | 3.9 | 61 | 7.6 | 0.26 | 12.61 | — | Chen et al.110 |
| Chitosan (MW = 190–310 kDa) | 0.11–0.12 | 140 | 48–5 | 15.1–16.4 | — | — | Haghju et al.112 |
| Sodium caseinate | 3.53 | 180 | 0.34 | 1 | 148 | — | Montes-de-Oca-Avalos et al.116 |
| Chitosan (MW = 186 kDa) | 1.73–1.97 | 86–100 | 17.7–25.9 | — | 14.94–19.49 | — | Wu et al.138 |
| Gelatin | — | 110–120 | 4.9 | 95.7 | 4.9 | — | Boelter and Brandelli113 |
| Casein | — | 75–90 | 1 | 5.3 | 143.7 | — | Boelter and Brandelli113 |
Acevedo-Fani et al.107 studied the incorporation of EO-loaded nanoemulsions prepared by microfluidization on sodium alginate films, and observed no significant difference in the tensile strength (about 5.5 MPa) of the films loaded with nanoemulsions containing thyme, lemongrass, or sage EO and the control (without charge). However, they found that the elongation at break for films produced with sage oil was around two times higher than the control films (around 78%), reinforcing the idea of the oil lubricant effect.
Otoni et al.70 also observed a plasticizing effect in pectin films with added cinnamaldehyde nanoemulsions. They observed that the addition of cinnamaldehyde nanoemulsions had no influence or slightly increased the tensile strength and Young's modulus values, as well as decreased the elongation values, with no effect of the oil droplet size into the emulsion. Furthermore, Mendes et al.53 observed that the incorporation of lemongrass essential oil (LEO)-loaded emulsions, regardless of the LEO content and droplet size, increased the extensibility of the thermoplastic starch films, suggesting that LEO acted as a plasticizer by increasing the flexibility of the biopolymer chains. Indeed, it is possible that LEO successfully interacts with fibers (gums), contributing to the emulsification of the system.
Some other authors have also found this same behavior. The addition of clove bud and oregano EO into methylcellulose films was studied by Otoni et al.108 using coarse and nanoemulsions. They reported that the EO reduced the Young's modulus and augmented the elongation at the break of the films. This also suggests a plasticizing effect, although it could be a single lubricant effect. Besides, films loaded with nanoemulsions of both EO presented significantly higher values of elongation at break compared to films with coarse emulsion, which indicates that the decrease of the droplet size was capable of producing more flexible films probably due to its better capacity in penetrating between adjacent biopolymer chains. On the other hand, the droplet size or EO addition did not influence the tensile strength of the films.
Otoni et al.109 studied the influence of the addition of micro- or nanoemulsions encapsulating carvacrol and cinnamaldehyde, produced using Acetem or Tween 60 as emulsifiers, on the mechanical properties of soy protein films. They reported a decrease in the tensile strength and Young's modulus of about 24 and 50%, respectively, for films loaded with nanoemulsions of carvacrol as compared to the control. However, the elongation at break increased two times. The reduction of the droplet size from micro- to nanoscale increased the elongation of the films with a reduction of the tensile strength and Young's modulus, reinforcing the hypothesis of the plasticizing effect.
Chen et al.110 also applied cinnamaldehyde nanoemulsions in chitosan films, observing that all films produced with nanoemulsions presented lower values of tensile strength and Young's modulus, and high values of elongation at break as compared to control films. Moreover, increasing the concentration of cinnamaldehyde nanoemulsions into films increased its tensile strength and Young's modulus. Robledo et al.111 also observed a tensile strength reduction in nanocomposite films produced with chitosan and quinoa protein loaded with thymol nanoemulsions.
Haghju et al.112 produced chitosan films with the addition of 0.5, 1.0, and 1.5% (w/w) of nettle extract (Urtica dioica L.), and observed a reduction on the tensile strength and an increase on the elongation at break of the chitosan films. In contrast, the addition of nettle extract encapsulated in liposomes led to lower values of tensile strength, and generally had no significant difference in the elongation at break. These authors suggested that the free nettle nanoemulsions acted as a plasticizer by reducing interactions among the macromolecule, and a discontinuity could explain the effect of nanoliposomes on the mechanical properties, which leads to a fragile structure.25
Boelter and Brandelli113 studied the addition of phosphatidylcholine liposomes encapsulating nisin into films based on a gelatin and casein blend. They observed that the addition of liposomes did not change the values of the tensile strength (∼1 MPa), Young's modulus (∼5 MPa) and elongation at break (∼140%) for the casein films. Nevertheless, gelatin films were less elastic, although they supported higher strength when compared to casein films. Furthermore, they were influenced by the addition of liposomes, which can be inferred from the decrease of the tensile strength from 8.5 to 4.9 MPa and the Young's modulus from 234 to 96 MPa. Alexandre et al.,26 working with gelatin-based films added with ginger EO nanoemulsions, also reported that the ginger EO nanoemulsions were able to increase the elongation at breakage of the films from 48 to 56%, and the Young's modulus from 6 to 8 MPa.
Finally, it is interesting to notice that the incorporation of micro- or nanoemulsions loaded with EO in films can affect its physical properties without a general rule, meaning that film characterizations must always be done when the films are developed with emulsions.
Water vapor-barrier properties
The water vapor permeability (WVP) of the active films should be highly considered for regulating the moisture passage from the food to the environment, protecting foods from dehydration, or even from the environment to food, avoiding food moisturizing. Hence, in these cases, the WVP of films should be as low as possible.114 However, packaging with high WVP could also be required in some cases, principally to package fresh fruits or vegetables.1The high WVP of biopolymers-based films can be linked to the hygroscopic character of biopolymers and plasticizers (usually, polyols). Therefore, the presence of the lipid fraction into the film structure would improve the water barrier properties owing to an increased tortuosity that creates a resistance to water vapor migration. It has been described that tortuosity is higher when the oil phase ratio increases or oil particle size is reduced.9 Nevertheless, Li et al.52 observed that the incorporation of a nanoemulsion containing thymol into gelatin-based films increased its WVP.
Acevedo-Fani et al.107 showed that the incorporation of Sage-EO (SG) nanoemulsions into alginate films decreased their WVP. In this same study, the incorporation of thyme (TH) or lemongrass (LG) EO did not significantly affect the WVP of the films, and were comparable to those of pure alginate films. Chen et al.110 reported that WVP in chitosan films increased as a consequence of the increase in the concentration of cinnamaldehyde oil nanoemulsions.
Otoni et al.109 worked with nanoemulsions loaded with thyme oil incorporated into soy protein-based films, and observed that a reduction of the droplet size significantly reduced the WVP. In contrast, no specific effect of EO on this barrier property was observed. Contrastingly, Otoni et al.70 observed that nanoemulsions of cinnamaldehyde EO improved the barrier to the water vapor of pectin/papaya puree films. Moreover, they observed that the decrease in the mean droplet size by increasing the homogenization speed did not necessarily significantly improve the barrier property of the films.
Haghju et al.112 determined the WVP of chitosan films laden with pure nettle extract and liposomes filled with nettle extract, and observed no significant effect of the free or encapsulated nettle extract on the WVP. A similar result was observed by Pérez-Córdoba and Sobral24 working with films based on gelatin, gelatin/chitosan blends, or gelatin/sodium caseinate blends incorporated with active compounds loaded nanoemulsions. However, they observed a positive effect on the WVP due to the increased affinity between the chitosan and the polar ends of nanoemulsions, causing a more obstructive pathway for water vapor to diffuse throughout the films.
Wu et al.25 observed that the addition of citrus EO chitosan-based films significantly reduced the WVP, with nanoemulsions leading to higher reductions than conventional emulsions. These authors attribute this behavior to the restricted movement of the water vapor molecules due to the reduced intermolecular spacing caused by the increased biopolymeric interactions between the biopolymers and the droplets. Nevertheless, the observed changes in WVP in all of these works were not sufficient to change the character of materials from low barrier to high barrier to water vapor, for instance.
Microstructure
Acevedo-Fani et al.107 used scanning electron microscopy (SEM) to study the effect of EO-loaded nanoemulsions in alginate films. These authors observed that the control films presented a homogenous microstructure, whereas the addition of nanoemulsions generally resulted in surface coarseness. In addition, the degree of roughness on the support contact side was lower in comparison to the airside of the films. This indicated that the oil droplet migration to the surface was probably caused by the drag force of water moving upward during drying. Pérez-Córdoba and Sobral24 have also observed similar behavior.Chen et al.110 had also used SEM analysis to study the morphology of the surface and cross-sectional areas of the films, and atomic force microscopy (AFM) to analyze the film's surface irregularities. They also observed that chitosan-based films loaded with cinnamaldehyde had rougher surfaces than pure chitosan films with the degree of roughness proportional to the oil concentration. This behavior was additionally verified via the cross-sectional SEM images, which revealed some cracks or bubble-like structures at low levels and smooth structures at high levels cinnamaldehyde, in which the oil droplets might have percolated out to the film surfaces after the desiccating step.
Similar phase separation and irregularities in the surface of casein and gelatin film were also observed by Boelter and Brandelli.113 The casein presented a smooth surface, whereas the films with added liposomes had a dotted, granular structure. Furthermore, the pure gelatin films were slightly fibrous, which became more fibrous and wrinkled with cracks upon the addition of liposomes. This change of structural characteristics was probably due to the incompatibility between the bioactive compounds and polymer matrices.
In methylcellulose films loaded with thyme EO,115 SEM analysis revealed that the vertical cross-section of the films exhibited nano-range and regular pores, which were indicative of the oil droplets embedded in the film matrices. However, this study did not report any leeching or aggregation of particles in the active films. Montes-de-Oca-Avalos et al.116 acquired both laser scanning confocal microscopy (LSCM) and SEM analysis on the airside of films made from coarse and nanoemulsions in the form of solution and gel. In general, the nanoemulsions (NE) exhibited stability to flocculation and coalescence about both solutions and gels. Moreover, they observed that the oil droplets leeched out in the coarse emulsions, but not in the coarse gels. Contrarily, Otoni et al.70 did not observe a difference in the film surfaces morphology due to the incorporation of clove bud and oregano EO in methylcellulose films, analyzed by SEM at a magnification of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×.
000×.
Dammak et al.21 produced active gelatin films incorporated with chitosan nanoparticles (ChiNP)-stabilized Pickering emulsions. They used AFM and dynamic light scattering (DLS) to confirm the size of the nanoparticles, revealing that ChiNPs were almost spherical in shape. Furthermore, they applied confocal laser scanning microscopy (CLSM) and SEM techniques to study the dispersion of emulsion droplets and droplet size into the gelatin film matrix, confirming the regular distributions of the droplets throughout the film matrix. Moreover, they observed that the droplet size distribution was similar to that of the fresh emulsion and films. Interestingly, these authors claimed that the incorporation of the Pickering emulsions into gelatin films was capable of producing films with good compatibility between the interface of oil droplets and the gelatin matrix (Fig. 6).
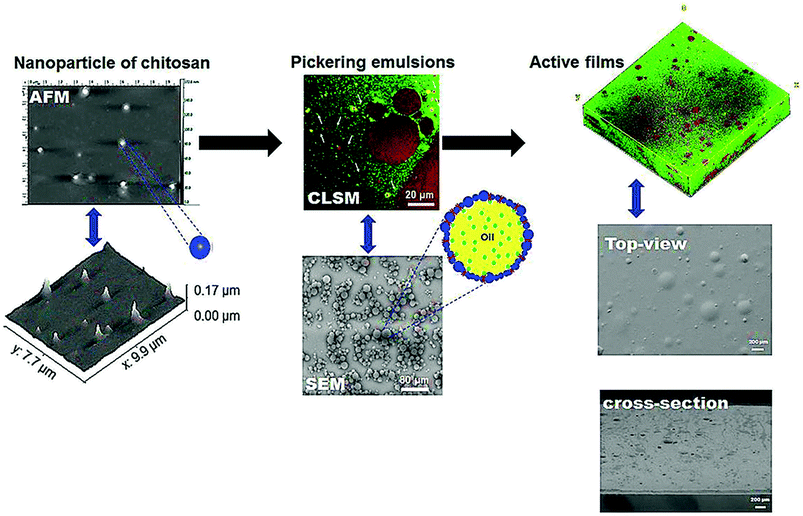 | ||
| Fig. 6 Microstructural images characterization of gelatin active films incorporated with Pickering emulsions encapsulating hesperidin. AFM: atomic force microscopy; CLSM: confocal laser scanning microscopy; SEM: scanning electronic microscopy.30 | ||
As observed from the previous studies, a general remark that can be drawn is that the incorporation of the emulsion-based systems leads to heterogeneity on surfaces during drying due to immiscibility and heterogeneity of the droplet size distribution. However, in most of the cases, emulsion-based systems markedly reduce the leeching of the directly incorporated EO and dramatically improved the chemical stability. It is indispensable to know that this homogeneity of the distribution is mainly related to the nature and concentration of the oil phase, emulsification technique, film matrix, and desiccating conditions.
Thermal properties
Another property that was extensively studied in films to understand the effect of the addition of nanoemulsions on the polymeric matrix is the thermal properties. Alexandre et al.26 produced gelatin films added with ginger EO nanoemulsions and montmorillonite. They reported that the differential scanning calorimetry (DSC) thermograms of all samples were quite similar, consisting of a typical first scan indicating a partially crystalline material and a second scan typical of amorphous material, where only the glass transition could be observed.114 These authors26 reported that the glass transition temperature (Tg) of the films was not significantly affected by any of the added compounds, varying between 51.7–56.6 °C and 50.5–52.8 °C for the dual scans, respectively.Pérez-Córdoba et al.72 studied the incorporation of nanoemulsions containing α-tocopherol/cinnamaldehyde, α-tocopherol/garlic oil and α-tocopherol/cinnamaldehyde/garlic oil in gelatin/chitosan blend films. They reported similar DSC curves for all of the films tested. According to these authors, the glass transition (Tg) was not affected by the formulation characteristics of the films, and it was about 46 °C and 10 °C for the first and second scans, respectively. These authors also found no influence of the nanoemulsions on the melting temperature (Tm) either, although the films incorporated with nanoemulsions revealed an extra marked endothermic peak around −18 °C, which was associated with the canola oil used for the encapsulation of the active compounds in the nanodroplets.72 Besides, these authors also reported a significant decrease in the melting enthalpy from 12.1 J g−1 to 9.0 J g−1 with the addition of the nanoemulsions. This is possibly due to the increasing inter-distances between the gelatin chains.
Haghju et al.112 worked with films of chitosan with the addition of nettle (Urtica dioica L.), and found that the nettle nanoemulsions acted as an oil agent and reduced interpolymeric interactions between chitosan. This decreased the glass temperature (from 71.3 to 60.5 °C) and the melting temperature (from 171.3 to 127.5 °C) when the nanoemulsions content increased from 0 to 1.5% (w/w). These same nanoemulsions, when encapsulated with liposome in chitosan films, were able to shift the Tg from 71.3 °C to 88 °C at the concentration of 0.5% (w/w). This is related to the interactions between chitosan and nanoliposomes in amorphous regions. It caused a reduction in Tg from 71.3 to 65.4 °C for the concentration of 1.5% (w/w), which was assigned to the low encapsulation efficiency.
X-ray diffraction
The crystallinity of active films is preferably evaluated using X-ray diffraction (XRD). Chen et al.110 evaluated the influence of the addition of cinnamaldehyde nanoemulsions on chitosan films, and found a broad band between 2θ = 7° and 35° with a maximum point at 23° and a shoulder at 12°. According to these authors, the addition of various amounts of nanoemulsions in the chitosan films caused specific changes in the level of crystallinity, regarding the ratio of aldehyde and amino group achieved. These authors also found an increase in d-spacing values and sharper reflection peaks with increasing cinnamaldehyde amount. Ghadetaj et al.23 studied films based on whey protein loaded with nanoemulsions of Grammosciadium ptrocarpum’ EO, and reported peaks around 2θ = 8° and 19° for the whey protein films, which classify this protein as a semi-crystalline polymer. Moreover, they reported that the addition of the nanoemulsions on the whey protein matrix did not significantly affect the crystallinity level of the films, which means that the structure of the film was preserved. The influence of montmorillonite and nanoemulsions of ginger EO on the crystallinity of the biopolymeric films was also studied by Alexandre et al.,26 observing that the ginger EO nanoemulsions had no substantial effect on the crystallinity level of these films.Pérez-Córdoba et al.72 found that gelatin–chitosan films presented distinguishing features in the pattern of XRD with two distinct peaks around 2θ = 10° and 20°. These authors also reported a peak around 2θ = 32°, which was related to hydrated chitosan crystals produced during the dissolution of chitosan in acetic acid solution or to the active compound chemical structure. According to the same authors, the incorporation of nanoemulsions containing α-tocopherol/cinnamaldehyde, α-tocopherol/garlic oil or α-tocopherol/cinnamaldehyde/garlic oil in the films slightly changed the peak intensity, although the profile of the diffraction spectra has been similar to that of the control films (without nanoemulsions incorporation).
Application of active films incorporated with emulsions-based systems
The application of active films needs to provide a controlled release of the bioactive compounds into foodstuff, and to protect them from the undesirable interactions that may promote its degradation. These actions contribute to decreasing the needed amount of food additives into foods for extending their shelf life, contributing to the development of healthier foods. Depending on the objectives of the film application, a fast release of the bioactive compounds may be desirable to act on the food bulk. Conversely, a slow-release rate could be necessary to maintain a critical concentration at the surface to avoid food deterioration.Antimicrobial films
The antimicrobial properties of some recently developed active films incorporated with emulsion-based systems for antimicrobial packaging application are presented in Table 4. Readers interested in the types of antimicrobial compounds and recent trends on the strategies used to encapsulate these antimicrobials for their stable inclusion into films must read the review prepared by Becerril et al.117| Film matrix | Bioactive compounds | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) UFC/g UFC/g |
Inhibition zone (mm2) | Ref. |
|---|---|---|---|---|
| a Total bacteria count.b Psychrotrophic bacteria count. | ||||
| Alginate | Thyme-EO | 1.5 (E. coli) | — | Acevedo-Fani et al.107 |
| Alginate | Lemongrass-EO | 7 (E. coli) | — | Acevedo-Fani et al.107 |
| Alginate | Sage-EO | 6 (E. coli) | — | Acevedo-Fani et al.107 |
| Low methyl ester pectin | Cinnamaldehyde | — | 24 (E. coli) | Otoni et al.108 |
| High methyl ester pectin | Cinnamaldehyde | — | 41 (E. coli) | Otoni et al.108 |
| Quinoa protein/chitosan | Thymol-EO | 4.7 (E. coli) | Robledo et al.111 | |
| Hydroxypropyl methyl cellulose | Thymus daenensis-EO | — | 9–13 (E. coli) | Moghimi et al.115 |
| Hydroxypropyl methyl cellulose | Thymus daenensis-EO | — | 23–48 (S. aureus) | Moghimi et al.115 |
| Hydroxypropyl methyl cellulose | Thymus daenensis-EO | — | 10–13 (MRSA18) | Moghimi et al.115 |
| Hydroxypropyl methyl cellulose | Thymus daenensis-EO | — | 11–14 (S. flexeneri) | Moghimi et al.115 |
| Sodium caseinate | Ginger-EO | 3–5 (molds and yeast) | 5.8–12 (S. Typhimurium) | Noori et al.132 |
| Sodium caseinate | Ginger-EO | 2.5–5.5 (psychrophilic bacteria) | 6.5–15 (L. monocytogenes) | Noori et al.132 |
| Sodium alginate and mandarin fiber | Oregano-EO | 4.5–6 (Staphylococcus aureus) | — | Artiga-Artigas et al.133 |
| Sodium alginate and mandarin fiber | Oregano-EO | 5.5–6.5 (psychrophilic bacteria) | — | Artiga-Artigas et al.133 |
| Sodium alginate and mandarin fiber | Oregano-EO | 0.7–3.0 (molds and yeasts) | — | Artiga-Artigas et al.133 |
| Modified chitosan (N-palmitoyl chitosan) | Carvacrol | — | 7.4–16.1 (E. coli and L. innocua) | Tastan et al.134 |
| Jujube gum | Nettle oil | 2–6 TBCa, 2–5 PBCb | — | Gharibzahedi and Mohammadnabi135 |
| Chitosan | Cinnamaldehyde | — | 13 (E. coli) | Chen et al.110 |
| Chitosan | Cinnamaldehyde | — | 12 (S. aureus) | Chen et al.110 |
| Chitosan | Cinnamaldehyde | — | 24 (C. albicans) | Chen et al.110 |
| Chitosan | Cinnamaldehyde | — | 7–15 (Staphylococcus aureus) | Sugumar et al.136 |
| Chitosan | Eucalyptus oil | — | 2–3 (S. Aureus) | Haghju et al.112 |
| Gelatin | Nisin | — | 10 (B. cereus) | Boelter and Brandelli113 |
| Gelatin | Nisin | — | 35 (C. perfringens) | Boelter and Brandelli113 |
| Gelatin | Nisin | — | 50 (L. monocytogenes) | Boelter and Brandelli113 |
Xu et al.118 used the liquid culture test to evaluate the antimicrobial activity of chitosan:gum Arabic (CS:GA) films emulsified with directly incorporated cinnamon EO (8% w/w) using Ultraturrax® at a rotation speed of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm during 4 min. These authors observed that upon increasing the GA proportion into the blend from 0 to 2, the antimicrobial activity of the CS:GA films against E. coli was further enhanced. This is because the addition of GA to the blend allowed higher retention of cinnamon oil and a slower release rate from the films.
000 rpm during 4 min. These authors observed that upon increasing the GA proportion into the blend from 0 to 2, the antimicrobial activity of the CS:GA films against E. coli was further enhanced. This is because the addition of GA to the blend allowed higher retention of cinnamon oil and a slower release rate from the films.
Arfat et al.119 studied the antimicrobial activity of fish protein isolate/fish skin gelatin blend-based films nanoemulsified with basil EO (BEO) and zinc oxide (ZnO) nanoparticles by using a microfluidizer at 150 MPa (3 passes). As expected by the authors, amongst all films, those loaded with the higher BEO and ZnO concentrations had the highest antimicrobial activity contra L. monocytogenes and P. aeruginosa. They assumed that the antibacterial action of the films improved due to the collective antimicrobial activities of BEO and ZnO.
The effect of incorporating nisin (1 mg mL−1) or nanoencapsulated nisin (1 mg mL−1) in soy lecithin liposomes, prepared by using a microfluidizer at 200 MPa (5 passes), on the antimicrobial activity of HPMC-based films was studied by Imran et al.120 Their results showed that the active HPMC film loaded with nisin produced a slightly more significant inhibition zone against L. monocytogenes than the encapsulated nisin, possibly due to the smooth release of nisin over the inoculated agar.
Chen et al.110 assessed the antimicrobial activity of chitosan films with added cinnamaldehyde nanoemulsion. Overall, the results showed that cinnamaldehyde chitosan films revealed better antimicrobial activity on molds (C. albicans) than on both bacterial strains, E. coli and S. aureus. They attributed this behavior to the high imine group content (1.5 and 2.0) in the active films because of the nanoemulsified cinnamaldehyde. Ghadetaj et al.23 also studied the antimicrobial activity of whey protein isolate (WPI) films loaded with free and nanoemulsified Grammosciadium ptrocarpum EO (GEO) (0.5, 1 and 1.5% w/w) and prepared via sonication at 20 kHz, 750 W for 4–5 min. These authors reported that the antimicrobial activity of the films against L. monocytogenes, E. coli, P. aeruginosa, and Salmonella typhimorium showed an increasing trend by increasing the oil concentration, probably due to the effect of phenolic compounds present in the GEO. As well as these authors, Wu et al.25 observed that active gelatin films loaded with nanoliposomes of cinnamon EO exhibited better antimicrobial properties against pathogens than gelatin films directly incorporated with cinnamon EO. However, Li et al.52 observed that films containing thymol nanoemulsions exhibited effective and prolonged inhibition activities against both Gram-positive (Bacillus subtilis) and Gram-negative (Escherichia coli O157:H7) bacteria. Therefore, nanoemulsions or nanoliposomes enhance the antimicrobial activity of the films, principally due to the release behavior of the essential oils.23
Almasi et al.73 produced active films based on calcium alginate containing emulsified thyme essential oil and acetic or propionic acids. These authors observed that when applied to ground meat, the antimicrobial efficiency of the microemulsion-films was much higher than that of the control films against coliforms, Staphylococcus aureus, yeast, mold, and lactic acid bacteria. On another side, Amjadi et al.121 produced films based on whey protein isolated incorporated with emulsified orange peel (Citrus sinensis) essential oil and observed that the antioxidant and antimicrobial activities of the active films loaded with nanoemulsions were significantly higher than those of films loaded with an emulsion.
Antioxidant films
The development of antioxidant films has become very popular since lipid oxidation is the primary source of food deterioration after microbial spoilage.81 The recent increase on the development of antioxidant films could be attributed to the presence of a wide variety of bioactive compounds (i.e., phenolic compounds, terpenoids flavonoids), principally from the EO and plant extracts, which could exert their antioxidant properties by various possible mechanisms.122 A less conventional antioxidant compound, the lipopeptides DCS1 were used as protectors against oil oxidation in an oil-in-water emulsion, and fat oxidation in beef meat with great results.123 Dammak et al.8 used the ABTS˙+ and DPPH˙ scavenging methods, and FRAP and reducing power assays to study the antioxidant activity of gelatin-based films with routine-loaded nanoemulsions, observing that those films presented high antioxidant capacity. These results corroborated other studies on chitosan/gelatin films incorporated with hesperidin-loaded Pickering emulsions,21 and with α-tocopherol, garlic EO, and cinnamaldehyde-loaded nanoemulsions.21Noronha et al.22 studied the active compound release, and DPPH˙ and ABTS˙+ radicals scavenging capacity of methylcellulose (MC) film with nanocapsules (NCs) suspensions containing α-tocopherol. A quick release of α-tocopherol was observed since the hydrophobic nature of NCs had a higher affinity to the food simulant (ethanol 95% v/v) compared to the film matrix (MC). These authors reported that the antioxidant capacity of the films considerably increased when the NCs concentration increased. Wrona et al.124 used the DPPH˙ scavenging method to evaluate the antioxidant activity of HPMC-based films loaded with poly(lactic acid) (PLA) nanoparticles containing green tea extract prepared by an emulsification-solvent evaporation technique. These authors reported that the smaller nanoparticles (47 nm) incorporated into the HPMC matrix provoked a higher DPPH˙ radical scavenging effect than those films containing the larger particles (117 nm). They suggested that small particles exhibited a higher release rate of active compounds.
Dammak et al.51 demonstrated the antioxidant activity of gelatin-based films loaded with routine nanoemulsions prepared by using a microfluidizer at a homogenization pressure of 100 MPa (3 passes). Their films revealed activity as a scavenger of both DPPH˙ and ABTS˙+ radicals, and the ferric reducing ability of plasma (FRAP), even at lower tested concentration. According to these authors,51 the scavenging capability and reductive power of the films were routine-loaded concentration-dependent. It was higher than the activity of β-carotene, used as a standard radical scavenger, suggesting that the routine has remarkable potency as hydrogen donors to react with free radicals, converting them into more stable agents. Recently, Pérez-Córdoba et al.72 using the DPPH˙ and ABTS˙+ scavenging methods, and FRAP assay also demonstrated the antioxidant activity of gelatin-chitosan-based films loaded with nanoemulsions encapsulating different active compounds (α-tocopherol/cinnamaldehyde/garlic oil) prepared by using a microfluidizer at pressures ranging from 69 to 100 MPa (3 passes). More recently, Lee et al.125 developed an active film based on hydroxypropyl methylcellulose (HPMC) loaded with oregano essential oil encapsulated into nanoemulsions, observing good antibacterial, antioxidant, and UV-barrier properties.
Chemical stability and biodegradability of active films loaded with nanoemulsions
No studies on chemical stability of films loaded with nanoemulsions were found in a recent research on the Web of Science. It is important to know this behavior before suggesting a practical application. Nevertheless, Chu et al.126 studied the retention of nanoemulsified cinnamon essential oil (CEO), a volatile compound, in pullulan-based active film during ambient storage (25 °C and 50% of relative humidity). They126 observed that incorporation of the CEO nanoemulsion with decreased droplet size could efficiently hinder the loss of CEO during both drying and preserving processes, which resulted from the homogeneous internal structures of the films.Regarding the effect of emulsions on the biodegradability of active films, Mendes et al.53 observed that the biodegradability of the cassava starch-based films determined in vegetal compost (soil) was not qualitatively affected by the addition of lemongrass essential oil emulsions. Similarly, Norcino et al.127 observed no effect of copaiba oil nanoemulsions on the biodegradability of pectin films determined by respirometry tests at 28 °C. Mendes et al.128 also did not observe an effect of the incorporation of nanoemulsified lemongrass essential oil on the biodegradability of films based on cassava starch, cocoa butter, and reinforced with brewery spent grain fibers.
Thus, these studies contributed to eliminating concerns on the supplementation of films with active ingredients that could be expected to have some biocidal effect on biodegradation media. Similar biodegradation behaviors were observed with active films incorporated with plant hydroethanolic extracts, which are rich in phenolics compounds having antioxidant and antimicrobial activities, carried out by compostability129 or respirometry.130,131
Conclusions and future directions
Due to the importance of active films for food preservation, scientific research has extensively been developed in this domain during the last decade. The studies have been focused mainly on the incorporation of natural non-polar bioactive compounds, namely essential oils extracted from plants, generally with lipophilic characters, causing various difficulties to incorporate them into hydrophilic biomaterial matrices, principally because its solvent is water.This review reports the new trends in this subject to develop a new generation of active films by using emulsions as a carrier of lipophilic compounds. The number of published research studies on this type of food has duplicated in the last five years, reaching almost 40.
The incorporation of the active film’ materials with structured emulsion-based systems would be able to handle and protect the desired bioactive principles within the film matrix in optimal conditions until their eventual release into the food product through either controlled release during storage or just before consumption. Emulsion-systems stabilized with a biopolymer (i.e., chitosan, gum Arabic) formed a layer around oil droplets, and provided higher thermal and chemical storage stabilities (lower degradation rate, and higher half-life) for the emulsified bioactive compounds. It can be considered that nanoemulsions are the most indicated system for active films development because, in principle, it will guarantee the good dispersion of bioactive compounds into the biopolymeric matrix. Nevertheless, the Pickering emulsion is also recommended because of its good stability regarding processing stresses, such as mixing and heating.
Release behaviors of these compounds can be adapted by the addition of varying proportions of structured emulsion-based systems. This can be attributed to differences in the biopolymer network structure attributable to the presence of these engineered interfaces of O/W emulsion droplets and the increased tortuosity of the road ahead, modifying the diffusion degree of bioactive compounds. Emulsions could be useful for the creation of delivery systems with controlled release properties triggered by environmental factors (i.e., pH-modulation). Engineering the interface of O/W emulsion droplets with different kinds of emulsifiers that modify its permeability is a novel strategy in the improvement of bioactive compound retention and stabilization. The most often applied emulsifiers are small-molecule surfactants, proteins, polysaccharides, and phospholipids.
Nevertheless, there has recently been considerable interest in identifying food-grade colloidal particles to stabilize food emulsions through the Pickering stabilization mechanism for higher stability. The primary challenge confronting the use of the emulsion-based systems in developing active films is the necessity of being sure to maintain their functionality with efficiency during the required time, and then become degraded only after that. Because of the strict structure–function relationship inherent in the biopolymeric films, it is possible to produce reasonable modifications that can successfully be used in specific applications. Furthermore, it is necessary to evaluate these possible applications in real systems, such as the effectiveness of a given active film will be influenced by interactions with the food product and the external ambient. Finally, no single structured design is suitable for all of the kinds of foods.
On the contrary, each designed structure possesses properties compatible with a given concrete application, hence the diversity of these systems. It can be inferred that encapsulation of lipophilic bioactive compounds in different emulsion-based systems, i.e., nanoemulsions, nanoliposomes, or lipid nanoparticles, might be a promising approach to overcome the issues associated with the direct application of those bioactive compounds into a film matrix.
The resulting active films exhibit better functionality regarding enhanced protection, increased stability, sustained release profile, improved bioavailability, and preserved precision targeting of the bioactive compounds. This review would open the way for producing active films for food packaging applications with a structured architecture network framework, aiming to improve their microstructural homogeneity and physicochemical performances. Nevertheless, overall, these films have a low potential for application in commercial scale, principally due to its sensitivity to water (as a vapor or liquid). Moreover, studies on the chemical stability and biodegradability of the films must be emphasized.
Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
This work was supported by the São Paulo Research Foundation (FAPESP) (CEPID FoRC 13/07914-8 and 15/02879-5); the Rio de Janeiro Research Foundation (FAPERJ) (E-26/203.049/2017, E-26/202.136/2018, E-26/010.000.984/2019 and E-26/201.790/2020); Brazil's Coordination for the Improvement of Higher Education Personnel (CAPES) (CAPES/FAPERJ E-45-PAPDRJ/2013 and E-26/202.136/2018); and the Brazilian National Council for Scientific and Technological Development (CNPq) (311422/2016 and 313119/2020-1).References
- K. Marsh and B. Bugusu, J. Food Sci., 2007, 72, R39–R55 CrossRef CAS PubMed.
- D. Dainelli, N. Gontard, D. Spyropoulos, E. Zondervan-van den Beuken and P. Tobback, Trends Food Sci. Technol., 2008, 19, S103–S112 CrossRef.
- M. Ozdemir and J. D. Floros, J. Food Process. Preserv., 2004, 44, 185–193 CAS.
- J. Gómez-Estaca, C. López-de-Dicastillo, P. Hernández-Muñoz, R. Catalá and R. Gavara, Trends Food Sci. Technol., 2014, 35, 42–51 CrossRef.
- S. Ganiari, E. Choulitoudi and V. Oreopoulou, Trends Food Sci. Technol., 2017, 68, 70–82 CrossRef CAS.
- C. Vilela, M. K. Hayouka, B. Röcker, S. Yildirim, M. D. C. Antunes, J. Nilsen-Nygaard, M. K. Pettersen and C. S. R. Freire, Trends Food Sci. Technol., 2018, 80, 212–222 CrossRef CAS.
- J. Bonilla and P. J. A. Sobral, Food Biosci., 2016, 16, 17–25 CrossRef CAS.
- I. Dammak, A. Bittante, R. V. Lourenco and P. J. A. Sobral, Int. J. Biol. Macromol., 2017, 101, 643–652 CrossRef CAS PubMed.
- J.-A. Q. Gallo, F. Debeaufort, F. Callegarin and A. Voilley, J. Membr. Sci., 2000, 180, 37–46 CrossRef.
- M. O. Nisperos-Carriedo, in Edible coatings and films to improve food quality, Technomic Publishing Company, Lancaster, 1st edn, 1994, pp. 305–336 Search PubMed.
- A. Gennadios, T. H. McHugh, C. L. Weller and J. M. Krochta, in Edible coatings and films to improve food quality, Technomic Publishing Company, Lancaster, 1st edn, 1994, pp. 210–278 Search PubMed.
- J. A. Torres, in Protein functionality in food systems, M. Dekker, New York, 1st edn, 1994, pp. 467–507 Search PubMed.
- J. Jeya Jeevahan, M. Chandrasekaran, S. P. Venkatesan, V. Sriram, G. Britto Joseph, G. Mageshwaran and R. B. Durairaj, Trends Food Sci. Technol., 2020, 100, 210–222 CrossRef CAS.
- R. Ahvenainen, Novel Food Packaging Techniques, Woodhead Publishing Ltd and CRC Press LLC, 1st edn, 2003 Search PubMed.
- Z. Fang, Y. Zhao, R. D. Warner and S. K. Johnson, Trends Food Sci. Technol., 2017, 61, 60–71 CrossRef CAS.
- N. Gontard, Les emballages actifs, Tec & Doc, 1st edn, 2000 Search PubMed.
- M. Kontominas, Bioactive Packaging of Foods: Quality and Safety Issues, Destech Publ. Inc., 1st edn, 2016 Search PubMed.
- M. L. Rooney, Active Food Packaging, Springer, 1st edn, 1995 Search PubMed.
- C. L. Wilson, Intelligent and Active Packaging for Fruits and Vegetables, CRC Press, 1st edn, 2007 Search PubMed.
- J. B. Lagos, F. C. Vargas, T. G. Oliveira, G. L. A. Makishi and P. J. A. Sobral, Curr. Opin. Food Sci., 2015, 5, 1–7 CrossRef.
- I. Dammak, R. V. Lourenço and P. J. A. Sobral, J. Food Eng., 2019, 240, 9–20 CrossRef CAS.
- C. M. Noronha, S. M. Carvalho, R. C. Lino and P. L. M. Barreto, Food Chem., 2014, 159, 529–535 CrossRef CAS PubMed.
- A. Ghadetaj, H. Almasi and L. Mehryar, Food Packag. Shelf Life, 2018, 16, 31–40 CrossRef.
- L. J. Pérez Córdoba and P. J. A. Sobral, J. Food Eng., 2017, 213, 47–53 CrossRef.
- J. Wu, H. Liu, S. Ge, S. Wang, Z. Qin, L. Chen, Q. Zheng, Q. Liu and Q. Zhang, Food Hydrocolloids, 2015, 43, 427–435 CrossRef CAS.
- E. M. C. Alexandre, R. V. Lourenço, A. M. Q. B. Bittante, I. C. F. Moraes and P. J. A. Sobral, Food Packag. Shelf Life, 2016, 10, 87–96 CrossRef.
- M. Mastromatteo, A. Danza, A. Conte, G. Muratore and M. A. Del Nobile, Int. J. Food Microbiol., 2010, 144, 250–256 CrossRef CAS PubMed.
- L. Atarés and A. Chiralt, Trends Food Sci. Technol., 2016, 48, 51–62 CrossRef.
- B. Shah, P. M. Davidson and Q. Zhong, Appl. Environ. Microbiol., 2012, 78, 8448–8453 CrossRef CAS PubMed.
- M. R. I. Shishir, L. Xie, C. Sun, X. Zheng and W. Chen, Trends Food Sci. Technol., 2018, 78, 34–60 CrossRef CAS.
- Q. Ma, P. M. Davidson and Q. Zhong, Food Chem., 2016, 206, 167–173 CrossRef CAS PubMed.
- V. Đorđević, B. Balanč, A. Belščak-Cvitanović, S. Lević, K. Trifković, A. Kalušević, I. Kostić, D. Komes, B. Bugarski and V. Nedović, Food Eng. Rev., 2014, 7, 452–490 CrossRef.
- K. Bhargava, D. S. Conti, S. R. da Rocha and Y. Zhang, Food Microbiol., 2015, 47, 69–73 CrossRef CAS PubMed.
- F. Donsì, A. Cuomo, E. Marchese and G. Ferrari, Innovative Food Sci. Emerging Technol., 2014, 22, 212–220 CrossRef.
- K. C. S. Galvão, A. A. Vicente and P. J. A. Sobral, Food Bioprocess Technol., 2018, 11, 355–367 CrossRef.
- T. Winuprasith and M. Suphantharika, Food Hydrocolloids, 2015, 43, 690–699 CrossRef CAS.
- D. J. McClements, Food Emulsions: Principles, Practices, and Techniques, CRC Press, 3th edn, 2015 Search PubMed.
- I. Dammak, P. J. A. Sobral, A. Aquino, M. A. Neves and C. A. Conte-Junior, Compr. Rev. Food Sci. Food Saf., 2020, 19, 2721–2746 CrossRef PubMed.
- S. U. Pickering, J. Chem. Soc., Trans., 1907, 91, 2001–2021 RSC.
- H. D. Silva, M. A. Cerqueira and A. A. Vicente, Food Bioprocess Technol., 2011, 5, 854–867 CrossRef.
- P. J. P. Espitia, C. A. Fuenmayor and C. G. Otoni, Compr. Rev. Food Sci. Food Saf., 2019, 18, 264–285 CrossRef CAS PubMed.
- J. Hategekimana, M. V. M. Chamba, C. F. Shoemaker, H. Majeed and F. Zhong, Colloids Surf., A, 2015, 483, 70–80 CrossRef CAS.
- K. A. Ishak and M. S. M. Annuar, Colloid Polym. Sci., 2016, 294, 1969–1981 CrossRef CAS.
- L. Fradette, B. Brocart and P. A. Tanguy, Chem. Eng. Res. Des., 2007, 85, 1553–1560 CrossRef CAS.
- I. N. Seekkuarachchi, K. Tanaka and H. Kumazawa, Ind. Eng. Chem. Res., 2006, 45, 372–390 CrossRef CAS.
- R. C. Santana, F. A. Perrechil and R. L. Cunha, Food Eng. Rev., 2013, 5, 107–122 CrossRef CAS.
- J. Carpenter and V. K. Saharan, Ultrason. Sonochem., 2017, 35, 422–430 CrossRef CAS PubMed.
- L. Lee and I. T. Norton, J. Food Eng., 2013, 114, 158–163 CrossRef CAS.
- N. Anton, J. P. Benoit and P. Saulnier, J. Controlled Release, 2008, 128, 185–199 CrossRef CAS PubMed.
- I. Dammak and P. J. A. Sobral, Food Bioprocess Technol., 2017, 10, 926–939 CrossRef CAS.
- I. Dammak, R. A. Carvalho, C. S. Trindade, R. V. Lourenco and P. J. A. Sobral, Int. J. Biol. Macromol., 2017, 98, 39–49 CrossRef CAS PubMed.
- X. Li, X. Yang, H. Deng, Y. Guo and J. Xue, Int. J. Biol. Macromol., 2020, 150, 161–168 CrossRef CAS PubMed.
- J. F. Mendes, L. B. Norcino, H. H. A. Martins, A. Manrich, C. G. Otoni, E. E. N. Carvalho, R. H. Piccoli, J. E. Oliveira, A. C. M. Pinheiro and L. H. C. Mattoso, Food Hydrocolloids, 2020, 100, 105428 CrossRef CAS.
- A. M. Chuah, T. Kuroiwa, I. Kobayashi and M. Nakajima, Colloids Surf., A, 2014, 440, 136–144 CrossRef CAS.
- J. Komaiko, A. Sastrosubroto and D. J. McClements, Food Chem., 2016, 203, 331–339 CrossRef CAS PubMed.
- C. C. Berton-Carabin, L. Sagis and K. Schroen, Annu. Rev. Food Sci. Technol., 2018, 9, 551–587 CrossRef CAS PubMed.
- I. Dammak and P. J. A. Sobral, J. Food Eng., 2018, 229, 12–20 CrossRef CAS.
- J. C. Cuevas-Bernardino, C. Lobato-Calleros, A. Román-Guerrero, J. Alvarez-Ramirez and E. J. Vernon-Carter, React. Funct. Polym., 2016, 103, 63–71 CrossRef CAS.
- P. Karthik, P. N. Ezhilarasi and C. Anandharamakrishnan, Crit. Rev. Food Sci. Nutr., 2017, 57, 1435–1450 CrossRef CAS PubMed.
- W. Dridi, C. Harscoat-Schiavo, J. Monteil, C. Faure and F. LealCalderon, Langmuir, 2018, 34, 9228–9237 CrossRef CAS PubMed.
- C. Arancibia, N. Riquelme, R. Zuniga and S. Matiacevich, Innovative Food Sci. Emerging Technol., 2017, 44, 159–166 CrossRef CAS.
- I. Dammak and P. J. A. Sobral, J. Food Eng., 2018, 237, 33–43 CrossRef CAS.
- D. J. McClements, E. A. Decker, Y. Park and J. Weiss, Crit. Rev. Food Sci. Nutr., 2009, 49, 577–606 CrossRef CAS PubMed.
- D. J. McClements, Soft Matter, 2011, 7, 2297–2316 RSC.
- C. Solans, P. Izquierdo, J. Nolla, N. Azemar and M. Garciacelma, Curr. Opin. Colloid Interface Sci., 2005, 10, 102–110 CrossRef CAS.
- T. Tadros, P. Izquierdo, J. Esquena and C. Solans, Adv. Colloid Interface Sci., 2004, 303, 108–109 Search PubMed.
- D. J. McClements and J. Rao, Crit. Rev. Food Sci. Nutr., 2011, 51, 285–330 CrossRef CAS PubMed.
- L. Salvia-Trujillo, R. Soliva-Fortuny, M. A. Rojas-Grau, D. J. McClements and O. Martin-Belloso, Annu. Rev. Food Sci. Technol., 2017, 8, 439–466 CrossRef CAS PubMed.
- M. I. Guerra-Rosas, J. Morales-Castro, M. A. Cubero-Márquez, L. Salvia-Trujillo and O. Martín-Belloso, Food Control, 2017, 77, 131–138 CrossRef CAS.
- C. G. Otoni, M. R. Moura, F. A. Aouada, G. P. Camilloto, R. S. Cruz, M. V. Lorevice, N. F. F. Soares and L. H. C. Mattoso, Food Hydrocolloids, 2014, 41, 188–194 CrossRef CAS.
- F. Liu, R. J. Avena-Bustillos, B.-S. Chiou, Y. Li, Y. Ma, T. G. Williams, D. F. Wood, T. H. McHugh and F. Zhong, Food Hydrocolloids, 2017, 62, 212–221 CrossRef CAS.
- L. J. Pérez-Córdoba, I. T. Norton, H. K. Batchelor, K. Gkatzionis, F. Spyropoulos and P. J. A. Sobral, Food Hydrocolloids, 2018, 79, 544–559 CrossRef.
- L. Almasi, M. Radi, S. Amiri and D. J. McClements, Food Hydrocolloids, 2021, 117, e106733 CrossRef.
- H. Almasi, S. Azizi and S. Amjadi, Food Hydrocolloids, 2020, 99, 105338 CrossRef CAS.
- B. Tonyali, A. McDaniel, A. Amamcharla, V. Trinetta and U. Yucel, Food Packag. Shelf Life, 2020, 24, 100484 CrossRef.
- T. Helgason, H. Salminen, K. Kristbergsson, D. J. McClements and J. Weiss, J. Colloid Interface Sci., 2015, 448, 114–122 CrossRef CAS PubMed.
- B. Mehrad, R. Ravanfar, J. Licker, J. M. Regenstein and A. Abbaspourrad, Food Res. Int., 2018, 105, 962–969 CrossRef CAS PubMed.
- H. Salminen, C. Gommel, B. H. Leuenberger and J. Weiss, Food Chem., 2016, 190, 928–937 CrossRef CAS PubMed.
- D. J. McClements, L. Bai and C. Chung, Annu. Rev. Food Sci. Technol., 2017, 8, 205–236 CrossRef PubMed.
- Y.-J. Jo, J.-Y. Chun, Y.-J. Kwon, S.-G. Min and M.-J. Choi, Int. J. Food Eng., 2015, 11, 31–39 CAS.
- Z. Hou, M. Zhang, B. Liu, Q. Yan, F. Yuan, D. Xu and Y. Gao, Food Hydrocolloids, 2012, 26, 205–211 CrossRef CAS.
- A. Acevedo-Fani, H. D. Silva, R. Soliva-Fortuny, O. Martín-Belloso and A. A. Vicente, Food Hydrocolloids, 2017, 71, 207–215 CrossRef CAS.
- J. A. Piornos, C. Burgos-Díaz, E. Morales, M. Rubilar and F. Acevedo, Food Hydrocolloids, 2017, 63, 139–148 CrossRef CAS.
- H. D. Silva, J. Poejo, A. C. Pinheiro, F. Donsì, A. T. Serra, C. M. M. Duarte, G. Ferrari, C. M. Cerqueira and A. A. Vicente, J. Funct. Foods, 2018, 48, 605–613 CrossRef CAS.
- J. Huang, Q. Wang, T. Li, N. Xia and Q. Xia, J. Sci. Food Agric., 2018, 98, 3513–3523 CrossRef CAS PubMed.
- L. Tessaro, C. G. Luciano, A. M. Q. B. Bittante, R. V. Lourenço, M. Martelli-Tosi and P. J. A. Sobral, Food Hydrocolloids, 2021, 113, e106523 CrossRef.
- L. Tessaro, R. V. Lourenço, M. Martelli-Tosi and P. J. A. Sobral, Int. J. Biol. Macromol., 2021, 186, 328–340 CrossRef CAS PubMed.
- L. Zhao, H. Xiong, H. Peng, Q. Wang, D. Han, C. Bai, Y. Liu, S. Shi and B. Deng, Eur. Food Res. Technol., 2011, 232, 647–654 CrossRef CAS.
- S. Vuuren and A. Viljoen, Planta Med., 2011, 77, 1168–1182 CrossRef PubMed.
- P. A. Yoshida, D. Yokota, M. A. Foglio, R. A. Rodrigues and S. C. Pinho, J. Microencapsulation., 2010, 27, 416–425 CrossRef CAS PubMed.
- D. Yokota, M. Moraes and S. C. Pinho, Braz. J. Chem. Eng., 2012, 29, 325–335 CrossRef CAS.
- Q. Lu, D. C. Li and J. G. Jiang, J. Agric. Food Chem., 2011, 59, 13004–13011 CrossRef CAS PubMed.
- B. Maherani, E. Arab-Tehrany, A. Kheirolomoom, F. Cleymand and M. Linder, Food Chem., 2012, 134, 632–640 CrossRef CAS PubMed.
- M. Moraes, J. M. P. Carvalho, C. R. Silva, S. Cho, M. R. Sola and S. C. Pinho, Int. J. Food Sci. Technol., 2013, 48, 274–282 CrossRef CAS.
- T. M. Taylor, P. M. Davidson, B. D. Bruce and J. Weiss, Crit. Rev. Food Sci. Nutr., 2005, 45, 587–605 CrossRef CAS PubMed.
- F. Ye, M. Miao, S. W. Cui, B. Jiang, Z. Jin and X. Li, Food Hydrocolloids, 2018, 76, 78–87 CrossRef CAS.
- F. Leal-Calderon and V. Schmitt, Curr. Opin. Colloid Interface Sci., 2008, 13, 217–227 CrossRef CAS.
- Y. Chevalier and M.-A. Bolzinger, Colloids Surf., A, 2003, 439, 23–34 CrossRef.
- M. Destribats, V. Lapeyre, E. Sellier, F. Leal-Calderon, V. Ravaine and V. Schmitt, Langmuir, 2012, 28, 3744–3755 CrossRef CAS PubMed.
- W. Richtering, Langmuir, 2012, 28, 17218–17229 CrossRef CAS PubMed.
- V. Schmitt and V. Ravaine, Curr. Opin. Colloid Interface Sci., 2013, 18, 532–541 CrossRef CAS.
- I. Dammak and P. J. A. Sobral, J. Food Eng., 2018, 229, 2–11 CrossRef CAS.
- M. Rai, P. Paralikar, P. Jogee, G. Agarkar, A. P. Ingle, M. Derita and S. Zacchino, Int. J. Pharm., 2017, 519, 67–78 CrossRef CAS PubMed.
- A. Sorrentino, G. Gorrasi and V. Vittoria, Trends Food Sci. Technol., 2007, 18, 84–95 CrossRef CAS.
- N. Pacheco, M. G. Naal-Ek, T. Ayora-Talavera, K. Shirai, A. Román-Guerrero, M. F. Fabela-Moróna and R. C. Cuevas-Bernardino, Int. J. Biol. Macromol., 2019, 125, 149–158 CrossRef CAS PubMed.
- M. F. Coronado-Jorge, C. H. C. Flaker, S. F. Nassar, I. C. F. Moraes, A. M. Q. B. Bittante and P. J. A. Sobral, J. Food Eng., 2014, 120, 81–87 CrossRef.
- A. Acevedo-Fani, L. Salvia-Trujillo, M. A. Rojas-Graü and O. Martín-Belloso, Food Hydrocolloids, 2015, 47, 168–177 CrossRef CAS.
- C. G. Otoni, S. F. O. Pontes, E. A. A. Medeiros and N. F. F. Soares, J. Agric. Food Chem., 2014, 62, 5214–5219 CrossRef CAS PubMed.
- C. G. Otoni, R. J. Avena-Bustillos, C. W. Olsen, C. Bilbao-Sáinz and T. H. McHugh, Food Hydrocolloids, 2016, 57, 72–79 CrossRef CAS.
- H. Chen, X. Hu, E. Chen, S. Wu, D. J. McClements, S. Liu, B. Li and Y. Li, Food Hydrocolloids, 2016, 61, 662–671 CrossRef CAS.
- N. Robledo, P. Vera, L. López, M. Yazdani-Pedram, C. Tapia and L. Abugoch, Food Chem., 2018, 246, 211–219 CrossRef CAS PubMed.
- S. Haghju, S. Beigzadeh, H. Almasi and H. Hamishehkar, J. Microencapsulation, 2016, 33, 438–448 CrossRef CAS PubMed.
- J. F. Boelter and A. Brandelli, Colloids Surf., B, 2016, 145, 740–747 CrossRef CAS PubMed.
- P. J. A. Sobral, F. C. Menegalli, M. D. Hubinger and M. A. Roques, Food Hydrocolloids, 2001, 15, 423–432 CrossRef CAS.
- R. Moghimi, A. Aliahmadi and H. Rafati, Carbohydr. Polym., 2017, 175, 241–248 CrossRef CAS PubMed.
- J. M. Montes-de-Oca-Avalos, D. Altamura, R. J. Candal, F. Scattarella, D. Siliqi, C. Giannini and M. L. Herrera, Food Res. Int., 2018, 105, 129–139 CrossRef CAS PubMed.
- R. Becerril, C. Nerín and F. Silva, Molecules, 2020, 25(5), 1134 CrossRef CAS PubMed.
- T. Xu, C. Gao, Y. Yang, X. Shen, M. Huang, S. Liu and X. Tang, Food Hydrocolloids, 2018, 84, 84–92 CrossRef CAS.
- Y. A. Arfat, S. Benjakul, T. Prodpran, P. Sumpavapol and P. Songtipya, Food Hydrocolloids, 2014, 41, 265–273 CrossRef CAS.
- M. Imran, A.-M. Revol-Junelles, N. René, M. Jamshidian, M. J. Akhtar, E. Arab-Tehrany, M. Jacquot and S. Desobry, Food Hydrocolloids, 2012, 29, 407–419 CrossRef CAS.
- S. Amjadi, H. Almasi, A. Ghadertaj and L. Mehryar, J. Food Process. Preserv., 2021, 45, e15196 CAS.
- A. Silva-Weiss, M. Ihl, P. J. A. Sobral, M. Gómez-Guillén and V. Bifani, Food Eng. Rev., 2013, 5, 200–216 CrossRef CAS.
- N. Jemil, M. Ouerfelli, M. P. Almajano, J. Elloumi-Mseddi, M. Nasri and N. Hmidet, Food Chem., 2020, 303, 125364 CrossRef CAS PubMed.
- M. Wrona, M. J. Cran, C. Nerín and S. W. Bigger, Carbohydr. Polym., 2017, 156, 108–117 CrossRef CAS PubMed.
- J. Y. Lee, C. V. Garcia, G. H. Shin and J. T. Kim, LWT–Food Sci. Technol., 2019, 106, 164–171 CrossRef CAS.
- Y. Chu, W. Cheng, X. Feng, C. Gao, D. Wu, L. Meng, Y. Zhang and T. Tang, Food Packag. Shelf Life, 2020, 25, e1005472 Search PubMed.
- L. B. Norcino, J. F. Mendes, C. V. L. Natarelli, A. Manrich, J. E. Oliveira and L. H. C. Mattoso, Food Hydrocolloids, 2020, 106, e105862 CrossRef.
- J. F. Mendes, L. B. Norcino, H. H. Martins, A. Manrich, C. G. Otoni, E. E. N. Carvalho, R. H. Piccolli, J. E Oliveira, A. C. M. Pinheiro and L. H. C. Mattoso, J. Food Sci., 2021, 86, 1979–1996 CrossRef CAS PubMed.
- J. Bonilla and P. J. A. Sobral, Int. J. Biol. Macromol., 2020, 151, 178–185 CrossRef CAS PubMed.
- J. Bonilla, R. B. Paiano, R. V. Lourenço, A. M. Q. B. Bittante and P. J. A. Sobral, Int. J. Biol. Macromol., 2020, 164, 1399–1412 CrossRef CAS PubMed.
- J. Bonilla, R. B. Paiano, R. V. Lourenço, A. M. Q. B. Bittante and P. J. A. Sobral, J. Polym. Environ., 2021, 29, 1380–1395 CrossRef CAS.
- S. Noori, F. Zeynali and H. Almasi, Food Control, 2018, 84, 312–320 CrossRef CAS.
- M. Artiga-Artigas, A. Acevedo-Fani and O. Martín-Belloso, Food Control, 2017, 76, 1–12 CrossRef CAS.
- Ö. Tastan, G. Ferrari, T. Baysal and F. Donsì, Food Hydrocolloids, 2016, 61, 756–771 CrossRef.
- S. M. T. Gharibzahedi and S. Mohammadnabi, Int. J. Biol. Macromol., 2017, 95, 769–777 CrossRef CAS PubMed.
- S. Sugumar, A. Mukherjee and N. Chandrasekaran, Int. J. Nanomed., 2015, 10, 67–75 CAS.
- M. L. Zambrano-Zaragoza, E. Gutiérrez-Cortez, A. Del Real, R. M. González-Reza, M. J. Galindo-Pérez and D. Quintanar-Guerrero, Food Res. Int., 2014, 62, 974–983 CrossRef CAS.
- C. Wu, L. Wang, Y. Hu, S. Chen, D. Liu and X. Ye, RSC Adv., 2016, 6, 20892–20900 RSC.
- H. Hashemi Gahruie, E. Ziaee, M. H. Eskandari and S. M. H. Hosseini, Carbohydr. Polym., 2017, 166, 93–103 CrossRef CAS PubMed.
- S. Ghani, H. Barzegar, M. Noshad and M. Hojjati, Int. J. Biol. Macromol., 2018, 112, 197–202 CrossRef CAS PubMed.
- N. Caro, E. Medina, M. Díaz-Dosque, L. López, L. Abugoch and C. Tapia, Food Hydrocolloids, 2016, 52, 520–532 CrossRef CAS.
- L. A. Castillo, S. Farenzena, E. Pintos, M. S. Rodríguez, M. A. Villar, M. A. Gárcia and O. V. López, Food Packag. Shelf Life, 2017, 14, 128–136 CrossRef.
- C. Chenwei, T. Zhipeng, M. Yarui, Q. Weiqiang, Y. Fuxin, M. Jun and X. Jing, Prog. Org. Coat., 2018, 123, 176–184 CrossRef.
- J. P. Correa, V. Molina, M. Sanchez, C. Kainz, P. Eisenberg and M. B. Massani, Food Packag. Shelf Life, 2017, 11, 31–39 CrossRef.
- R. Battisti, N. Fronza, Á. Vargas, S. M. D. Silveira, M. S. P. Damas and M. G. N. Quadri, Food Packag. Shelf Life, 2017, 11, 115–124 CrossRef.
- M. Rocha, A. Alemán, V. P. Romani, M. E. López-Caballero, M. C. Gómez-Guillén, P. Montero and C. Prentice, Food Hydrocolloids, 2018, 81, 351–363 CrossRef.
- O. Moreno, À. Gil, L. Atarés and A. Chiralt, LWT–Food Sci. Technol., 2017, 84, 189–195 CrossRef CAS.
- Z. Dong, F. Xu, I. Ahmed, Z. Li and H. Lin, Food Control, 2018, 92, 37–46 CrossRef CAS.
- I. Echeverría, M. E. López-Caballero, M. C. Gómez-Guillén, A. N. Mauri and M. P. Montero, Int. J. Food Microbiol., 2018, 266, 142–149 CrossRef PubMed.
- S. Kumar, A. Shukla, P. P. Baul, A. Mitra and D. Halder, Food Packag. Shelf Life, 2018, 16, 178–184 CrossRef.
- G. B. Lomate, B. Dandi and S. Mishra, Food Packag. Shelf Life, 2018, 16, 211–219 CrossRef.
- M. Pabast, N. Shariatifar, S. Beikzadeh and G. Jahed, Food Control, 2018, 91, 185–192 CrossRef CAS.
- L. J. Pérez-Córdoba, PhD thesis, University of São Paulo, 2018.
| This journal is © The Royal Society of Chemistry 2021 |