A silk-based sealant with tough adhesion for instant hemostasis of bleeding tissues†
Shumeng
Bai
a,
Xueliang
Zhang
a,
Pingqiang
Cai
bc,
Xiaowei
Huang
d,
Yuqing
Huang
d,
Rui
Liu
a,
Mengya
Zhang
a,
Jibin
Song
 d,
Xiaodong
Chen
d,
Xiaodong
Chen
 *bc and
Huanghao
Yang
*ad
*bc and
Huanghao
Yang
*ad
aCollege of Biological Science and Engineering, Fuzhou University, Fuzhou 350108, People's Republic of China. E-mail: hhyang@fzu.edu.cn
bInnovative Center for Flexible Devices (iFLEX), School of Materials Science & Engineering, Nanyang Technological University, 50 Nanyang Avenue, Singapore 639798, Singapore. E-mail: chenxd@ntu.edu.sg; Web: http://www.ntu.edu.sg/home/chenxd/
cMax Planck - NTU Joint Lab for Artificial Senses, Nanyang Technological University, 50 Nanyang Avenue, Singapore 639798, Singapore
dMOE Key Laboratory for Analytical Science of Food Safety and Biology, Fujian Provincial Key Laboratory of Analysis and Detection Technology for Food Safety, State Key Laboratory of Photocatalysis on Energy and Environment, College of Chemistry, Fuzhou University, Fuzhou 350108, People's Republic of China
First published on 28th June 2019
Abstract
Marine mussels harness catechol-rich foot proteins with hierarchically assembled nanostructures to achieve robust adhesion in the dynamic underwater environment. Herein, mimicking the chemical composition and hierarchical nanostructure of mussel foot proteins, a silk-based sealant (named SFT) with superior wet adhesion and instant hemostasis was developed by introducing tannic acid (TA) into silk fibroin (SF). The presence of TA containing abundant phenolic moieties triggered the conformational transition of SF from random coil to β-sheet and the hierarchical assembly of nanofibrillar structures, imparting SFT with high intrinsic toughness (123.1 kJ m−3). The phenolic moiety, with strong binding affinity to nucleophiles in tissue biomolecules, also endowed SFT with tough adhesion to wet tissues (strength ≈134.1 kPa) even in the presence of blood and robust tissue motions (e.g., bleeding heart) and exhibited instant hemostatic capability (within 30 s). Given the unprecedented combination of tough adhesion and instant homeostasis, the engineered SFT can fulfil unmet challenges and serve as a promising surgical sealant for suturelessly sealing ruptured tissues in wet and dynamic biological environments.
New conceptsAdhesion to wet tissue surfaces, especially in highly dynamic biological environments, is important in clinical fields but remains extremely challenging. Currently available sealants are cytotoxic, adhere weakly to tissues, or cannot be used in wet and dynamic environments within the body. To address these challenges, marine mussels, which secrete catechol-rich foot proteins to maintain strong adhesion under seawater, provide an ideal model for researchers to design high-performance adhesive systems. However, the existing biomimetic technologies focus on imitating the catechol feature of the mussel foot proteins, while the importance of their hierarchically assembled nanostructures for robust underwater adhesion is neglected. We envisioned that simultaneously mimicking the chemical composition and hierarchically assembled nanostructures is a promising strategy to enhance the wet adhesion properties of bio-inspired materials. Here, we reported a facile method for developing strong underwater sealants with hierarchically assembled nanostructures by introducing tannic acid into silk fibroin to address the aforementioned limitations. The co-assembly behavior of tannic acid with the natural biomolecules can open up a new avenue for the one-step development of functional nanoscale materials and hierarchical bio-assemblies. |
Introduction
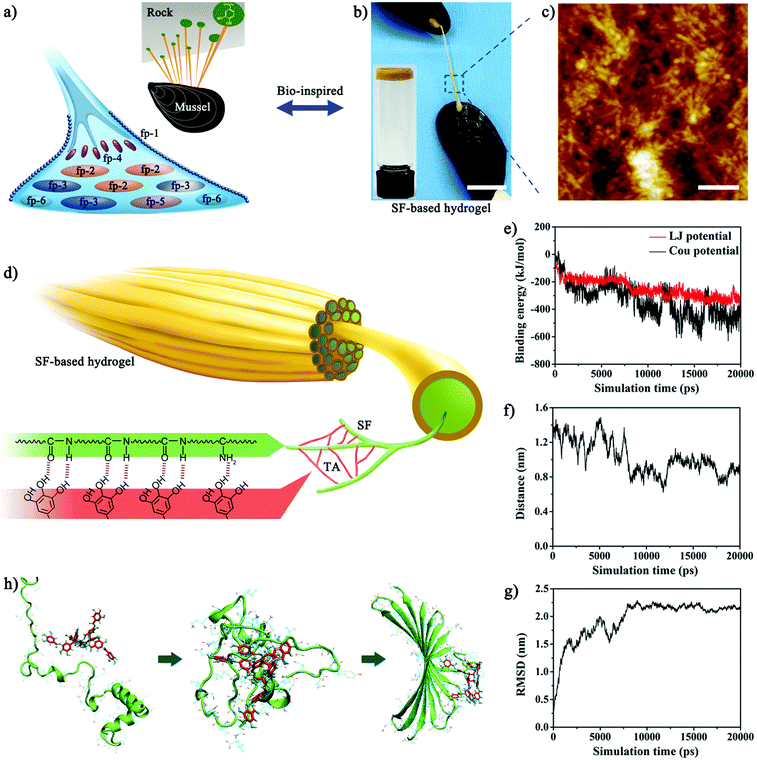
Surgical techniques for the instant sealing of ruptured tissues are of significant clinical importance.1,2 Conventional techniques (e.g., the use of sutures and staples), though extensively used to reconnect incisions, cannot provide efficient waterproof sealing, therefore leading to blood leakage.3 Surgical sealants have received considerable attention as promising alternatives for improved sealing and ease of operability but provide negligible traumatic closure.4,5 However, current clinically available sealants, such as cyanoacrylate and fibrin sealants, are far from ideal due to their toxicity, low adhesion strength to wet or bleeding tissues and low tolerance to mechanical perturbations.5–8 For instance, cyanoacrylate immediately solidifies upon exposure to blood, but it becomes more rigid than native tissues and its toxic degraded products limit clinical translation.7,8 The fibrin sealant provides low adhesion strength to bleeding tissues and cannot withstand the forces in dynamic conditions (e.g., shear of blood flow, beating heart).5 Thus, there is an unmet need for developing new highly biocompatible sealants with uncompromised adhesion even to wet bleeding tissues.In nature, sessile marine organisms adhere to diverse surfaces and maintain strong adhesion under seawater.9,10 Especially, mussels secrete catechol-rich foot proteins to glue byssal plaques onto rocks (Fig. 1a), which supplies a novel strategy for designing high-performance adhesive systems.11–13 Inspired by mussels, phenolic chemistry has gained extensive interest in the recapitulation of similar wet adhesion.14–16 Mimicking the chemical composition of mussel foot proteins, various adhesive materials have been synthesized through the covalent tethering of phenolic moieties to the backbone of polymers.17–20 However, such phenol-conjugated composites do not fully satisfy the wet bonding requirements to be classified as ideal medical sealants due to time-consuming synthesis, low yield of production and additional UV photo-activation.17,21 Recently, studies in nanoscience have emerged indicating that the hierarchically assembled nanostructures of mussel foot proteins also account for important elements of the mussel's underwater adhesion.11,22–25 Researchers have designed biomimetic adhesives through the hierarchical self-assembly of mussel foot proteins from nanofibers to agglomerates.22 Notwithstanding the exciting success, the lack of a facile method for developing strong underwater adhesives with hierarchically assembled nanostructures has limited their translation into clinical practice.
FDA-approved silk fibroin (SF), a natural biopolymer, has been extensively utilized in biomedical applications, including sutures and adhesives, due to its good biocompatibility and biodegradability and tunable mechanical properties.26–29 However, SF-based materials have not yet been fully used as surgical sealants due to their unsuitable mechanical and adhesive properties in wet and dynamic biological environments.30–32 Recently, the co-assembly behaviors of tannic acid (TA) with other polymer matrices has opened up new avenues for the one-step development of natural biomolecule-based adhesives.33,34 Here, we demonstrated the use of TA to crosslink SF in aqueous conditions, allowing the self-assembly into a novel SF-based hydrogel sealant (named SFT) (Fig. 1b). TA with abundant phenolic moieties triggered the conformational transition of SF from random coil to β-sheet and the formation of a hierarchical nanofibrillar structure (Fig. 1c). The nanofibrillar hydrogel network with β-sheet conformation imparted SFT with high intrinsic toughness and robust underwater adhesion performance (Fig. S1, ESI†), which was similar to that of mussel foot proteins. We engineered this SF-based sealant for the rapid and effective closure of ruptured bleeding tissues in vivo. In addition, the SFT sealant maintained good biocompatibility and biodegradability as well as outstanding antimicrobial activity. The ease of this instant co-assembly strategy and tough adhesion to mechanically robust bleeding tissues will broaden the clinical applications of SF-based materials in wound closure and surgical sealing.
Results and discussion
The polyphenol group of TA has high binding affinity with nucleophiles (e.g., amido bond, amines, and thiol) and can thereby tightly bind to biological molecules such as proteins and peptides.35,36 Since SF is abundant in nucleophiles, we hypothesized that strong cross-linked networks could be instantly formed between SF and TA, resulting in a hierarchical supramolecular material (Fig. 1d). Consistent with the hypothesis, molecular dynamics simulations also suggested a co-assembly process (Fig. S2, ESI†). Upon mixing, the binding energy, interaction distance, and root mean square deviation (RMSD) curves reached a plateau state and stabilized within about 20 ns (Fig. 1e–g), indicating the rapid interaction between SF and TA. The van der Waals forces (Lennard-Jones potential energy) first drove the initial attraction and attachment of SF and TA. As SF and TA molecules approached each other, electrostatic forces gradually increased and became dominant. Furthermore, this assembly process was energetically favourable and strong hydrogen bonds were formed between TA and amino acids containing hydroxyl or amino groups, as evident from the binding energy of TA and key amino acids for the last 1 ns during equilibrium (Fig. S3, ESI†). In addition, the simulation of spatial conformation elaborated that TA induced and promoted the protein folding behavior (Fig. 1g and h). Amino acids of the SF protein at positions 45 to 65 were strongly recruited by TA through van der Waals and electrostatic forces, which subsequently folded around TA and formed similar β-sheet structures (Fig. S4, ESI†). As the reaction proceeded, the amino acid pair 39/63 approached TA molecules through electrostatic interaction and attracted each other through π–π stacking interaction. This pair of 39/63 amino acids was thereby attached by hydrogen bonds in β-sheet conformation, which were far from each other in the initial random coil conformation of the SF protein. Meanwhile, the corresponding pair of 50/52 amino acids reached the corner position and connected the two segments in β-sheet conformation. Given the multi-aromatic ring structure, TA could hold on to consecutive amino acids in the SF protein through electrostatic interactions and hydrogen bonds during the co-assembly process, subsequently changing the conformation of SF to form stable β-sheet structures through π–π stacking interactions.The synthesis procedure of SFT was extremely facile and was achieved by spontaneous gelation upon mixing aqueous SF (5 wt%) and TA (0.3 g mL−1) solutions. The rheological measurements revealed that SFT exhibited typical gel characteristics as G′ was greater than G′′ after gelation (Fig. S5, ESI†). The increasing trend in the G′ value indicated the formation of hydrogels composed of noncovalent bonds such as hydrogen bonds.34 The dynamic time sweep experiment also suggested the good stability of SFT. Additionally, no complicated synthetic procedure was involved; thus, the amount of the prepared SFT could be easily scaled up. It was worth noting that the co-assembly of TA and SF led to the formation of hierarchically assembled nanofibers. The mean diameter of the nanofibers reached as high as ∼30 nm, which was close to the geometric confinement of silk fibrils (50 ± 30 nm) for achieving outstanding mechanical properties.37,38 As control, pure SF displayed nanoparticle structures (Fig. S6, ESI†), indicating that the intermolecular interactions between SF and TA induced the structural transformation from nanoparticles to nanofibers. The nanofibrillar structure is also shown in SEM images (Fig. S7, ESI†). More importantly, the nanofibers further aggregated into nanofibrillar bundles and lamellas and finally formed porous macroscopic materials, which confirmed the hierarchically assembled structures of SFT. This hierarchical supramolecular organization of nanofibrillar structures endowed SFT with collective molecular motions and therefore robust macroscopic mechanical functions.39 Interestingly, the assembled formation of the nanofibrillar structure only occurred when the concentration of the TA solution was 0.3 g mL−1. TA solutions with a lower or higher concentration than 0.3 g mL−1 were difficult to co-assemble with SF to form the nanofibrillar structure. In natural silk fibers, silk molecules self-assemble into organized nanostructures through noncovalent interactions, which further aggregate into microstructures and macroscopic materials.40 pH is thought to be one of the important factors affecting the assembly process. Under 0.3 g mL−1 TA solution, the pH value of the mixture was fairly close to the theoretical isoelectric point of silk fibroin (pI ≈ 4.5), which facilitated the hierarchical assembly of neighbouring silk molecules by suppressing the electrostatic repulsion.41,42 The unsuitable pH conditions caused by TA solutions with other concentrations prevented the assembly behaviours of SF.
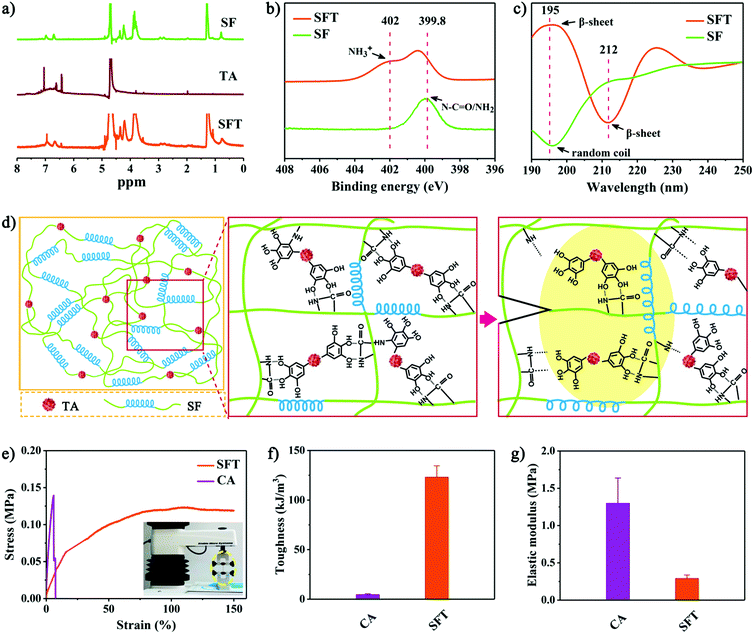
The co-assembly of SF and TA into SFT was also experimentally confirmed. Nuclear magnetic resonance (NMR) spectra showed similar peaks in SFT and TA (Fig. 2a), suggesting that phenolic oxidation seemed greatly suppressed to retain the durable adhesive bonding capability of TA.33 X-ray photoelectron spectra (XPS) exhibited slight increase in the N1s signal of SF during the assembly process (Fig. 2b). Prior to assembly, the peak at 399.8 eV for amide (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)/amine (NH2) was detected,43 which increased to 402.0 eV for the protonated amine (NH3+) after interaction with TA, suggesting the protonation of the amide/amine group of SF. The change in chemical protonation also indicated the aforementioned conformational transition. Circular dichroism (CD) spectra demonstrated the conformational transition of SF in the assembly process (Fig. 2c). The CD spectrum of the original SF solution showed a random coil conformation with negative ellipticity at ∼195 nm.44 After gelation, positive ellipticity at ∼195 nm and negative ellipticity at ∼212 nm appeared, revealing induction of β-sheet conformation. Fourier transform infrared (FTIR) spectroscopy confirmed the structural changes in the SF protein (Fig. S8, ESI†). A slight decrease from 1543 cm−1 (corresponding to random coil) to 1512 cm−1 (corresponding to β-sheet) was observed.45 This showed that the intermolecular interactions between SF and TA triggered the conformational transition of SF from random coil to β-sheet.
O)/amine (NH2) was detected,43 which increased to 402.0 eV for the protonated amine (NH3+) after interaction with TA, suggesting the protonation of the amide/amine group of SF. The change in chemical protonation also indicated the aforementioned conformational transition. Circular dichroism (CD) spectra demonstrated the conformational transition of SF in the assembly process (Fig. 2c). The CD spectrum of the original SF solution showed a random coil conformation with negative ellipticity at ∼195 nm.44 After gelation, positive ellipticity at ∼195 nm and negative ellipticity at ∼212 nm appeared, revealing induction of β-sheet conformation. Fourier transform infrared (FTIR) spectroscopy confirmed the structural changes in the SF protein (Fig. S8, ESI†). A slight decrease from 1543 cm−1 (corresponding to random coil) to 1512 cm−1 (corresponding to β-sheet) was observed.45 This showed that the intermolecular interactions between SF and TA triggered the conformational transition of SF from random coil to β-sheet.
As a multiblock copolymer, SF comprises hydrophilic blocks, which form amorphous random coils and α-helices, as well as hydrophobic blocks, which tend to form hydrogen-bonded β-sheets. The nanoscale confinement of β-sheet crystals in silk has been reported to impart the SF-based materials with great stiffness, resilience and fracture toughness.37,46 We therefore proposed that the hierarchically assembled nanofibrillar structures in SFT with β-sheet conformation could act as the matrix to dissipate energy through hysteresis after deformation (the loose β-sheet helix-like architecture in the yellow zone of Fig. 2d). Together with the polyphenol–nucleophile interfacial effect, such inherent structural features of β-sheet-rich SFT achieved significantly improved mechanical toughness of 123.1 ± 11.5 kJ m−3 (Fig. 2e and f). As one clinical counterpart, cyanoacrylate has toughness of 4.6 ± 0.8 kJ m−3, which is much lower than that of SFT. Although cyanoacrylate showed high elastic modulus of 1.3 ± 0.3 MPa, the elastic modulus of SFT was only 0.3 ± 0.1 MPa (Fig. 2g), which better approximates the modulus of living tissues.47,48 These remarkable mechanical performances of SFT appear promising for tissue sealing.
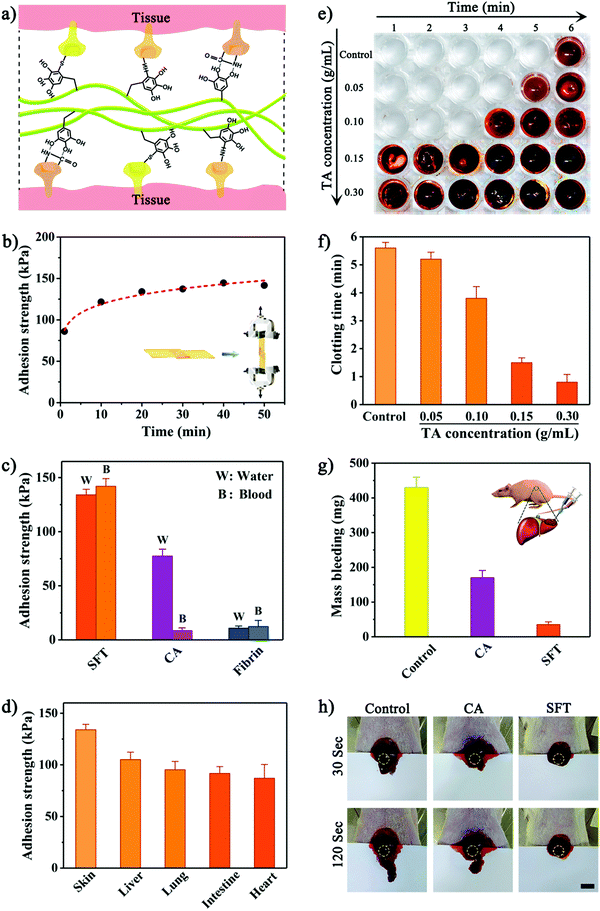
In addition to intrinsic toughness, we tested the adhesion strength of SFT on wet tissues according to ASTM standard methods.6,8 Lap shear, peeling adhesion, and wound closure tests were performed using wet porcine skins as substrates for simulating the native properties of human tissues (Fig. S9–S11, ESI†). It is worth noting that TA tended to interact with Glu, Lys, Thr and other amino acids containing hydroxyl or amino groups to form strong hydrogen bonds. For van der Waals interactions, two interactive models were mainly involved (Fig. S12, ESI†): π–π stacking of TA and peptide bonds (e.g., Gly, Ala, and Ser) and π–π stacking of TA and side-chain alkanes or rigid planar structures of amino acids (e.g., Arg, Lys, and Phe). Therefore, the abundant polyphenol groups endowed SFT with strong adhesion to tissue surfaces by binding with tissue proteins (Fig. 3a). The maximum adhesion efficacy was achieved by the optimized formulation of SFT produced using 5 wt% SF solution with 0.3 g mL−1 TA solution, which was due to the unique hierarchically assembled nanofibrillar structure at this reaction ratio. Together with its remarkable mechanical performances, a strong adhesion of SFT was instantly formed on the wet porcine skin with strength of 86.1 ± 6.4 kPa within 1 min (Fig. 3b). Importantly, SFT exhibited time-dependent yet rapid increase in the adhesion strength, which reached 134.1 ± 5.2 kPa in 20 min. This robust and progressive adhesion allowed the application of the SFT material in a facile manner, promoting its clinical translation.8 In contrast, fibrin glue weakly adhered to tissues, whereas cyanoacrylate immediately solidified upon contact with wet tissues, which made their application and repositioning challenging.
Tolerance to blood exposure is another key parameter of surgical sealants. It was observed that SFT retained strong adhesion in the presence of blood (Fig. 3c). Actually, SFT exhibited higher adhesion strength to tissues wetted with blood than that with water, which might be ascribed to the additional interactions between TA and proteins in blood.49 On the contrary, the adhesion strength provided by cyanoacrylate significantly declined by ∼89% upon exposure to blood.
Additionally, SFT was applicable to a variety of wet tissues (Fig. 3d). It adhered strongly to porcine liver, lung, intestine, and heart, offering substantial advantages for the anchoring and integration of various tissues. Meanwhile, the cytocompatibility of SFT was evaluated (Fig. S13, ESI†). Human synovial fibroblast (HSF) cells exhibited unaffected proliferation and normal fusiform morphology after incubation with SFT. However, cyanoacrylate significantly inhibited cellular growth and the HSF cells displayed anomalous morphology. Similar results were provided by a CCK-8 assay, confirming good biocompatibility of SFT.
Besides high tolerance to blood exposure, instant hemostasis of surgical sealants is equally significant in clinical applications. Because of its robust coagulating effect through binding blood molecules, we hypothesized that SFT could be used as a promising hemostatic agent to effectively seal bleeding. The in vitro hemostatic ability of SFT was evaluated by investigating the clotting time of whole blood (Fig. 3e and Fig. S14, ESI†). Under natural conditions, blood begins to coagulate after 5–6 minutes.50 A similar clotting time (5.6 ± 0.2 minutes) was observed for the control experiment (Fig. 3f). The presence of SFT reduced the blood clotting time to 0.8 ± 0.3 minutes, which might be due to the strong interactions between the phenolic moiety in SFT and nucleophiles in blood proteins that accelerate platelet aggregation and activation of clotting factors.49 The reduced blood clotting time for SFT can be beneficial for rapid in vivo hemostasis. A rat liver bleeding model was established to investigate the hemostatic sealing effect (Fig. 3g). After adhering and completely covering the haemorrhaging site, the SFT material significantly reduced blood loss to 35.2 ± 8.6 mg, which was considerably lower than the level after cyanoacrylate treatment (170.5 ± 21.1 mg). It is worth mentioning that the liver treated with SFT instantly stopped bleeding (Fig. 3h and Fig. S15, ESI†), indicating rapid local hemostasis to avoid prolonged blood leakage from the bleeding tissues.
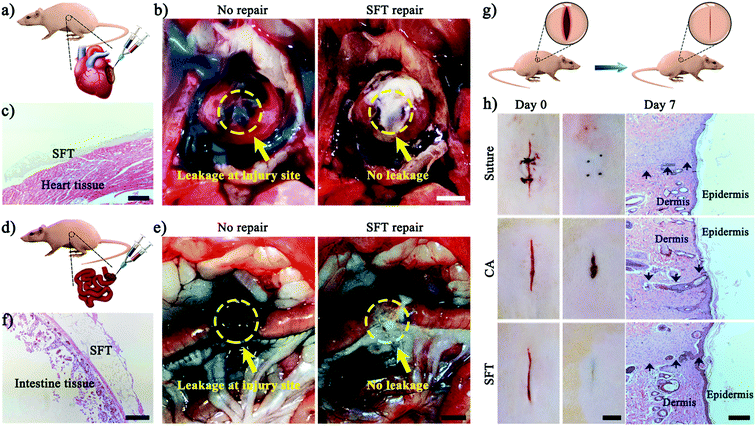
Subsequently, we demonstrated that the instant hemostatic capacity and tough adhesion of SFT also tolerated robust mechanical perturbations such as a beating heart and intestine filled with biofluids, which could be sealed (Fig. 4a–f). Fresh blood was gushing from the lesion site; we then applied SFT. Immediate wound sealing was achieved without secondary haemorrhage (Fig. 4b, Movie S1, ESI†), confirming the efficacy of SFT for sealing severely bleeding tissues within highly dynamic environments. H&E staining showed that SFT tightly adhered to the surface of the heart (Fig. 4c), providing a strong sealing effect. Moreover, SFT also achieved complete sealing of severely leaking intestinal tissues (Fig. 4d, Movie S2, ESI†). The test dye injected through the intestine leaked, confirming injury diagnosis (Fig. 4e). After the application of SFT at the incision site, we re-injected the test dye to observe the efficacy. No leak was observed from the sealed site and SFT remained intact and formed a tight connection with the intestinal tissue (Fig. 4f).
A surgical sealant with appropriate biodegradability and antimicrobial performance can facilitate the restoration of wounded tissues. Therefore, we further characterized the in vivo biodegradation of SFT (Fig. S16, ESI†). After subcutaneous implantation into rats for 45 days, the in vivo degradation ratio reached up to 80.4% by weight and 84.7% by volume. H&E staining revealed that the biodegradation of SFT materials enabled the ingrowth of autologous cells and gradual replacement of the material by native tissues. These results demonstrated the high degree of the in vivo biodegradation of SFT materials. Although H&E staining showed the occurrence of an inflammatory response of the hydrogel, it is encouraging to note that the inflammatory response gradually reduced and became mild after implantation for 45 days, indicating that SFT could be used as a biocompatible temporary agent for in vivo applications.51,52 It is worth noting that SFT also exhibited an outstanding antibacterial performance (Fig. S17, ESI†). S. aureus is a Gram-positive bacterium and one of the most common clinical pathogens responsible for serious nosocomial infections. LIVE/DEAD BacLight staining results revealed that S. aureus showed red fluorescence with agglomeration after incubation with SFT, suggesting that most of the bacteria were killed or underwent lysis.53 Scanning electron microscopy (SEM) also showed that the bacteria incubated with SFT completely lost their cellular integrity. Moreover, zone of inhibition tests showed the potential antibacterial activity of SFT for the Gram-negative bacteria E. coli (Fig. S18, ESI†).16,54,55
Besides being efficient in sealing wounds on tissue surfaces, SFT could also be used for the effective repair of cutaneous wounds due to its aqueous formulation before gelation and its biodegradability. As shown in a rat skin incision model (Fig. 4g), wound incisions treated with SFT remained closed for 7 days, achieving high efficiency in wound healing (Fig. 4h). Moreover, the SFT sealing treatment facilitated wound closure and re-epithelialization with a smooth appearance with no further actions needed, while the suturing treatment required suture removal for repositioning by specialized personnel.4 In contrast, the wound incisions treated with cyanoacrylate failed and showed a delayed reconnecting process, which could be attributed to the immediate solidification of cyanoacrylate upon contact with blood. Furthermore, the results of the wound contraction area were employed to quantitatively evaluate the therapeutic effects of SFT (Fig. S19, ESI†).51,56,57 After treatment for 7 days, the SFT group presented about 95% wound contraction, which was fairly close to that of the suturing treatment group. It is worth noting that the SFT group exhibited about 37.6% higher wound contraction than that of the cyanoacrylate group, demonstrating its facilitating effect on the wound healing process. H&E staining histology examination also confirmed the wound healing effect (Fig. 4h). The skin sections treated with SFT had the majority of the epidermal layers restored. These results demonstrated that SFT effectively sealed skin incisions without interfering with the healing process.
Conclusions
In conclusion, we developed a novel SF-based hydrogel sealant with adequate wet adhesion strength and instant hemostatic capacity under both wet and dynamic biological environments. Inspired by mussels and computational simulations, we introduced TA to drive the hierarchical assembly and simultaneous β-sheet conformational transition of SF. The resulting hierarchical nanofibrillar structure with β-sheet conformation endowed the SF-based sealant with high mechanical toughness (123.1 kJ m−3). Optimized SFT formulation achieved robust wet adhesion (134.1 kPa) and instant hemostasis (within 30 s). In vivo experiments in rat models showed rapid sealing of severely bleeding tissues within wet and highly dynamic environments. Moreover, the engineered SFT exhibited excellent biocompatibility and biodegradability as well as effective antibacterial protection. These merits of the STF sealant meet the key challenges for broader clinical applications such as surgery and regenerative medicine. We envision that simultaneously mimicking the chemical composition and hierarchically assembled nanostructures is a promising strategy to enhance the wet adhesion properties of bio-inspired materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 51603041, U1505221), Natural Science Foundation of Fujian Province of China (No. 2017Y0058), the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT15R11). The authors also thank the financial support by NTU-Northwestern Institute for Nanomedicine, Singapore.References
- C. Ghobril and M. W. Grinstaff, Chem. Soc. Rev., 2015, 44, 1820 RSC.
- C. Heinzmann, C. Weder and L. M. de Espinosa, Chem. Soc. Rev., 2016, 45, 342 RSC.
- N. Annabi, A. Tamayol, S. R. Shin, A. M. Ghaemmaghami, N. A. Peppas and A. Khademhosseini, Nano Today, 2014, 9, 574 CrossRef CAS PubMed.
- A. Meddahi-Pellé, A. Legrand, A. Marcellan, L. Louedec, D. Letourneur and L. Leibler, Angew. Chem., Int. Ed., 2014, 53, 6369 CrossRef PubMed.
- N. Lang, M. J. Pereira, Y. Lee, I. Friehs, N. V. Vasilyev, E. N. Feins, K. Ablasser, E. D. O’Cearbhaill, C. Xu, A. Fabozzo, R. Padera, S. Wasserman, F. Freudenthal, L. S. Ferreira, R. Langer, J. M. Karp and P. J. del Nido, Sci. Transl. Med., 2014, 6, 218ra6 CrossRef PubMed.
- N. Annabi, Y. N. Zhang, A. Assmann, E. S. Sani, G. Cheng, A. D. Lassaletta, A. Vegh, B. Dehghani, G. U. Ruiz-Esparza, X. Wang, S. Gangadharan, A. S. Weiss and A. Khademhosseini, Sci. Transl. Med., 2017, 9, eaai7466 CrossRef PubMed.
- A. M. Behrens, N. G. Lee, B. J. Casey, P. Srinivasan, M. J. Sikorski, J. L. Daristotle, A. D. Sandler and P. Kofinas, Adv. Mater., 2015, 27, 8056 CrossRef CAS PubMed.
- J. Li, A. D. Celiz, J. Yang, Q. Yang, I. Wamala, W. Whyte, B. R. Seo, N. V. Vasilyev, J. J. Vlassak, Z. Suo and D. J. Mooney, Science, 2017, 357, 378 CrossRef CAS PubMed.
- P. B. Messersmith, Science, 2008, 319, 1767 CrossRef CAS PubMed.
- A. R. Scott, Nature, 2015, 519, S12 CrossRef CAS PubMed.
- Q. Lin, D. Gourdon, C. Sun, N. Holten-Andersen, T. H. Anderson, J. H. Waite and J. N. Israelachvili, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 3782 CrossRef CAS PubMed.
- J. J. Wilker, Nat. Mater., 2014, 13, 849 CrossRef CAS PubMed.
- J. Horsch, P. Wilke, M. Pretzler, M. Seuss, I. Melnyk, D. Remmler, A. Fery, A. Rompel and H. G. Börner, Angew. Chem., Int. Ed., 2018, 57, 15728 CrossRef CAS PubMed.
- J. Saiz-Poseu, J. Mancebo-Aracil, F. Nador, F. Busqué and D. Ruiz-Molina, Angew. Chem., Int. Ed., 2019, 58, 696 CrossRef CAS PubMed.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426 CrossRef CAS PubMed.
- Y. Liang, X. Zhao, T. Hu, B. Chen, Z. Yin, P. X. Ma and B. Guo, Small, 2019, 15, 1900046 CrossRef PubMed.
- J. Xu, X. Li, J. Li, X. Li, B. Li, Y. Wang, L. Wu and W. Li, Angew. Chem., Int. Ed., 2017, 56, 8731 CrossRef CAS PubMed.
- G. P. Maier, M. V. Rapp, J. H. Waite, J. N. Israelachvili and A. Butler, Science, 2015, 349, 628 CrossRef CAS PubMed.
- M. A. Rahim, S. L. Kristufek, S. Pan, J. J. Richardson and F. Caruso, Angew. Chem., Int. Ed., 2019, 58, 1904 CrossRef CAS PubMed.
- K. A. Burke, D. C. Roberts and D. L. Kaplan, Biomacromolecules, 2016, 17, 237 CrossRef CAS PubMed.
- Y. Hong, F. Zhou, Y. Hua, X. Zhang, C. Ni, D. Pan, Y. Zhang, D. Jiang, L. Yang, Q. Lin, Y. Zou, D. Yu, D. E. Arnot, X. Zou, L. Zhu, S. Zhang and H. Ouyang, Nat. Commun., 2019, 10, 2060 CrossRef PubMed.
- C. Zhong, T. Gurry, A. A. Cheng, J. Downey, Z. Deng, C. M. Stultz and T. K. Lu, Nat. Nanotechnol., 2014, 9, 858 CrossRef CAS PubMed.
- B. K. Ahn, J. Am. Chem. Soc., 2017, 139, 10166 CrossRef CAS PubMed.
- E. Filippidi, T. R. Cristiani, C. D. Eisenbach, J. H. Waite, J. N. Israelachvili, B. K. Ahn and M. T. Valentine, Science, 2017, 358, 502 CrossRef CAS PubMed.
- A. S. Viana and R. Santos, Beilstein J. Nanotechnol., 2018, 9, 2277 CrossRef CAS PubMed.
- F. G. Omenetto and D. L. Kaplan, Science, 2010, 329, 528 CrossRef CAS PubMed.
- D. N. Rockwood, R. C. Preda, T. Yücel, X. Wang, M. L. Lovett and D. L. Kaplan, Nat. Protoc., 2011, 6, 1612 CrossRef CAS PubMed.
- B. Zhu, H. Wang, W. R. Leow, Y. Cai, X. J. Loh, M. Y. Han and X. Chen, Adv. Mater., 2016, 28, 4250 CrossRef CAS PubMed.
- X. W. Huang, J. J. Wei, M. Y. Zhang, X. L. Zhang, X. F. Yin, C. H. Lu, J. B. Song, S. M. Bai and H. H. Yang, ACS Appl. Mater. Interfaces, 2018, 10, 35495 CrossRef CAS PubMed.
- M. A. Serban, B. Panilaitis and D. L. Kaplan, J. Biomed. Mater. Res., Part A, 2011, 98A, 567 CrossRef CAS PubMed.
- T. Yucel, N. Kojic, G. G. Leisk, T. J. Lo and D. L. Kaplan, J. Struct. Biol., 2010, 170, 406 CrossRef CAS PubMed.
- G. G. Leisk, T. J. Lo, T. Yucel, Q. Lu and D. L. Kaplan, Adv. Mater., 2010, 22, 711 CrossRef CAS PubMed.
- K. Kim, M. Shin, M. Y. Koh, J. H. Ryu, M. S. Lee, S. Hong and H. Lee, Adv. Funct. Mater., 2015, 25, 2402 CrossRef CAS.
- M. Shin, J. H. Ryu, J. P. Park, K. Kim, J. W. Yang and H. Lee, Adv. Funct. Mater., 2015, 25, 1270 CrossRef CAS.
- J. Shin, J. S. Lee, C. Lee, H. J. Park, K. Yang, Y. Jin, J. H. Ryu, K. S. Hong, S. H. Moon, H. M. Chung, H. S. Yang, S. H. Um, J. W. Oh, D. I. Kim, H. Lee and S. W. Cho, Adv. Funct. Mater., 2015, 25, 3814 CrossRef CAS.
- R. Wang, J. Li, W. Chen, T. Xu, S. Yun, Z. Xu, Z. Xu, T. Sato, B. Chi and H. Xu, Adv. Funct. Mater., 2017, 27, 1604894 CrossRef.
- T. Giesa, M. Arslan, N. M. Pugno and M. J. Buehler, Nano Lett., 2011, 11, 5038 CrossRef CAS PubMed.
- S. Bai, X. Zhang, Q. Lu, W. Sheng, L. Liu, B. Dong, D. L. Kaplan and H. Zhu, Biomacromolecules, 2014, 15, 3044 CrossRef CAS PubMed.
- J. Chen, F. K. C. Leung, M. C. A. Stuart, T. Kajitani, T. Fukushima, E. van der Giessen and B. L. Feringa, Nat. Chem., 2018, 10, 132 CrossRef CAS PubMed.
- B. Drucker and S. G. Smith, Nature, 1950, 165, 196 CrossRef CAS.
- A. E. Terry, D. P. Knight, D. Porter and F. Vollrath, Biomacromolecules, 2004, 5, 768 CrossRef CAS PubMed.
- J. Zhang, R. Hao, L. Huang, J. Yao, X. Chen and Z. Shao, Chem. Commun., 2011, 47, 10296 RSC.
- M. Shin, S. G. Park, B. C. Oh, K. Kim, S. Jo, M. S. Lee, S. S. Oh, S. H. Hong, E. C. Shin, K. S. Kim, S. W. Kang and H. Lee, Nat. Mater., 2017, 16, 147 CrossRef CAS PubMed.
- S. Bai, S. Liu, C. Zhang, W. Xu, Q. Lu, H. Han, D. L. Kaplan and H. Zhu, Acta Biomater., 2013, 9, 7806 CrossRef CAS PubMed.
- S. Manchineella, G. Thrivikraman, K. K. Khanum, P. C. Ramamurthy, B. Basu and T. Govindaraju, Adv. Healthcare Mater., 2016, 5, 1222 CrossRef CAS PubMed.
- S. Keten, Z. Xu, B. Ihle and M. J. Buehler, Nat. Mater., 2010, 9, 359 CrossRef CAS PubMed.
- J. Xie, Q. Zhang, T. Zhu, Y. Zhang, B. Liu, J. Xu and H. Zhao, Acta Biomater., 2014, 10, 2463 CrossRef CAS PubMed.
- P. Cai, B. Hu, W. R. Leow, X. Wang, X. J. Loh, Y. L. Wu and X. Chen, Adv. Mater., 2018, 30, 1800572 CrossRef PubMed.
- M. V. Lomova, A. I. Brichkina, M. V. Kiryukhin, E. N. Vasina, A. M. Pavlov, D. A. Gorin, G. B. Sukhorukov and M. N. Antipina, ACS Appl. Mater. Interfaces, 2015, 7, 11732 CrossRef CAS PubMed.
- A. K. Gaharwar, R. K. Avery, A. Assmann, A. Paul, G. H. Mckinley, A. Khademhosseini and B. D. Olsen, ACS Nano, 2014, 8, 9833 CrossRef CAS PubMed.
- X. Zhao, B. Guo, H. Wu, Y. Liang and P. X. Ma, Nat. Commun., 2018, 9, 2784 CrossRef PubMed.
- Z. Deng, T. Hu, Q. Lei, J. He, P. X. Ma and B. Guo, ACS Appl. Mater. Interfaces, 2019, 117, 6796 CrossRef PubMed.
- X. W. Huang, J. J. Wei, T. Liu, X. L. Zhang, S. M. Bai and H. H. Yang, Nanoscale, 2017, 9, 17193 RSC.
- Y. Liang, X. Zhao, P. X. Ma, B. Guo, Y. Du and X. Han, J. Colloid Interface Sci., 2019, 536, 224 CrossRef CAS PubMed.
- R. Dong, X. Zhao, B. Guo and P. X. Ma, ACS Appl. Mater. Interfaces, 2016, 8, 17138 CrossRef CAS PubMed.
- J. Qu, X. Zhao, Y. Liang, T. Zhang, P. X. Ma and B. Guo, Biomaterials, 2018, 183, 185 CrossRef CAS PubMed.
- X. Zhao, H. Wu, B. Guo, R. Dong, Y. Qiu and P. X. Ma, Biomaterials, 2017, 122, 34 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9nh00317g |
| This journal is © The Royal Society of Chemistry 2019 |